Abstract
3-Nitro-1H-1,2,4-triazole-based amides with a linear, rigid core and 3-nitrotriazole-based fluconazole analogs were synthesized as dual functioning antitrypanosomal agents. Such compounds are excellent substrates for type I nitroreductase (NTR) located in the mitochondrion of trypanosomatids and, at the same time, act as inhibitors of the sterol 14α-demethylase (T. cruzi CYP51) enzyme. Because combination treatments against parasites are often superior to monotherapy, we believe that this emerging class of bifunctional compounds may introduce a new generation of antitrypanosomal drugs. In the present work, the synthesis and in vitro and in vivo evaluation of such compounds is discussed.
Keywords: Bifunctional nitrotriazoles, carbinols, fuconazole analogs, T. cruzi CYP51, type I nitroreductase, antitrypanosomal agents, Chagas disease
Introduction
About one sixth of the world's population, most living in developing countries, suffer from a series of infections collective known as neglected tropical diseases (NTDs).1 In addition to killing more than 500,000 people each year, these illnesses impair physical and cognitive development of children, make it difficult to farm or earn a living, and limit productivity in the workplace. Two NTDs are American trypanosomiasis (also known as Chagas disease), which is causes by the protozoan parasite Trypanosoma cruzi and is endemic in 21 countries across Latin America, and human African trypanosomiasis (HAT) (also known as African sleeping sickness), which is prevalent across sub-Saharan Africa and caused by T. brucei rhodesiense and T. brucei gambiense Although the number of incidences of both infections has significantly declined in the past 20 years due to implementation of vector control initiatives, the diseases still remain a major global health issue, especially since the number of cases in non-endemic sites (United States, Australia, Europe and Japan) is rising, primarily due to international population migration and transfusion of contaminated blood.2-4
The therapeutic options for Chagas are currently limited to two drugs, both nitroheterocyclic compounds, nifurtimox (Nfx; Lampit®) and benznidazole (Bnz; Rochagan®), while the success of treatment depends on the phase of the disease. Thus, cure rates of 65-80% can be achieved in the acute phase, whereas only 15-30% cure rates have been reported among adults in the chronic stage.5, 6 Besides the low efficacy in patients with chronic disease, safety and tolerability issues are also major drawbacks for these drugs. Therefore, the development of new, safer and more effective therapeutic treatments remains a key challenge for Chagas disease control.
Currently, inhibitors of the fungal sterol 14α-demethylase enzyme (CYP51) represent a new form of treatment against Chagas disease and demonstrated promising efficacy in preclinical studies.7, 8 Additional pathogen-specific drug discovery efforts have resulted in inhibitors for the orthologous enzyme T. cruzi CYP51 (TcCYP51).9-12 One such compound, VNI, showed parasitological cures in both the acute and chronic in vivo Chagas model.11 However, the antifungal agent posaconazole revealed ∼80% treatment failure in clinical trials in human patients with chronic Chagas disease while Bnz was proven more efficacious.13 In addition, in newly designed in vitro assays, nitroheterocyclic compounds were more efficacious trypanocidal agents than four different CYP51 inhibitors, including antifungal drugs posaconazole and ravuconazole and two pyridine-based compounds,14 suggesting that inhibitors of sterol biosynthesis may not be as efficient as single chemotherapeutic agents against Chagas disease as they are against fungal infections. Interestingly, a recent study with Bnz and posaconazole administered concomitantly or in sequence suggested a positive interaction between the two drugs, at least in an acute murine infection model.15
We have recently demonstrated that another class of nitroheterocyclic compounds, 3-nitro-1H-1,2,4-triazoles, are very potent antichagasic agents both in vitro and in vivo, whereas several analogs showed very good in vitro efficacy against T. brucei rhodesiense as well.16-19 These studies clearly demonstrated that 3-nitrotriazole-based compounds are significantly more potent and less toxic than their 2-nitroimidazole-based counterparts,19, 20 with part of the trypanocidal activity being dependent on the parasite's expression of an oxygen-insensitive type I nitroreductase (NTR), an enzyme absent from most other eukaryotes.16, 17, 19 This same enzyme, via a series of 2 electron reduction reactions which lead to the production of toxic metabolites, is responsible for the trypanocidal activity of Nfx, Bnz and other nitroheterocyclic prodrugs in general.21-24
In this work we examined the possibility of synthesizing dual-functioning 3-nitrotriazole-based compounds; such compounds could potentially act as prodrugs via their reducible nitro-group and, at the same time, be inhibitors of CYP51 enzyme via their triazole-based scaffold.25 The synthesis of bifunctional agents has the advantage of combining the beneficial effects of nitroheterocyclics with the beneficial effects of ergosterol biosynthesis inhibitors in one molecule. To accomplish this task, we synthesized and evaluated in vitro and in vivo linear, rigid 3-nitrotriazole-based amides and 3-nitrotriazole-based carbinols (analogs of fluconazole), as potential antitrypanosomal/antichagasic agents, NTR substrates and TcCYP51 inhibitors.
Based on our previous work,16-19 we have realized that most 3-nitrotriazole-based analogs are very good substrates of NTR enzyme. Therefore, our goal was to obtain 3-nitrotriazole-based structures demonstrating affinity for CYP51. Early studies with 3-nitrotriazole-based amides having a flexible core showed lack of affinity for TcCYP51. Therefore, we assumed a more rigid, linear core should potentially provide a better fit in the active site of this enzyme. Thus, amides 2-8 were synthesized (Table 1). We chose the class of amides because they demonstrated better ADME properties than other 3-nitrotriazole-based compounds.19 Alternatively, the core of the CYP51 inhibitor fluconazole was used as a good starting point, and compounds 18-21 were synthesized (Table 1). Carbinols 14-17 were synthesized as structures that combine the linearity and rigidity of the amides 2-8 and share some of the features of the fluconazole core.
Table 1.
In vitro antiparasitic activity, host toxicity and physical properties of tested compounds.
| ID No | T.b.rhod.a IC-50 μM | SI | T.cruzib IC-50 μM | SI | Cytotox. L6c IC-50 μM | Chemical Structure | Bnz/comp | clogP | PSA (Å2) |
|---|---|---|---|---|---|---|---|---|---|
| Melars. | 0.007 ±0.001 | ||||||||
| Bnz | 2.18 ± 0.08 | ||||||||
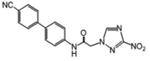
| 2 | 0.336 | 490 | 0.138 | 1194 | 164.7 |
|
10.77 | 2.75 | 129.42 |
| 3 | 4.060 | 24 | 0.102 | 973 | 98.8 |
|
7.24 | 3.23 | 118.52 |
| 4 | 73.870 | 16.58 | >16.5 | > 273.6 |
|
0.04 | 2.33 | 131.41 | |
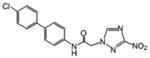
| 5 | 0.901 | 140 | 0.045 | 2797 | 125.9 |
|
45.1 | 2.716 | 108.87 |
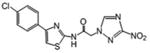
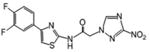
| 6 | 16.066 | 0.459 | 192 | 88.1 |
|
4.42 | 1.625 | 121.23 | |
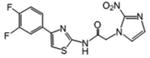
| 7 | 10 | 3.986 | 15.6 | 62.1 |
|
0.51 | 2.436 | 108.87 | |
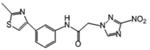
| 8 | 7.035 | 10 | 0.552 | 122 | 67.3 |
|
3.67 | 1.183 | 121.23 |
| 9 | 17.015 | 0.554 | 186 | 103.0 |
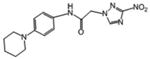
|
3.66 | 0.901 | 112.11 | |
| 10 | 34.067 | 1.194 | 205.6 | 245.5 |
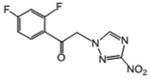
|
1.29 | 1.85 | 93.6 | |
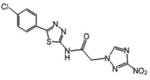
| 11 | 1.75 | 125 | 0.188 | 1164 | 219.5 |
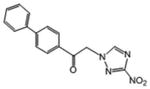
|
11.9 | 3.21 | 93.6 |
| 12 | 51.71 | 22.775 | 4.9 | 112.6 |
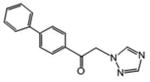
|
2.393 | 45.03 | ||
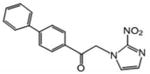
| 13 | 76.55 | 14.229 | 9.7 |
|
3.048 | 84.48 | |||
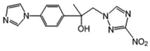
| 14 | 1.838 | >173 | 3.29 | >97 | >318.5 |
|
0.68 | 0.54 | 114.58 |
| 15 | 153.16 | >2.43 | 157.2 | >2.4 | >371.7 |
|
-0.09 | 68.76 | |
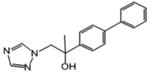
| 16 | 65.6 | 1.74 | 0.706 | 161.9 | 114.3 |
|
3.17 | 2.74 | 50.94 |
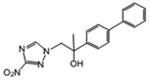
| 17 | 1.09 | 78.9 | 0.102 | 844 | 86.0 |
|
21.94 | 3.36 | 96.76 |
| 18 | 26.610 | 1.174 | 220 | 258.4 |
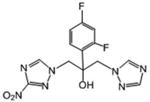
|
1.81 | 1.18 | 127.47 | |
| 19 | 2.877 | 44 | 0.033 | 3807.7 | 126.6 |
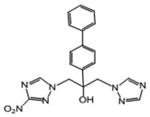
|
67.82 | 2.54 | 127.47 |
| 20 | 220.86 | 5.786 | >49.4 | > 286.0 |
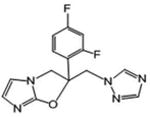
|
0.35 | 1.25 | 52.79 | |
| 21 | 11.596 | 0.339 | 428 | 145.1 |
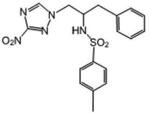
|
1.7 | 3.55 | 122.7 | |
| 22 | 76.25 | 3.64 | 187.5 | 1.48 | 277.3 |
|
0.77 | 30.35 | |
| Fluconazole | 9.967 | >29.51 | >294.1 |
|
0.56 | 81.65 | |||
| 23 | 1.99 | 122 | 0.438 | 556 | 243.5 |
|
3.6 | 1.74 | 122.7 |
T.b. rhodesiense, strain STIB 900 trypomastigotes;
T. cruzi, strain Tulahuen C4 amastigotes;
SI is the ratio: IC50 in L6 cells/IC50 in each parasite.
Cytotoxicity in host L6 cells.
Reference drugs: Melarsoprol (Melars) and Benznidazole (Bnz). The IC50 value of each reference drug is the mean from multiple measurements in parallel with the compounds of interest. Active and moderately active compounds with acceptable selectivity are colored green and pink, respectively; compounds with an unacceptable selectivity are colored blue whereas inactive compounds are colorless. PSA: polar surface area. All physical properties were predicted by using the Marvin Calculator (www.chemaxon.com). Values are means of 2 to 3 measurements. The SD was < 5%. Data for compound 23 are taken from ref. [17].
Results and Discussion
Chemistry
The structures of synthesized compounds are shown in Table 1. Their synthesis is straightforward and based on well-established chemistry outlined in Scheme 1. Thus, amides 2-9 were obtained by nucleophilic substitution of chloroacetamides 1a-g with the potassium salt of 3-nitrotriazole or 2-nitroimidazole under refluxing conditions. Similarly, nitro(triazole/ imidazole)-ketones 10-13 were synthesized by nucleophilic substitution of α-bromoketones. Carbinols 14-19 were obtained in one step by using a modified Corey-Chaykovsky reaction;26 in this one-pot reaction, trimethylsulfoxonium iodide was used in a 2-propanol: aqueous KOH (1:1) solution and the appropriate ketone was added to form in situ an oxirane; then 3-nitrotriazole was added followed by 1-h refluxing. Although the yields by using this method were low, the method is attractive due to its simplicity and short reaction time. The obtained carbinols 14-19 were used as racemic mixtures. When 2-nitroimidazole was used in the modified Corey-Chaykovsky reaction, the bicyclic compound 20 was formed as the final product, presumably via an intramolecular aromatic nucleophilic substitution of the nitro-group by the hydroxyl-group. The sulfonamide 21 was formed by nucleophilic attack of the sodium salt of 3-nitrotriazole with the appropriate aziridine-sulfonamide (Scheme 1).
Scheme 1. Preparation of compounds 2-21.
i) Et3N, CH2Cl2, RT; ii) 3-Nitro-1,2,4-triazole/2-nitroimidazole, KOH, CH3CN, reflux 9h; iii) 3-Nitro-1,2,4-triazole/2-nitroimidazole, KOH, CH3CN, α-bromoketone, reflux 5-6 h; iv) KOH in H2O:2-propanol, reflux 1h; v) NaH (60%), propanol-1, reflux 6 h.
Biological Evaluation
Anti-parasitic activity
Compounds were tested for antitrypanosomal activity against T. cruzi amastigotes and bloodstream form (BSF) of T. b. rhodesiense. Dose response curves were constructed from which the concentration of compound that inhibits parasite growth by 50 % (IC50) was calculated for each parasite (Table 1). In addition, compounds were tested for toxicity in L6 rat skeletal myoblasts, the host cells for T. cruzi amastigotes, in order to calculate a selectivity index for each parasite (SI = IC50L6/IC50parasite) (Table 1). The TDR (Special Programme for Research and Training in Tropical Diseases, World Health Organization) criteria for activity were adapted to interpret the data.27 Thus, for T. cruzi amastigotes, an IC50 of <4.0 μM, between 4.0-60 μM or >60 μM, designates ‘active’, ‘moderately active’ or ‘inactive’ compounds, respectively, whereas for BSF T. b. rhodesiense, IC50 values of <0.5 μM, between 0.5-6.0 μM or > 6.0 μM identify ‘active’, ‘moderately active’ or ‘inactive’ compounds, respectively. In addition, SI values should ideally be ≥50 and ≥100 for T. cruzi and T. b. rhodesiense, respectively.27
All the 3-nitrotriazole-based rigid amides (2, 3, 5, 6, 8, 9) except 4, and all the 3-nitrotriazole-based carbinols (14, 17-19), as well as the fluconazole-like 3-nitrotriazole-based sulfonamide 21, were active antichagasic agents with acceptable selectivity indices (Table 1). Only 3 compounds (two 3-nitrotriazole-based amides and one 3-nitrotriazole-based carbinol) were active (2) or moderately active (5, 14) anti-HAT agents with acceptable SI values. In addition, ketone 11, the precursor of carbinol 19 showed a moderate anti-HAT activity with acceptable selectivity. As observed before, compound 7, the 2-nitroimidazole-based analog of 6, was borderline active against T. cruzi amastigotes but with an unacceptable SI value (Table 1).
Analysis of antichagasic activity
Linear, rigid amides 2-9
Although a detailed SARs evaluation cannot be conducted due to the limited number of compounds studied, we made the following observations: A chloro-substituent in the para position of a diphenyl-core in the rigid amide 5 increased the efficacy against T. cruzi by a factor of 3 compared to the cyano-substituted analog 2. Although 5 was slightly more toxic to L6 cells than compound 2, its SI for T. cruzi was also increased about 2-fold compared to 2 due to its better anti-chagasic efficacy; this cannot be attributed to lipophilicity since both compounds share similar clogP values, but perhaps to its lower PSA value (Table 1). Chloro-substitution in the phenylthiazole-core of 3 was also beneficial for its antichagasic activity and selectivity compared to the difluoro-substitution in the analog 6. However, in this case, increased antichagasic efficacy may be related to the increased lipophilicity of 3 compared to 6. Reversing the order between the thiazole and phenyl rings in amide 8 slightly decreased antichagasic activity compared to 6 and once again, this reduction may be related to slightly lower lipohilicity compared to 6. Furthermore, this reversal lead to a slightly more toxic compound (compare 8 to 3 and 6). Replacing the terminal phenyl ring in the biphenyl core of 2 or 5 with a piperidinic group in 9, resulted in reduced efficacy against T. cruzi, which seems to be attributed to a decreased clogP value (Table 1). Compound 4 with a thiodiazole ring in the rigid core failed to show either good antichagasic activity or acceptable selectivity. However, compound 4 has significant solubility problems and may have not been evaluated properly. Although compound 7, the 2-nitroimidazole analog of 6, showed borderline antichagasic activity according to the TDR criteria, it was the most toxic rigid amide in L6 cells and failed to show an acceptable SI for T. cruzi. Compound 7 was about 8.7-fold less active against T. cruzi compared to its 3-nitrotriazole-analog 6, even though the latter was 1.5 times less lipophilic, suggesting that the reason for the reduced antichagasic activity of 7 was related to the 2-nitroimidazole ring. All 3-nitrotriazole-based rigid amides, except 4, were more potent antichagasic agents than Bnz, with compound 5 being 45-fold more potent than Bnz (Table 1).
Carbinols and their precursors
With regard to the carbinols in Table 1, which were racemic mixtures, we observe the following: Lipophilicity and the presence of the nitro-group in the triazole-ring played a significant role in the antichagasic activity. Thus, the 3-nitrotriazole-based carbinol 14, with a terminal imidazole ring in the core, was significantly less lipophilic than carbinol 17, with a terminal phenyl ring in the core, and 32-fold less potent against T. cruzi amastigotes than 17 (Table 1). On the other hand, carbinol 15, the analog of 14 without the 3-nitro group in the triazole ring, had a negative clogP value and was inactive against T. cruzi. Interestingly, carbinol 16, the analog of 17 without the 3-nitro group in the triazole ring, was still active against T. cruzi, most likely because of its relatively high lipophilicity. However, the lack of the nitro-group resulted in a 7-fold reduction in antichagasic potency compared to 17. Carbinol 18 is the mono-nitrotriazole analog of fluconazole. This compound was 36-fold less potent than its more lipophilic analog 19, which has an additional phenyl ring in the side chain. However, compound 18 was 8.5-fold more potent than fluconazole, which is less lipophilic and lacks the nitro-group (Table 1). Carbinol 19 was the most potent antichagasic compound tested and demonstrated the highest SI value. In addition, 19 was about 68-fold more potent than Bnz (Table 1). The bicyclic compound 20 was formed in situ from the corresponding 2-nitroimidazole analog of fluconazole presumably by intramolecular nucleophilic aromatic substitution of the nitro-group by the hydroxyl group. This compound, although slightly more lipophilic than 18, was about 5-fold less potent against T. cruzi, presumably because it lacks the nitro group. In addition, compound 20 was moderately active and marginally selective (SI > 49.4) for T. cruzi parasite. In compound 21 we replaced the hydroxyl-group with a tosylamino-group. Compound 21 was selectively active against T. cruzi, but its potency was about 3-fold less compared to carbinol 17 with a similar lipophilicity. An increased PSA value compared to 17 may have played a role for this reduction in this case.
Some ketones, precursors of the carbinols in Table 1, were also evaluated for antichagasic activity. Interestingly, the 3-nitrotriazole-based ketones 10 and 11, precursors of carbinols 18 and 19, respectively, were found to be selectively active against T. cruzi (Table 1). The pattern of lipophilicity and the presence of the nitro-group in the triazole ring were consistent with our predictions for efficacy. Thus, the more lipophilic 11 was more potent than 10, whereas ketone 12, the analog of 11 without the nitro-group, was only moderately active against T. cruzi and did not fulfill the TDR selectivity criteria. Once again, the 2-nitroimidazole analog of 11, ketone 13, was only moderately active against T. cruzi parasites, but also the most toxic compound to the host cells. From this evaluation, it appears that 3-nitrotriazole-based ketones represent another class of antichagasic agents. Finally, compound 22, the precursor of carbinols 14 and 15, was inactive against T. cruzi (Table 1).
Analysis of anti-HAT activity
Linear, rigid amides 2-9
With regard to the anti-HAT activity of amides 2-9, biphenyl amides 2 and 5 (which were active and moderately active, respectively) were more potent against T. b. rhodesiense than phenylthiazole-amides 3, 6, 7 and 8. In fact, none of the latter demonstrated an acceptable selective anti-HAT activity (Table 1). The phenyl-thiodiazoleamide 4 did not show any anti-HAT activity, in part due to solubility issues as mentioned earlier, whereas the piperidinophenylamide 9 was inactive, perhaps because of planarity issues in the side chain. Interestingly, the 2-nitroimidazole-based phenylthiazole-amide 7 demonstrated a lower IC50 value against T. b. rhodesiense than its 3-nitrotriazole-based analog 6, possibly due to the higher lipophilicity of the first. Nonetheless, both were inactive against this parasite.
Carbinols and their precursors
Only carbinol 14 was moderately active with acceptable selectivity against T. b. rhodesiense. Carbinol 17 with increased lipophilicity was more active than 14 but not selective enough for this parasite. Similarly, carbinol 19 was more active than the less lipophilic analog 18, but also lacked an acceptable selectivity for T. b. rhodesiense. Linear, rigid carbinols lacking the nitro-group (15, 16) were inactive against T. b. rhodesiense. Interestingly, the precursor of carbinol 19, the lipophilic enough 3-nitrotriazole-based ketone 11, was moderately active and had an acceptable selectivity for T. b. rhodesiense.
Involvement of type I Nitroreductase
Representative 3-nitrotriazole-based linear rigid amides and carbinols, with IC50 values against T. cruzi ranging from 33 nM to 3.29 μM, were evaluated as substrates of recombinant TbNTR and TcNTR enzymes (Table 2). Enzyme specific activity was measured as oxidized NADH per min per mg of protein. All tested nitrotriazoles were excellent substrates of Tb and TcNTR enzymes. Thus, tested nitrotriazoles were metabolized by Tb and TcNTR at rates approximately 2-fold higher than Bnz, whereas TbNTR can metabolize nitrotriazoles (and Bnz) at rates 2 to 3-fold greater than those of TcNTR. In addition, blood stream form T. b. brucei parasites overexpressing TbNTR were 2 to 3-fold more susceptible to compound 2 compared to parasites not induced to express elevated levels of TbNTR (IC50: 0.36 ± 0.01 vs 0.84 ± 0.16). However, no correlation existed between parasitic IC50 values (Table 1) and activation rate by TcNTR or TbNTR, suggesting that antiparasitic activity was not exclusively dependent on NTR-induced activation.
Table 2.
Enzymatic activity of recombinant TbNTR and TcNTR toward Bnz and representative 3-nitrotriazoles from Table 1.
| compound | Specific activity values (nmol NADH oxidized per min per mg NTR) | |
|---|---|---|
|
| ||
| TbNTR | TcNTR | |
| Bnz | 1540 ± 67 | 680 ± 8 |
| 2 | 3172 ± 11 | 888 ± 24 |
| 14 | 3456 ± 6 | 1067 ± 16 |
| 18 | 3063 ± 43 | 1178 ± 10 |
| 19 | 2681 ± 21 | 1160 ± 19 |
A precipitation issue occurred with compound 2 in the assay buffer used. Enzyme assays were carried out in triplicate and repeated twice.
TcCYP51 inhibition
Azole-based scaffolds are effective inhibitors of the T. cruzi enzyme sterol 14α-demethylase (TcCYP51).25 By using eburicol as substrate, TcCYP51 is a central enzyme in parasitic sterol biosynthesis, an essential pathway in all life stages of T. cruzi for the formation of ergosterol and related structures from lanosterol.28, 29 Being azoles, our compounds could be potential inhibitors of TcCYP51. Moreover, reduction of the nitro group by P450 reductase (which normally reduces the Fe3+ to Fe2+ in the heme cofactor of CYP51) could potentially cause irreversible binding to the protein. Benznidazole was found to be an irreversible inhibitor of TcCYP51, although with weak affinity for the enzyme.30 Binding of a hererocyclic compound to CYP51 can be quantified spectroscopically by measuring a dose-response type 2 shift that occurs upon coordination of a Lewis-base atom of the ligand to the heme iron of the enzyme.9 The first 3-nitrotriazole-based compound we tested as a potential TcCYP51 ligand was chlorothiophene-2-sulfonamide 23, with a flexible core (Table 1). The biological properties of 23 have been described before17 and are included in Table 1 for reference. This compound displayed a week affinity toward TcCYP51, providing a high Kd value of 65.2 ± 5.3 (Table 3). Therefore, we assumed that compounds with a more rigid core could better fit in the active site of the enzyme and thus act as stronger inhibitors. Indeed, compound 2 with a linear rigid core inhibited the enzyme with an initial I/E2 ratio (molar ratio of inhibitor/enzyme which causes a 2-fold decrease in enzyme activity) of 4 in a 5 min reaction. However, this ratio was increased to 36 in the 1 h reaction, indicating that inhibition by 2 was reversible. The 3-nitro-triazole-fluconazole analog 18 binds to TcCYP51 and induces the same typical type 2 spectral response as fluconazole (Fig. 1), indicating at least initial coordination of the triazole N4 to the P450 heme iron.9 However, 18 was a weaker binder than fluconazole (Kd 0.606 versus 0.230 μM) (Table 3). To the contrary, carbinol 19, an analog of fluconazole with an elongated rigid side chain, elicited the spectral response in TcCYP51 with an apparent Kd 10-fold stronger than fluconazole. In the reconstituted CYP51 reaction, 19 produced I/E2 ratios of <1 and 3, in 5 min and 1 h reactions, respectively (Table 3 and Fig. 2). Furthermore, for carbinol 19 at high I/E ratios (≥ 20), the inhibition seems to be irreversible, although further experiments are needed for verification (Fig. 2). Correlation exists between in vitro antichagasic potency and inhibition of TcCYP51 for the nitro-compounds 2, 19 and 23. However, compound 18 was more potent in killing T. cruzi amastigotes than fluconazole (Tables 1), although the latter was a stronger TcCYP51 inhibitor (Table 3). This might be related to the nitro-group of 18, making this compound bifunctional. Crystallography studies could shed more light on the orientation of these bifunctional agents in the TcCYP51 active site.
Table 3. Binding of compounds to recombinant T. cruzi CYP51.
| Compound | Kd (μM) | I/E2 (5 min) | I/E2 (1 h) |
|---|---|---|---|
| 2 | ND | 4 | 36 |
| 18 | 0.606 ± 0.023 | 5 | 32 |
| 19 | 0.023 ± 0.012 | <1 | 3 |
| 23 | 65.2 ± 5.3 | ND | ND |
| Fluconazole | 0.230 ± 0.029 | <1 | 16 |
| Bnz* | ND | 43.2 | 45 |
Kd: Dissociation constant. I/E2: Molar ratio of inhibitor/enzyme which causes a 2-fold decrease in enzyme activity in 5 min and 1 hour reactions. ND: Not determined.
Values for Bnz were taken from Ref. [30].
Fig 1.
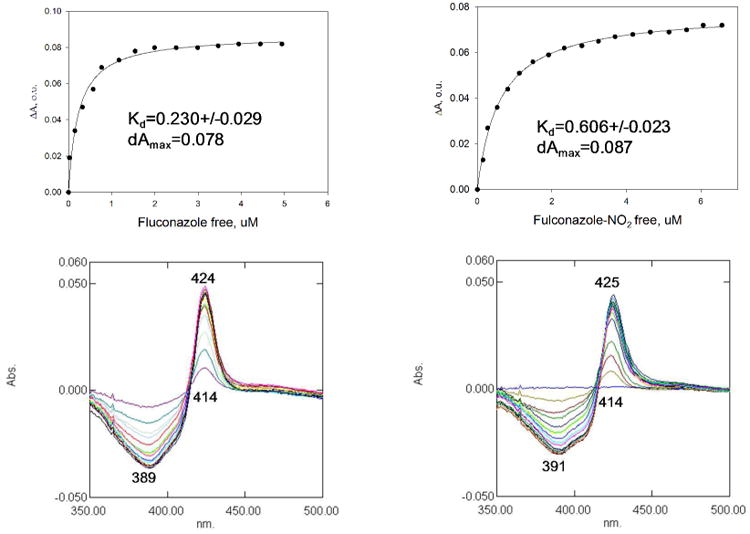
Binding of fluconazole and compound 18 (NO2-fluconazole) to T. cruzi CYP51. Both compounds produce typical type 2 spectral response in T. cruzi CYP51, indicating at least initial coordination of triazole N4 to the P450 heme iron. However, compound 18 is a weaker binder.
Fig 2.
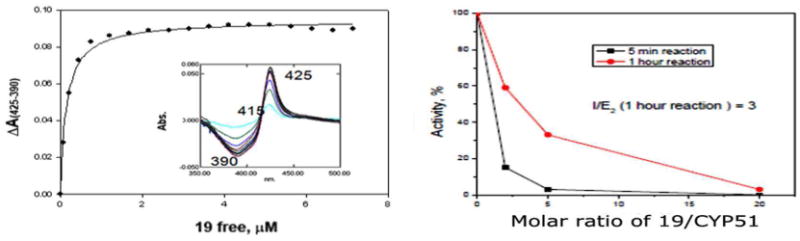
Binding (left panel) and inhibition (right panel) of T. cruzi CYP51 by compound 19 in 5 min and 1 h reactions. The same results were obtained independently of the sequence that substrate eburicol (S) or inhibitor (I) were added, namely: Reaction mixture + I/+NADPH, 5 min incubation at 37°C /+S or, Reaction mixture + I +S /+NADPH.
In vivo antichagasic activity
Compounds 2, 5, 18 and 19 were evaluated for in vivo anti-chagasic activity by using an acute T. cruzi infected murine model as described before.19 Bnz was used in parallel as a positive control. Infected mice (5 mice/group) were treated i.p. for up to 10 consecutive days with each compound, at 15 mg/kg/day, except for compound 5 which was administered at 13 mg/kg/day. The Parasite Index (PI), which is an indicator for parasite clearance, was calculated after 5 and 10 days of treatment.19 The data from these studies are summarized in Fig. 3. All tested compounds reduced the PI to < 4% after 10-day treatment with statistical significance compared to the 10-day untreated control. The linear rigid amides 2 and 5 were able to reduce parasites to undetectable levels even after 5 days of treatment. However, only compound 5 yielded a statistically significant different PI value compared to untreated control after 5 days of treatment (P<0.0001), whereas for Bnz and compound 2 (treated in the same experiment) the corresponding P value was 0.080 and 0.0819, respectively. Carbinols 18 and 19 provided a PI value of 24.9 and 42.04, respectively, after 5-day administration but without statistical significance compared to the untreated control (P = 0.1680 and 0.2706, respectively). Bnz in this latter experiment provided a PI value of 13.38 after 5-day treatment, also not statistically different from the untreated control (P = 0.2706). None of the tested compounds was toxic to mice at the given doses and administration schedule.
Fig 3.
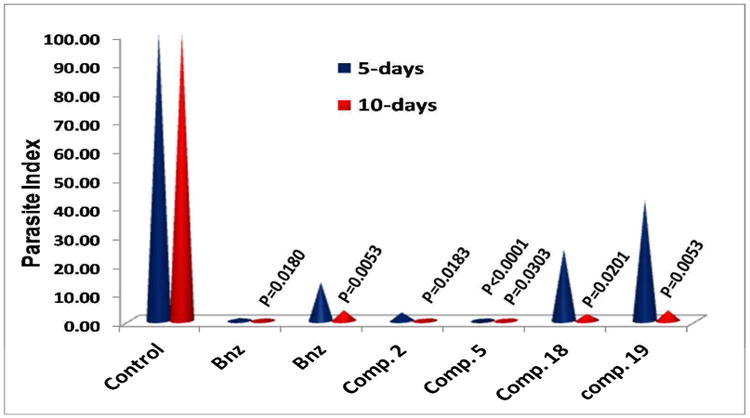
In vivo evaluation of the antichagasic efficacy of compounds 2, 5, 18 and 19 in the acute murine model. Benznidazole (Bnz) data from 2 experiments in parallel were included. All compounds except 5 were administered (i.p.) at 15 mg/kg/day. Compound 5 was administered (i.p.) at 13 mg/kg/day. The P values refer to comparisons between treated groups and control group. When P values are not given, there was no statistical difference. Groups of 5 mice/group were used. Occasionally, 10 mice were used in the control group.
In vitro ADME studies
To understand better the in vivo activity of compounds 2 and 19 and explain the discrepancy between in vitro and in vivo potency, we performed some in vitro ADME studies (Table 4). Compound 2 represents the linear rigid amides and 19 the carbinols. Both compounds demonstrate high Caco 2 permeability31 and similar metabolic stability in the presence of murine or human microsomes. Both compounds were quite stable, explaining their good in vivo activity after 10-day dosing. The fact that compound 19 was acting slower than 2 may be related to its stronger binding to TcCYP51, a membrane protein, whereas type I NTR is mitochondrial based. Thus, if compound metabolites formed through NTR-activation are the most toxic chemical species for T. cruzi, compound 2 would be expected to act faster than 19. Obviously, additional pharmacokinetic parameters (such as volume distribution and protein binding) which can affect the in vivo behavior of these compounds cannot be excluded.
Table 4.
In vitro ADME data for compounds 2 and 19.
| Compound | mean A->B Papp (×10-6cm s-1) | Test species | Mean remaining parent, with NADPH (%) | Mean remaining parent, free of NADPH (%) |
|---|---|---|---|---|
| 2 | 18.7 | Human | 96.8 | 84.8 |
| Mouse | 79.4 | 110 | ||
| 19 | 16.5 | Human | 90.7 | 106 |
| Mouse | 77.6 | 102 | ||
| Verapamile | Human | 8.4 | 89.4 | |
| Mouse | 6.0 | 96.5 | ||
| Warfarin | 25.3 | Human | 102 | 96.7 |
| Mouse | 104 | 101 | ||
| Ranitidine | 0.24 |
Papp: Caco 2 apparent permeability. Permeability ranking: Low: <0.5; Moderate: 0.5 - 5; High: > 5. For permeability measurements 10 μM concentration was used and 2 h assay time. For metabolic stability, 1 μM concentration and 0 and 60 min incubation time was used. Test species represent either human or mouse microsomes.
It is concluded from the above data that 3-nitrotriazole-based linear rigid amides and carbinols are potent antichagasic agents via their dual functioning properties as substrates for trypanosomal type I NTR and as inhibitors of CYP51. In addition, these compounds demonstrate excellent Caco 2 permeability and metabolic stability in the presence of human or murine microsomes. Carbinols were slower in clearing the parasites in vivo compared to the linear rigid amides, however, both type of compounds were able to clear T. cruzi after 10-day treatment. This is apparent in the images shown in Fig. 4. We have also shown previously that 3-nitrotriazole-based compounds are not mutagenic compared to their 2-nitroimidazole-based analogs,19 and do not cause developmental toxicity in zebrafish.20
Fig 4.
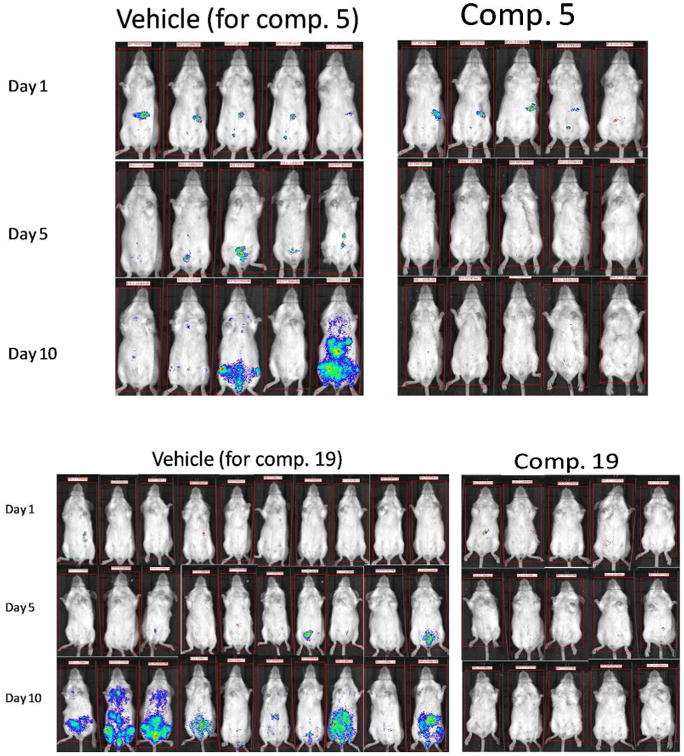
Images from mice treated with vehicle or compounds 5 and 19.
In comparison with some monofunctional 3-nitrotriazole-based analogs, there is no substantial difference in antichagasic in vitro activity. Thus, we have seen in vitro IC50 values against T. cruzi amastigotes at low nM concentrations with several 3-nitrotriazole-based amides/sulfonamides or piperazines which, having a more flexible core, did not demonstrate affinity for TcCYP51.17-19 In the present work, the bifunctional compounds which have been tested in vivo were able to completely clear the parasites after 10-day treatment, something that was not always achievable with monofunctional 3-nitrotriazole-based derivatives.19 However, since in vitro activity does not always translate to in vivo efficacy, due to pharmacokinetic/ pharmacodynamic factors,19 and since activity in the acute model does not guarantee cures, future experiments in a chronic murine model are necessary to determine the validity of these bifunctional compounds for the treatment of Chagas disease.
Experimental
Chemistry
General
All starting materials and solvents were purchased from Sigma-Aldrich (Milwaukee, WI), were of research-grade quality and used without further purification. Solvents used were anhydrous and the reactions were carried out under a nitrogen atmosphere and exclusion of moisture. Melting points were determined by using a Mel-Temp II Laboratory Devices apparatus (Holliston, MA) and are uncorrected. Proton NMR spectra were obtained on a Varian Inova-500 or an Agilent Hg-400 spectrometer at 500 or 400 MHz, respectively, and were referenced to Me4Si or to the corresponding solvent if the solvent was not CDCl3. High-resolution electrospray ionization (HRESIMS) mass spectra were obtained on a Agilent 6210 LC-TOF mass spectrometer at 11000 resolution. Thin-layer chromatography was carried out on aluminum oxide N/UV254 or polygram silica gel G/UV254 coated plates (0.2 mm, Analtech, Newark, DE). Chromatography was carried out on preparative TLC alumina GF (1000 microns) or silica gel GF (1500 microns) plates (Analtech). All compounds were purified by preparative TLC chromatography on silica gel or alumina plates and also checked by HPLC (≥ 95% purity).
Synthesis of chlorides 1a-g
Some of the chlorides 1a-g are known in the literature and others are unknown or known through libraries but without any reference. The synthesis and spectroscopic data of 1a-g are provided in the supporting material.
General Synthetic Procedure of amides 2-9
The potassium salt of 3-nitro-1,2,4-triazole or 2-nitroimidazole (1 eq) was formed in CH3CN (6-10 mL), by refluxing with KOH (1.2 eq) for 30 min. To this suspension 1a-g (1.1 eq) was added and the reaction mixture was refluxed under a nitrogen atmosphere for 9 h. In certain cases, 1a-g was added in CH3CN solution. The reaction mixture was checked by TLC for completion of the reaction and the solvent was evaporated. The residue was dissolved in ethyl acetate and the inorganic salts were filtered away. Upon preparative TLC (silica gel or alumina, depending on the mobility of the major band; ethyl acetate-petroleum ether), the desired product was obtained usually as a powder. Purity was checked also by HPLC and it was > 95%.
N-[4-(4-cyanophenyl)phenyl]-2-(3-nitro-1H-1,2,4-triazol-1-yl)acetamide (2)
White microcrystallic powder (80%): mp 235 °C (dec); 1H NMR (500 MHz, (CD3)2CO) δ: 9.84 (br s, 1H), 8.76 (s, 1H), 7.90-7,75 (m, 8H), 5.46 (s, 2H). HRESIMS calcd for C17H13N6O3 and C17H12N6NaO3 m/z [M+H]+ and [M+Na]+ 349.1044 and 371.0863, found 349.1056 and 371.0873.
N-[4-(4-chlorophenyl)-1,3-thiazol-2-yl]-2-(3-nitro-1H-1,2,4-triazol-1-yl)acetamide (3)
White powder (64%): mp > 235 °C; 1H NMR (500 MHz, (CD3)2CO) δ: 8.82 (s, 1H), 7.95 (d, J=8.5 Hz, 2H), 7.64 (s, 1H), 7.46 (d, J=8.5 Hz, 2H), 5.70 (s, 2H). HRESIMS calcd for C13H10ClN6O3S and C13H9ClN6NaO3S m/z [M+H]+ and [M+Na]+ 365.0218, 367.0191 and 387.0038, 389.0011, found 365.0231, 367.0200 and 387.0038, 389.0012.
N-[5-(4-chlorophenyl)-1,3,4-thiadiazol-2-yl]-2-(3-nitro-1H-1,2,4-triazol-1-yl) acetamide (4)
White powder (63%): mp > 235 °C; 1H NMR (500 MHz, DMSO-6d) δ: 8.93 (s, 1H), 7.96 (d, J=8.5 Hz, 2H), 7.60 (d, J=8.5 Hz, 2H), 5.57 (s, 2H). HRESIMS calcd for C14H11ClN4O4S m/z [M+H]+ 366.0184, 368.0148, found 366.0178, 368.0158.
N-(4′-chloro-[1,1′-biphenyl]-4-yl)-2-(3-nitro-1H-1,2,4-triazol-1-yl)acetamide (5)
Off white powder (77%): mp > 245 °C; 1H NMR (500 MHz, CD3OD + a drop of DMSO-6d) δ: 8.73 (s, 1H), 7.69 (d, J=9.0 Hz, 2H), 7.61 (dd, J=9.0, 4.0 Hz, 4H), 7.43 (d, J=8.0 Hz, 2H), 5.32 (s, 2H). HRESIMS calcd for C16H11ClN5O3 m/z [M-H]- 356.05556, found 356.0563.
N-(4-(3,4-difluorophenyl)thiazol-2-yl)-2-(3-nitro-1H-1,2,4-triazol-1-yl)acetamide (6)
White powder (60%): mp 169-171 oC; 1H NMR (500 MHz, (CD3)2CO) δ: 11.71 (br s, 1H), 8.80 (s, 1H), 7.83 (ddd, J=14, 7.5, 2 Hz, 1H), 7.76 (m, 1H), 7.64 (s, 1H), 7.37 (dd, J= 19, 8.5 Hz, 1H), 5.69 (s, 2H). HRESIMS calcd for C13H9F2N6O3S m/z [M+H]+ 367.0419, found 367.0426.
N-(4-(3,4-difluorophenyl)thiazol-2-yl)-2-(2-nitro-1H-imidazol-1-yl)acetamide (7)
Off white powder (55%): mp 180-183 °C (dec); 1H NMR (500 MHz, (CD3)2CO) δ: 11.70 (br s, 1H), 7.82 (ddd, J=12, 7.5, 2.5 Hz, 1H), 7.77 (m, 1H), 7.63 (s, 1H), 7.61 (s, 1H), 7.36 (ddd, J= 16.5, 10.5, 8.5 Hz, 1H), 7.22 (s, 1H), 5.70 (s, 2H). HRESIMS calcd for C14H10F2N5O3S and C14H9F2N5NaO3S m/z [M+H]+ and [M+Na]+ 366.0467 and 388.0286 found 366.0466 and 388.0281.
N-(3-(2-methylthiazol-4-yl)phenyl)-2-(3-nitro-1H-1,2,4-triazol-1-yl)acetamide (8)
White, sticky solid (73%): mp 114-115 °C (dec); 1H NMR (400 MHz, CDCl3 + 2 drops DMSO-6d) δ: 10.39 (s, 1H), 8.61 (s, 1H), 8.11 (s, 1H), 7.62 (d, J=7.6 Hz, 2H), 7.39 (s, 1H), 7.36 (t, J=8.0 Hz, 1H), 5.27 (s, 2H), 2.75 (s, 3H). HRESIMS calcd for C14H13N6O3S m/z [M+H]+ 345.0764, found 345.0776.
2-(3-nitro-1H-1,2,4-triazol-1-yl)-N-(4-(piperidin-1-yl)phenyl)acetamide (9)
Orange microcrystals (79%): mp 180-182 °C (dec); 1H NMR (400 MHz, CDCl3 + 1 drop DMSO-6d) δ: 9.19 (br s, 1H), 8.44 (s, 1H), 7.37 (d, J=9.0 Hz,2H), 6.87 (d, J=9.0 Hz, 2H), 5.10 (s, 2H), 3.10 (t, J=5.5 Hz, 4H), 1.68 (m, 4H), 1.57 (m, 2H). HRESIMS calcd for C15H19N6O3 m/z [M+H]+ 331.1513, found 331.1523.
General Synthetic Procedure of ketones 10-13
As in the case of amides 2-9, the potassium salt of 3-nitro-1,2,4-triazole or 2-nitroimidazole or 1,2,4-triazole (1 eq) was formed in CH3CN (6-10 ml), by refluxing with KOH (1.2 eq) for 30 min and the appropriate α-bromoketone (1 eq) was added to this suspension. The reaction mixture was refluxed under nitrogen atmosphere for 5-6 h. The procedure for separation of the desired ketones 10-13 was analogous to that used for amides 2-9.
1-(2,4-difluorophenyl)-2-(3-nitro-1H-1,2,4-triazol-1-yl)ethan-1-one (10)
White crystals (85%): mp 102-104 °C; 1H NMR (500 MHz, CDCl3) δ: 8.30 (s, 1H), 8.09 (dd, J=9.0, 7.0 Hz, 1H), 7.10 (td, J=9.5, 2.5 Hz, 1H), 7.02 (td, J=9.0, 2.5 Hz, 1H), 5.69 (d, J=3.0 Hz, 2H). HRESIMS calcd for C10H7F2N4O3 m/z [M+H]+ 269.0481, found 269.0487.
1-(4-phenylphenyl)-2-(1H-1,2,4-triazol-1-yl)ethan-1-one (11)
White powder (95%): mp 175-177 °C; 1H NMR [500 MHz, (CD3)2CO] δ: 8.67 (s, 1H), 8.16 (d, J=8.5, 2H), 7.84 (d, J=8.5, 2H), 7.70 (d, J=7.5, 2H), 7.48 (t, J=7.5, 2H), 7.41 (t, J=7.5, 1H), 6.15 (s, 2H). HRESIMS calcd for C16H13N4O3 and C16H12N4NaO3 m/z [M+H]+ and [M+Na]+ 309.0982 and 331.0802 found 309.0983 and 331.0804.
1-(4-phenylphenyl)-2-(1H-1,2,4-triazol-1-yl)ethan-1-one (12)
White microcrystals (65%; higher yield based on recovered ketone): mp 159-161 °C; 1H NMR (400 MHz, CDCl3) δ: 8.28 (s, 1H), 8.07 (d, J=8.8 Hz, 2H), 8.03 (s, 1H), 7.76 (d, J=8.8 Hz, 2H), 7.64 (d, J=6.8 Hz, 2H), 7.52-7.44 (m, 3H), 5.71 (s, 2H). HRESIMS calcd for C16H14N3O m/z [M+H]+ 264.1131, found 264.1154.
2-(2-nitro-1H-imidazol-1-yl)-1-(4-phenylphenyl)ethan-1-one (13)
White powder (60 %): mp 163-164 °C; 1H NMR [500 MHz, (CD3)2CO] δ: 8.20 (d, J=9.0 Hz, 2H), 7.92 (d, J=9.0 Hz, 2H), 7.78 (d, J=7.0 Hz, 2H), 7.57 (d, J=1.0 Hz, 1H), 7.53 (t, J=7.5 Hz, 2H), 7.46 (t, J=7.5 Hz, 1H), 7.23 (d, J=1.0 Hz, 1H), 6.26 (s, 2H). HRESIMS calcd for C17H14N3O3 m/z [M+H]+ 308.1030, found 308.1028.
General Synthetic Procedure of carbinols 14-19 and compound 20
The Corey-Chaykovsky reaction was applied for this synthesis and a one-pot reaction was adapted from an international patent26 because of its short time, albeit with low yields. Briefly, in a 2-necked 25 mL round bottom flask, KOH (2.4 eq) was dissolved in 3 mL distilled water and an equal volume of 2-propanol was added. Trimethylsulfoxonium iodide (1.2 eq) was added and stirred for 15 min under a nitrogen atmosphere. Then, the appropriate ketone (1 eq) was added and stirring was continued for 15 min. Finally 3-nitro-1,2,4-triazole or 2-nitroimidazole or 1,2,4-triazole (1.2 eq) was added and the reaction mixture was refluxed for 1 h. 2-Propanol was evaporated and the aqueous solution was neutralized with 1% HCl. This solution was then extracted with dichloromethane and the organic layer washed with NaHCO3 solution. The organic layer was dried over Na2SO4 and chromatographed by preparative TLC (silica gel, ethyl acetate plus 1% MeOH). When 2-nitroimidazole was used in the reaction mixture, a nucleophilic aromatic substitution of the nitro-group by the hydroxyl took place and the bicyclic-compound 20 was obtained as the final product.
2-[4-(1H-imidazol-1-yl)phenyl]-1-(3-nitro-1H-1,2,4-triazol-1-yl)propan-2-ol (14)
Off white powder (15%): mp 133-134 °C; 1H NMR [500 MHz, (CD3)2CO] δ: 8.47 (s, 1H), 8.06 (s, 1H), 7.72 (d, J=8.5 Hz, 2H), 7.60 (d, J=8.5 Hz, 2H), 7.58 (s, 1H), 7.10 (s, 1H), 5.09 (s, 1H), 4.69 (d, J=4.5 Hz, 2H), 1.68 (s, 3H). HRESIMS calcd for C14H15N6O3 m/z [M+H]+ 315.1200, found 315.1207.
2-[4-(1H-imidazol-1-yl)phenyl]-1-(1H-1,2,4-triazol-1-yl)propan-2-ol (15)
White powder (32%): mp 138-140 °C; 1H NMR [500 MHz, (CD3)2CO] δ: 8.20 (s, 1H), 8.05 (s, 1H), 7.80 (s, 1H), 7.66 (d, J=8.5 Hz, 2H), 7.56 (d, J=8.5 Hz, 2H), 7.10 (s, 1H), 4.89 (s, 1H), 4.54 (d, J=4.0 Hz, 2H), 1.57 (s, 3H).HRESIMS calcd for C14H16N5O m/z [M+H]+ 270.1349, found 270.1363.
2-(4-phenylphenyl)-1-(1H-1,2,4-triazol-1-yl)propan-2-ol (16)
White powder (37%; based on recovered ketone the yield was 50%): mp 118-120 °C; 1H NMR [500 MHz, (CD3)2CO] δ: 1H 8.20 (s, 1H), 7.81 (s, 1H), 7.67-7.61 (m, 6H), 7.45 (t, J=7.5 Hz, 2H), 7.35 (t, J=7.5 Hz, 1H), 4.79 (s, 1H), 4.43 (s, 2H), 1.55 (s, 3H). HRESIMS calcd for C17H18N3O m/z [M+H]+ 280.1444, found 280.1453.
1-(3-nitro-1H-1,2,4-triazol-1-yl)-2-(4-phenylphenyl)propan-2-ol (17)
White powder (19%): mp was not determined; 1H NMR (500 MHz, CDCl3) δ: 8.17 (s, 1H), 7.63-7.58 (m, 4H), 7.51-7.44 (m, 4H), 7.38 (t, J=7.0 Hz, 1H), 4.52 (d, J=3.5 Hz, 2H), 1.67 (s, 3H). HRESIMS calcd for C17H16N4NaO3 m/z [M+Na]+ 347.1115, found 347.1124.
2-(2,4-difluorophenyl)-1-(3-nitro-1H-1,2,4-triazol-1-yl)-3-(1H-1,2,4-triazol-1-yl)-propan-2-ol (18)
White powder (25%): mp 154-156 °C; 1H NMR (400 MHz, CDCl3) δ: 8.32 (s, 1H), 7.94 (s, 1H), 7.87 (s, 1H), 7.42 (ddd, J=8.8, 6.4, 2.4 Hz, 1H), 6.88-6.79 (m, 2H), 5.66 (s, 1H), 4.99 (d, J=14.4 Hz, 1H), 4.76 (d, J=14.4 Hz, 1H), 4.64 (d, J=14.0 Hz, 1H), 4.34 (d, J=14.0 Hz, 1H). HRESIMS calcd for C13H19F2N7O3 m/z [M+H]+ 352.09697, found 352.0980.
1-(3-nitro-1H-1,2,4-triazol-1-yl)-2-(4-phenylphenyl)-3-(1H-1,2,4-triazol-1-yl)propan-2-ol (19)
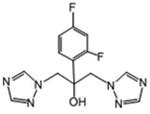
White powder (33%): mp 90-93 °C (dec); 1H NMR [500 MHz, (CD3)2CO] δ: 8.46 (s, 1H), 8.23 (s, 1H), 7.86 (s, 1H), 7.65-7.60 (m, 6H), 7.45 (t, J=8.0, 2H), 7.36 (t, J=7.5, 1H), 5.57 (s, 1H), 4.98 (d, J=14.0, 1H), 4.95 (d, J=14.0, 1H), 4.91 (d, J=14.0, 1H), 4.79 (d, J=14.0, 1H). HRESIMS calcd for C19H18N7O3 m/z [M+H]+ 392.1466, found 392.1469.
1-{[2-(2,4-difluorophenyl)-2H,3H-imidazo[2,1-b][1,3]oxazol-2-yl]methyl}-1H-1,2,4-triazole (20)
White powder (29 %): mp 134-137 °C (dec); 1H NMR [500 MHz, (CD3)2CO] δ: 8.35 (s, 1H), 7.72 (s, 1H), 7.49 (ddd, J=17.5, 9.0, 6.5 Hz, 1H), 7.21 (ddd, J=11.5, 9.0, 2.5 Hz, 1H), 7.05 (dddd, J=11.5, 9.0, 3.0, 1.0 Hz, 1H), 6.66 (d, J=1.5 Hz, 1H), 6.51 (d, J=1.5 Hz, 1H), 5.04 (d, J=15.0 Hz, 1H), 4.95 (d, J=15.0 Hz, 1H), 4.88 (dd, J=11.0, 2.0 Hz, 1H), 4.46 (dd, J=11.0, 2.0 Hz, 1H). HRESIMS calcd for C14H12F2N5O and C14H11F2N5NaO m/z [M+H]+ and [M+Na]+ 304.1004 and 326.0824, found 304.1006 and 326.0829.
4-Methyl-N-[1-(3-nitro-1H-1,2,4-triazol-1-yl)-3-phenylpropan-2-yl]benzene-1-sulfonamide (21)
3-Nitro-1,2,4-triazole (0.68 mmol) was dissolved in dry 1-propanol (5 mL) and then NaH (60%, 1.05 eq) was added. Then the commercially available (Aldrich) S-(+)-2-benzyl-1-(p-tosylaziridine) (0.68 mmol) was added and the reaction mixture was refluxed for 6 h. The reaction mixture was chromatographed on silica gel plates with ethyl acetate-petroleum ether (60:40) as eluent. White crystals (36 %): mp 147-148 °C; 1H NMR (400 MHz, CDCl3) δ: 8.18 (s, 1H), 7.43 (d, J=8.0 Hz, 2H), 7.26 (m, 3H), 7.18 (d, J=8.0 Hz, 2H), 7.00 (m, 2H), 4.64 (d, J=7.2 Hz, 1H), 4.54 (dd, J=14.0, 4.0 Hz, 1H), 4.20 (dd, J=14.8, 6.8 Hz, 1H), 3.83 (m, 1H), 2.96 (dd, J=14.8, 6.8 Hz, 1H), 2.75 (dd, J=14.4, 7.2 Hz, 1H), 2.42 (s, 3H). HRESIMS calcd for C18H20N5O4S m/z [M+H]+ 402.1231, found 402.1235.
1-(4-(2-methyloxiran-2-yl)phenyl)-1H-imidazole (22)
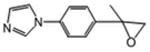
This compound was isolated during the synthesis of compound 14. White crystals (17%): mp 79-81 °C; 1H NMR [rr500 MHz, (CD3)2CO] δ: 8.05 (s, 1H), 7.58 (d, J=8.5 Hz, 2H), 7.56 (s, 1H), 7.53 (d, J=8.5 Hz, 2H), 3.00 (d, J=5.5 Hz, 1H), 2.77 (d, J=5.5 Hz, 1H), 1.71 (s, 3H). HRESIMS calcd for C12H13N2O m/z [M+H]+ 201.1022, found 201.1024.
Biological evaluation
In vitro screening
In vitro activity against T. cruzi, T. b. rhodesiense, and cytotoxicity assessment using L6 cells (rat skeletal myoblasts) was determined using a 96-well plate format as previously described.31 Data were analyzed with the graphic program Softmax Pro (Molecular Devices, Sunnyvale, CA, USA), which calculated IC50 values by linear regression from the sigmoidal dose inhibition curves.
Enzymatic activity studies with Type I NTR
Recombinant TbNTR was prepared and assayed as previously described.33,34 The activity of purified his-tagged TbNTR was assessed spectrophotometrically at 340 nm using various nitrotriazole substrates (100 μM) and NADH (100 μM) and expressed as nmol NADH oxidized min-1 mg-1 of enzyme.
Testing T. cruzi CYP51 as a potential inhibition target
Selected compounds (2, 18 and 19) were evaluated as CYP51 ligand/inhibitors using purified full-length T. cruzi CYP51 protein sample.28 Binding affinities of the compounds were estimated using spectral titration. The experiments were performed on a dualbeam Shimadzu UV-240IPC spectrophotometer in the wavelength range 350-450 nm at 25 °C in 2 mL tandem cuvettes at 2 μM P450 and ligand concentrations ranging from 0.5 to 10 μM. The apparent dissociation constants were calculated by plotting the absorbance changes in the difference spectra (ΔA425–390) upon titration against free ligand concentration and fitting the data to a rectangular hyperbola in Sigma Plot Statistics. Inhibitory potencies of the compounds were compared in the reconstituted CYP51 reaction 5 min and 1 h, at 50/2/1 molar ratio substrate/inhibitor/enzyme. P450 concentration in the reaction mixture was 1 μM. The reaction products were analyzed on a reverse-phase HPLC system (Waters) equipped with a C18Nova Pak column and a β-RAM detector (INUS Systems, Inc.).9
In vivo antichagasic activity assessment of selected compounds
For in vivo studies, a Brazilian strain trypomastigotes from transgenic T. cruzi parasites expressing firefly luciferase were used as described before.19 Briefly, parasites were injected in Balb/c mice (105 trypomastigotes per mouse) and three days later mice were anesthesized by inhalation of isofluorane, followed by an injection with 150 mg/kg of D-luciferin potassium-salt in PBS. Mice were imaged 5 to 10 min after injection of luciferin with an IVIS 100 (Xenogen, Alameda, CA) and the data acquisition and analysis were performed with the software LivingImage (Xenogen) as described before.35 Treatment with test compounds was started 4 days after infection at a specific concentration (usually 15 mg/kg/day × 10 days) by i.p. injection. The vehicle control was 2% methylcellulose + 0.5% Tween 80 and groups of 5 mice/group were used. In the vehicle control group 10 mice were used. Mice were imaged on days 5 and 10 as above. Parasite index was calculated as the ratio of parasite levels in treated mice compared to the control group and multiplied by 100. The ratio of parasite levels is calculated for each animal dividing the luciferase signal after treatment by the luciferase signal on the first imaging (before treatment). Mean values of all animals in each group ± SD were then used to calculate the parasite index.35
In vitro ADME studies
ADME in vitro studies were performed by APREDICA (Watertown, MA). Samples were analyzed by LC/MS/MS using an Agilent 6410 mass spectrometer coupled with an Agilent 1200 HPLC and a CTC PAL chilled autosampler, all controlled by MassHunter software (Agilent). After separation on a C18 reverse phase HPLC column (Agilent, Waters, or equivalent) using an acetonitrile-water gradient system, peaks were analyzed by mass spectrometry (MS) using ESI ionization in MRM mode.
Microsomal stability screen
Each test compound was dissolved in DMSO and incubated (37 °C) at 1 μM final concentration with 0.3 mg/mL of microsomal protein in 100 mM potassium phosphate, 3 mM MgCl2, pH 7.4, in the presence or absence of 2 mM NADPH (to detect NADPH-free degradation) for up to 60 min as has been described before.19 Data were reported as % remaining parent compound.
Caco-2 monolayer permeability studies
Caco-2 cells grown in tissue culture flasks were trypsinized, suspended in medium, and the suspensions were applied to wells of a collagen-coated BioCoat Cell Environment in 96-well format. The cells were allowed to grow and differentiate for three weeks, feeding at 2-day intervals. For Apical to Basolateral (A→B) permeability, the test agent was added to the apical (A) side at 10 μM final concentration and amount of permeation was determined on the basolateral (B) side after 2 h assay time as described before.19 Data were expressed as permeability (Papp): Papp = (dQ/dt)/C0A, where dQ/dt is the rate of permeation, C0 is the initial concentration of test agent, and A is the area of the monolayer.31
Supplementary Material
Acknowledgments
The authors thank M. Cal, S. Keller and M. Jud (Swiss TPH) for parasite assay results and Dr. Ana Rodriguez (New York University School of Medicine) for obtaining the in vivo data. This work was supported in part by internal funds of the Radiation Medicine Department at NorthShore University HealthSystem. Experiments on T. cruzi CYP51 were funded by NIH (GM067871, G.I.L.). In vitro screenings against parasites were funded by DNDi. For that project, DNDi received funding from the following donors: Department for Internationl Development (DFID), UK; Bill & Melinda Gates Foundation (BMGF), USA; Reconstruction Credit Institution-Federal Ministry of Education and Research (KfW-BMBF), Germany; and Directorate-General for International Cooperation (DGIS), the Netherlands. Benjamín Aguilera-Venegas acknowledges financial support by FONDECYT Postdoctorado 3130364. The donors had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations Used
- NTD
Neglected tropical diseases
- T. cruzi
Trypanosoma cruzi
- T. brucei
Trypanosoma brucei
- HAT
human African trypanosomiasis
- Nfx
nifurtimox (4-(5-nitrofurfurylindenamino)-3-methylthio-morpholine-1,1-dioxide)
- Bnz
benznidazole (N-benzyl-2-(2-nitro-1H-imidazol-1-yl)acetamide)
- NTR
type I nitroreductase
- TcNTR
T cruzi NTR
- TbNTR
T brucei NTR
- CYP51
sterol 14α-demethylase enzyme
- TcCYP51
T cruzi CYP51
- IC50
concentration for 50% growth inhibition
- SI
selectivity index
- SARs
structure-activity relationships
- TDR
Tropical Diseases Research (http//www.who.int/tdr/en/)
Footnotes
Ethical Statement: Animal studies were approved by the Institutional Animal Care and Use Committee of New York University School of Medicine (protocol #81213). This protocol adheres to the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
Supporting Information Available: “Synthesis, spectroscopic data and references of precursor chloroacetamides 1a-g”.
References
- 1.Molyneux DH, Hotez PJ, Fenwick A. “Rapid-Impact Interventions”: How a Policy of Integrated Control for Africa's Neglected Tropical Diseases Could Benefit the Poor. PLoS Medicine. 2005;2:e336. doi: 10.1371/journal.pmed.0020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease (review) Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 3.Leslie M. Infectious diseases. A tropical disease hits the road. Science. 2011;333:934. doi: 10.1126/science.333.6045.934. [DOI] [PubMed] [Google Scholar]
- 4.Gautret P, Clerinx J, Caumes E, Simon F, Jensenius M, Loutan L, Schlagenhauf P, Castelli F, Freedman D, Miller A, Bronner U, Parola P. Euro Surveill. 2009;14:19327. [PubMed] [Google Scholar]
- 5.Bern C. Antitrypanosomal therapy for chronic Chagas' disease. N Engl J Med. 2011;364:2527–2534. doi: 10.1056/NEJMct1014204. [DOI] [PubMed] [Google Scholar]
- 6.Fabbro DL, Streiger ML, Arias ED, Bizai ML, del Barco M, Amicone NA. Trypanocide treatment among adults with chronic Chagas disease living in Santa Fe city (Argentina), over a mean follow-up of 21 years: parasitological, serological and clinical evaluation. Rev Soc Bras Med Trop. 2007;40:1–10. doi: 10.1590/s0037-86822007000100001. [DOI] [PubMed] [Google Scholar]
- 7.Urbina JA. Ergosterol biosynthesis and drug development for Chagas disease. Mem Inst Oswaldo Cruz. 2009;104(Suppl1):311–318. doi: 10.1590/s0074-02762009000900041. [DOI] [PubMed] [Google Scholar]
- 8.Keenan M, Abbott MJ, Alexander PW, Armstrong T, Best WM, Berven B, Botero A, Chaplin JH, Charman SA, Chatelain E, von Geldern TW, Kerfoot M, Khong A, Nguyen T, McManus JD, Morizzi J, Ryan E, Scandale I, Thompson RA, Wang SZ, White KL. Analogues of fenarimol are potent inhibitors of Trypanosomal cruzi and are efficacious in a murine model of Chagas disease. J Med Chem. 2012;55:4189–4204. doi: 10.1021/jm2015809. [DOI] [PubMed] [Google Scholar]
- 9.Lepesheva GI, Ott RD, Hargrove TY, Kleshchenko YY, Schuster I, Nes WD, Hill GC, Villalta F, Waterman MR. Sterol 14alpha-demethylase as a potential target for antitrypanosomal therapy: enzyme inhibition and parasite cell growth. Chem Biol. 2007;14:1283–1293. doi: 10.1016/j.chembiol.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hargrove TY, Kim K, Soeiro MDC, da Silva CF, Batista DDJ, Batista MM, Yazlovitskaya EM, Waterman MR, Sulikowski GA, Lepesheva GI. Cyp51 structures and structure-based development of novel, pathogen-specific inhibitory scaffolds. Int J Parasitol Drugs Drug Resist. 2012;2:178–186. doi: 10.1016/j.ijpddr.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villalta F, Dobish MC, Nde PN, Kleshchenko YY, Hargrove TY, Johnson CA, Waterman MR, Johnston JN, Lepesheva GI. VNI Cures Acute and Chronic Experimental Chagas Disease. J Infect Dis. 2013;208:504–511. doi: 10.1093/infdis/jit042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andriani G, Amata E, Beatty J, Clements Z, Coffey BJ, Courtemanche G, Devine W, Erath J, Juda CE, Wawrzak Z, Wood JT, Lepesheva GI, Rodriguez A, Pollastri MP. Antitrypanosomal lead discovery: Identification of a ligand-efficient inhibitor of Trypanosoma cruzi CYP51 and parasite growth. J Med Chem. 2013;56:2556–2567. doi: 10.1021/jm400012e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina I, Prat JG, Salvador F, Treviño B, Sulleiro E, Serre N, Pou D, Roure S, Cabezos J, Valerio L, Blanco-Grau A, Sánchez-Montalvá A, Vidal X, Pahissa A. Randomized trial of posaconazole and benznidazole for chronic Chagas' disease. N Engl J Med. 2014;370:1899–1907. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 14.Moraes CB, Giardini MA, Kim H, Franco CH, Araujo-Junior AM, Schenkman S, Chatelain E, Freitas-Junior LH. Nitroheterocyclic compounds are more efficacious than CYP51 inhibitors against Trypanosoma cruzi: implications for Chagas disease drug discovery and development. Scientific Reports. 2014;4:4703–4714. doi: 10.1038/srep04703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diniz LdF, Urbina JA, de Andrade IM, Mazzeti AL, Martins TAF, Caldas IS, Talvani A, Ribeiro I, Bahia MT. Benznidazole and posaconazole in experimental Chagas disease: Positive interaction in concomitant and sequential treatments. PLoS Negl Trop Dis. 2013;7:e2367. doi: 10.1371/journal.pntd.0002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadopoulou MV, BourdinTrunz B, Bloomer WD, McKenzie C, Wilkinson SR, Prasittichai C, Brun R, Kaiser M, Torreele E. Novel 3-nitro-1H-1,2,4-triazole-based aliphatic and aromatic amines as anti-Chagasic agents. J Med Chem. 2011;54:8214–8223. doi: 10.1021/jm201215n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papadopoulou MV, Bloomer WD, Rosenzweig HS, Chatelain E, Kaiser M, Wilkinson SR, McKenzie C, Ioset JR. Novel 3-nitro-1H-1,2,4-triazole-based amides and sulfonamides as potential anti-trypanosomal agents. J Med Chem. 2012;55:5554–5565. doi: 10.1021/jm300508n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papadopoulou MV, Bloomer WD, Rosenzweig HS, Kaiser M, Chatelain E, Ioset JR. Novel 3-nitro-1H-1,2,4-triazole-bearing piperazines and 2-amino-benzothiazoles as anti-Chagasic agents. Bioorg Med Chem. 2013;21:6600–6607. doi: 10.1016/j.bmc.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadopoulou MV, Bloomer WD, Rosenzweig HS, Ashworth R, Wilkinson SR, Kaiser M, Andriani G, Rodriguez A. Novel 3-nitro-1H-1,2,4-triazole-based compounds as potential anti-Chagasic drugs: In vivo studies. Future Med Chem. 2013;5:1763–1776. doi: 10.4155/fmc.13.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchanan-Kilbey G, Djumpah J, Papadopoulou MV, Bloomer WD, Hu L, Wilkinson SR, Ashworth R. Evaluating the developmental toxicity of trypanocidal nitroaromatic compounds on zebrafish. Acta Tropica. 2013;128:701–705. doi: 10.1016/j.actatropica.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson SR, Taylor MC, Horn D, Kelly JM, Cheeseman I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. PNAS. 2008;105:5022–5027. doi: 10.1073/pnas.0711014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alsford S, Eckert S, Baker N, Glover L, Sanchez-Flores A, Leung KF, Turner DJ, Field MC, Berriman M, Horn D. High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature. 2010;482:232–236. doi: 10.1038/nature10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker N, Alsford S, Horn D. Genome-wide RNAi screens in African trypanosomes identify the nifurtimox activator NTR and the eflornithine transporter AAT6. Mol Biochem Parasitol. 2011;176:55–57. doi: 10.1016/j.molbiopara.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkinson SR, Bot C, Kelly JM, Hall BS. Trypanocidal activity of nitroaromatic prodrugs: current treatments and future perspectives. Curr Top Med Chem. 2011;11:2072–2084. doi: 10.2174/156802611796575894. [DOI] [PubMed] [Google Scholar]
- 25.Urbina JA. Specific treatment of Chagas disease: current status and new developments. Curr Opin Infect Dis. 2001;6:733–741. doi: 10.1097/00001432-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Kim YF, Yoon GJ, Park MH. Preparation of fluconazole. From PCT Int Appl. 1998 WO 9832744 A1 19980730. Language: English. [Google Scholar]
- 27.Nwaka S, Ramirez B, Brun R, Maes L, Douglas F, Ridley R. Advancing drug innovation for neglected diseases—criteria for lead progression. PLoS Negl Trop Dis. 2009;3:e440. doi: 10.1371/journal.pntd.0000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lepesheva GI, Zaitseva NG, Nes WD, Zhou W, Arase M, Liu J, Hill GC, Waterman MR. CYP51 from Trypanosoma cruzi: a phyla-specific residue in the B′ helix defines substrate preferences of sterol 14alpha-demethylase. J Biol Chem. 2006;281:3577–8. doi: 10.1074/jbc.M510317200. [DOI] [PubMed] [Google Scholar]
- 29.Lepesheva GI, Hargrove TY, Anderson S, Kleshchenko Y, Furtak V, Wawrzak Z, Villalta F, Waterman MR. Structural insights into inhibition of sterol 14alpha-demethylase in the human pathogen Trypanosoma cruzi. J Biol Chem. 2010;285:25582–25590. doi: 10.1074/jbc.M110.133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lepesheva GI, Hargrove TY, Kleshchenko Y, Nes WD, Villalta F, Waterman MR. CYP51: A major drug target in cytochrome P450 superfamily. Lipids. 2008;43:1117–1125. doi: 10.1007/s11745-008-3225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart BH, Chan OH, Lu RH, Reyner EL, Schmid HL, Hamilton HW, Steinbaugh BA, Taylor MD. Comparison of intestinal permeabilities determined in multiple in vitro and in situ models: Relationship to absorption in humans. Pharm Res. 1995;12:693–699. doi: 10.1023/a:1016207525186. [DOI] [PubMed] [Google Scholar]
- 32.Orhan I, Sener B, Kaiser M, Brun R, Tasdemir D. Inhibitory activity of marine sponge-derived natural products against parasitic protozoa. Mar Drugs. 2010;8:47–58. doi: 10.3390/md8010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall BS, Wu X, Hu L, Wilkinson SR. Exploiting the drug-activating properties of a novel trypanosomal nitroreductase. Antimicrob Agents Chemother. 2010;54:1193–1199. doi: 10.1128/AAC.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall BS, Meredith EL, Wilkinson SR. Targeting the substrate preference of a type I nitroreductase to develop anti-trypanosomal quinone-based prodrugs. Antimicrob Agents Chemother. 2012;56:5821–5830. doi: 10.1128/AAC.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andriani G, Chessler ADC, Courtemanche G, Burleigh BA, Rodriguez A. Activity in vivo of anti-trypanosoma cruzi compounds selected from a high throughput screening. PLoS Negl Trop Dis. 2011;5:e1298. doi: 10.1371/journal.pntd.0001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.