Abstract
Whole-parasite malaria vaccines have shown promise in clinical trials. We recently reported the first human trial of a malaria vaccine based on Plasmodium falciparum genetically attenuated parasites (PfGAP). Herein we report for the first time that PfGAP induces prolonged functional humoral responses in humans. Six volunteers were exposed to 5 bites of PfGAP-infected mosquitoes followed by approximately 200 bites. Plasma collected from all volunteers 3 months after the last exposure efficiently inhibits invasion of hepatocytes by P. falciparum sporozoites. The level of inhibition observed is comparable to that attained using plasma collected after 4–5 intravenously administrations of high numbers of irradiated sporozoites, validating the potential of PfGAP malaria vaccines. Our data highlight the role of antibody responses in pre-erythrocytic stages of human malaria, and suggests that to be protective, malaria vaccines might need to elicit long-lasting functional antibodies in addition to cellular responses.
Keywords: Malaria, Vaccines, Whole-parasite immunization, PfGAP, ISI
1. Introduction
Malaria remains the second leading cause of death from infectious disease worldwide, causing annually 219 million cases and 660,000 deaths, mostly in children under the age of five [1]. Recent reductions in malaria incidence and mortality have been attributed to interventions such as insecticide-treated bed nets, indoor spraying, early diagnosis and treatment; however many believe that eradication requires a vaccine that interrupts malaria transmission.
Pre-erythrocytic malaria vaccines aim to prevent clinical disease and transmission by allowing parasite development in the liver, eliciting a host immune response, while precluding erythrocyte invasion and thus any clinical symptoms. The most effective malaria vaccines are those that use whole parasites (reviewed in [2]). Murine models of pre-erythrocytic malaria vaccines revealed the importance of cellular responses: under certain immunization regimens robust CD8T responses have been shown to be necessary and sufficient for protection [3]. However, it is unclear whether cellular responses are sufficient to generate protection in humans, and the results from human clinical studies suggest that humoral immune responses also play a significant role. For example, while protection elicited by the RTS,S [4] and PfSPZ [5] malaria vaccines correlates with the titer of antibodies against circumsporozoite protein (CSP), these titers are not entirely predictive of protection. Similarly, although anti-CSP antibodies elicited by whole parasite immunization inhibit hepatocytes invasion by sporozoites [6], it is not known whether this is sufficient to confer protection. Antigens other than CSP might also be involved in the humoral response against malaria. For example, titers of antibodies against non-CSP malaria sporozoite proteins such as AMA-1 and SSP2 have been shown to be higher in volunteers protected from challenge after exposure to irradiated sporozoites, as compared to not-protected volunteers [7].
Our previous studies in murine models have shown that a P. yoelii genetically attenuated parasite (PyGAP) vaccine that harbors simultaneous deletions of the p52 and p36 genes cause developmental arrest in early liver stages and induce protection [8]. We recently reported the first in-human proof-of-concept, safety and immunogenicity clinical trial based on genetically attenuated Plasmodiumfalciparum sporozoites (PfGAP), which carry a double deletion of the P. falciparum orthologues of P. yoelii p52 and p36 genes [9,10] (clinical trial number NCT01024686). We now report the first assessment of functional antibodies obtained form these human volunteers vaccinated with PfGAP.
2. Materials and methods
2.1. Vaccination
Details of the PfGAP vaccination trial (NCT01024686) were previously reported [9]. Six malaria-naïve adults were exposed to 5 PfGAP-infected mosquito bites, followed by exposure to approximately 200 (range 190–263) PfGAP-infected mosquito bites 42 days later (Fig. 1A). Plasma samples were collected before exposure, 42 days after the first exposure, and 90 days after the second exposure. Banked plasma samples from a previously described Phase I malaria vaccine trial (NCT01058226) were used as a control [11]. Six malaria-naïve adults were exposed to 5 bites of wild type P. falciparum-infected mosquito (Fig. 1B). Plasma samples were collected before and 56 days after exposure. Volunteers were checked twice daily for microscopic determination of parasitemia by thick blood smears. All participants developed parasitemia (as determined by the presence of two unequivocal parasites) between days 9 and 14, and were treated with a standard oral regimen of chloroquine [11]. Both studies were approved by the Western Institutional Review Board. Written informed consent for use of samples in these studies was obtained from each donor.
Fig. 1.
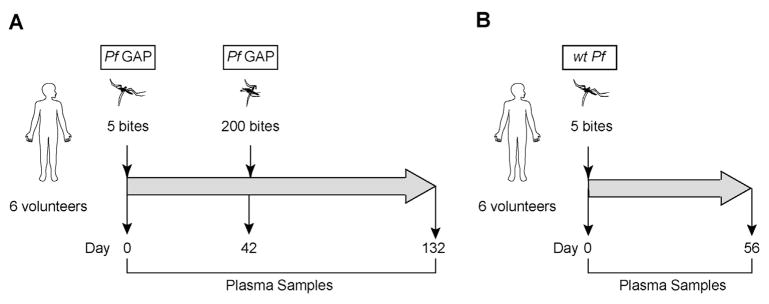
Experimental design. (A) Six volunteers were immunized with 5 bites of mosquitoes infected with PfGAP, followed by 200 bites 42 days later. Plasma samples were taken on Day 0 (pre-immunization); on Day 42 (42 days after the PfGAP5 immunization but before the PfGAP200 immunization), and on Day 132 (90 days after the PfGAP200 immunization). (B) Six volunteers were immunized with 5 bites of mosquitoes infected with wtPf; plasma samples were taken on Day 0 (pre-immunization), and on Day 56 after the wtPf5 immunization.
2.2. Enzyme-linked immunosorbent assay (ELISA)
Immulon4 plates (Thermo) were coated with E. coli-expressed full-length CSP recombinant protein at 1 μg/ml in Phosphate Buffer Saline (PBS, Sigma), incubated overnight at 4 °C, washed with wash buffer (0.05% Tween-20 in PBS), and blocked with 5% nonfat dry milk (BD Biosciences) for 2 h at RT. Plasma was added at 1:200 dilution and incubated for 2 h at RT. Alkaline-phosphatase labeled goat anti-human IgG (K + L) was added at 1:800 dilution and incubated for 2 h. Plates were developed with phosphatase substrate tablets (Sigma). After 20 min, OD was measured at 405 nm, with 650 nm correction, using a Spectramax® M2 plate reader (Molecular Devices).
2.3. Inhibition of sporozoite invasion (ISI)
Immortalized HC04 human hepatocyte cells (MRA-975, MR4) were cultured in D10 media (DMEM (Gibco) supplemented with 10% (v/v) FBS (Gemini Bio Products), 200 mM L-glutamine (Gibco) and 1% (v/v) Pen-strep (Gibco)) at 37 °C in 5% CO2, and split at 90% confluence. NF54 P. falciparum sporozoites were freshly dissected from Anopheles stephensi mosquitoes on ice as described [12] and incubated for 30 min with monoclonal antibodies against PfCSP (2A10, MR4) or human plasma. Sporozoites were added to HCO4 cells and incubated for 90 min before washing, permeabilizing, and staining cells with anti-CSP antibodies.
3. Results and discussion
We recently published the first in-human evaluation of PfGAP administered by exposure to 5 bites of infected mosquitoes, followed by approximately 200 bites (Fig. 1A) [9]. High titers of anti-CSP antibodies were detected in all six volunteers after this immunization regimen [9]. In comparison, anti-CSP antibodies were not detected before or after exposure to 5 bites of PfGAP-infected mosquitoes, or in samples from a trial in which volunteers were exposed to 5 bites of wild type P. falciparum-infected mosquitoes (wtPf, Fig. 1B) [11].
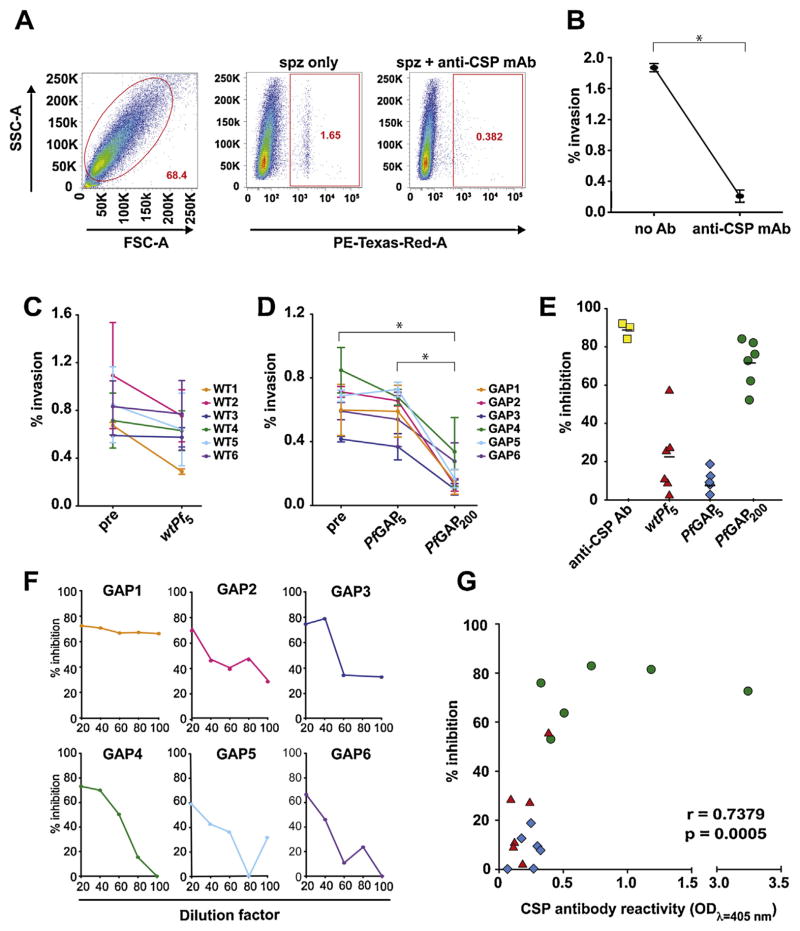
In this study, we assessed the functional activity of the antibody response to PfGAP using our recently published flow cytometry-based inhibition of sporozoite invasion (ISI) assay that quantitatively monitors the antibody-mediated inhibition of invasion in a medium-throughput and reproducible manner [13]. Pre-incubation of sporozoites with anti-CSP monoclonal antibody resulted in an average 89% (range 84–92%) statistically significant reduction of their ability to invade hepatocytes in culture (Fig. 2A and B). After optimizing this assay for plasma and serum samples, we used it to test the plasma of volunteers exposed to either wtPf or PfGAP. Using a 1:20 dilution of plasma from volunteers exposed to 5 bites of mosquitoes infected with wtPf, we saw a 20% average (range 2–57%) reduction of hepatocyte invasion by sporozoites, as compared to the reduction elicited by pre-exposure plasma from the same volunteers (wtPf5, Fig. 2C and E). Similarly, exposure to 5 bites of PfGAP infected mosquitoes resulted in a 10% average (range 0–18%) reduction of invasion (PfGAP5, Fig. 2D and E). These differences were not statistically significant. Importantly, upon exposure to ~200 bites of PfGAP-infected mosquitoes, the plasma from these volunteers yielded a 71.4% average (range 52–84%) statistically significant reduction of invasion (PfGAP200, Fig. 2D and E). By serially diluting the plasma in 2-fold increments, we saw a positive correlation between the plasma concentration and the magnitude of inhibition (Fig. 2F), suggesting that the functional antibody response observed is dose-dependent.
Fig. 2.
Plasma from PfGAP-exposed volunteers inhibits sporozoite invasion of hepatocytes. (A) Representative staining showing the live HCO4 gate, and CSP-positive staining in cells incubated with sporozoites (spz only), or with sporozoites and anti-CSP monoclonal antibodies (spz + anti-CSP mAb). (B–D) Inhibition of sporozoite invasion assay. (B) Percentage of HC04 cells infected with P. falciparum sporozoites in the absence of antibodies (no Ab) or after incubation with anti-CSP monoclonal antibodies (anti-CSP mAb). (C) Percentage of HC04 cells infected with P. falciparum sporozoites after incubation with plasma obtained from six volunteers (WT1 to WT6) before (pre) and after exposure to 5 wtPf-infective bites (wtPf5). (D) Percentage of HC04 cells infected with P. falciparum sporozoites after incubation with plasma obtained from six volunteers (GAP1–GAP6) before (pre) and after exposure to 5 (PfGAP5) or 200 (PfGAP200) PfGAP-infective bites. Each line represents the mean percentage of infected cells from three independent experiments run in triplicate. Error bars in parts B–D represent the standard deviation. Statistics was determined using one way ANOVA; asterisks indicate p < 0.05. (E) Quantitation of ISI assay data from parts B to D, shown as percentage inhibiton of hepatocyte invasion by sporozoites. The baseline for each datapoint corresponds to pre-exposure values. The markers correspond to anti-CSP monoclonal antibodies (yellow squares), wtPf5 (red triangles), PfGAP5 (blue diamonds) and PfGAP200 (green circles). Values with negative inhibition were set to zero. (F) Titration of plasma from volunteers exposed to ~200 bites of PfGAP-infected mosquitoes (from 1:20 to 1:100), shown as percentage inhibiton of hepatocyte invasion by sporozoites. (G) Correlation between the percent inhibition and the titer of anti-CSP antibodies, as determined by ELISA. Sample symbols are as in part E.
To address this possibility that parasite proteins other than CSP may be targeted by the immune response of the host [7], we analyzed plasma samples by ELISA to determine the presence of antibodies against PE antigens LSA-1, LSA-3, STARP and AMA-1. We were unable to detect the presence of antibodies to any of these antigens (data not shown). Although these negative data do not rule out the involvement of other antigens, it is possible that several doses of PfGAP vaccination may be required to boost levels of antibodies to non-immunodominant proteins to detectable levels. However, the observation that CSP antibody titers from plasma of immunized volunteers are highly correlated with the level of inhibition detected in the ISI assay (Fig. 2G), confirms previous reports suggesting that anti-CSP antibodies play a major role in the inhibition of hepatocyte invasion by sporozoites [6]. Because one of the six volunteers exposed to 200 bites of PfGAP-infected mosquitoes (GAP1) developed a breakthrough infection [9], we were unable to correlate the inhibition of hepatocyte infection observed in our study with protection. However, a recent study using whole irradiated parasites suggests that the ISI assay is indicative of protection [5].
To date, sterile protection against malaria infection in humans has only been achieved through whole-parasite vaccination [14,15]. We have shown that PyGAP consistently induce long-term sterile protection in murine models [8]. In this study, we report for the first time that exposure of volunteers to ~200 bites of PfGAP-infected mosquitoes generates a strong functional humoral response, and that plasma collected 3 months after the last exposure to PfGAP efficiently inhibits sporozoite invasion of hepatocytes. Importantly, this inhibition is comparable to that reported from plasma collected 14 days after intravenous immunization with 4–5 doses of 135,000 irradiated sporozoites [5], suggesting that a complete immunization regimen of 3–5 doses of PfGAP could provide a more efficacious method of inducing long-term functional antibody responses.
Analysis of malaria vaccine trial samples show that protection from infection induced by RTS,S and irradiated sporozoites depends on both anti-CSP antibodies and CSP-specific CD4+T cells [5,16]. This suggests that a successful malaria vaccine should simultaneously target both humoral and cellular responses, similarly to what has been proposed by HIV researchers [17]. Importantly, vaccination with PfGAP induces both arms of the immune response [9]. In conclusion, our data serves as a model of immune responses against malaria infection, with the goal of designing better and more efficient vaccines. Future experiments will investigate whether the functional antibody response that we observe is sufficient to confer long-term protection against wild type malaria parasites, and will attempt to identify new sporozoite surface antigens with ISI function.
Acknowledgments
Recombinant CSP was a gift from Patrick Duffy (NIAID, NIH). Homo sapiens HC04 cells (MRA-975, deposited by Jetsumon Sattabongkot) cells were obtained through MR4 as part of the BEI Resources Repository. Monoclonal 2A10 antibody (Mus musculus (B cell); Mus musculus (myeloma) 2A10, MRA-183, deposited by E. Nardin) was obtained through MR4 as part of the BEI Resources Repository. We are grateful to William W. Betz, Mark F. Kennedy, Heather Kain and Jen C.C. Hume of the Seattle Biomedical Research Institute Insectary Facility for mosquito and sporozoite production, to Angela Talley and Patrick Duffy for serum samples, and to Marissa Vignali for editing the manuscript.
Funding: This work was supported by grant U19 AI089986-01 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, by grant W81XWH-11-2-0184 from the U.S. Department of Defense and a Ruth L. Kirschstein National Research Service Award (F32 AI091129).
Footnotes
Conflict of interest statement: The authors declare no conflict of interest.
References
- 1.WHO. World Malaria Report. Geneva: World Health Organization; 2013. [Google Scholar]
- 2.Butler NS, Vaughan AM, Harty JT, Kappe SH. Whole parasite vaccination approaches for prevention of malaria infection. Trends in Immunology. 2012;33:247–54. doi: 10.1016/j.it.2012.02.001. http://dx.doi.org/10.1016/j.it.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Trimnell A, Takagi A, Gupta M, Richie TL, Kappe SH, Wang R. Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes. Journal of Immunology. 2009;183:5870–8. doi: 10.4049/jimmunol.0900302. http://dx.doi.org/10.4049/jimmunol.0900302. [DOI] [PubMed] [Google Scholar]
- 4.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–20. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 5.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341:1359–65. doi: 10.1126/science.1241800. http://dx.doi.org/10.1126/science.1241800 [Epub 2013 Aug 8] [DOI] [PubMed] [Google Scholar]
- 6.Chappel JA, Hollingdale MR, Kang AS. IgG(4) Pf NPNA-1 a human anti-Plasmodium falciparum sporozoite monoclonal antibody cloned from a protected individual inhibits parasite invasion of hepatocytes. Human Antibodies. 2004;13:91–6. [PubMed] [Google Scholar]
- 7.Trieu A, Kayala MA, Burk C, Molina DM, Freilich DA, Richie TL, et al. Sterile protective immunity to malaria is associated with a panel of novel P. falciparum antigens. Molecular and Cellular Proteomics. 2011:10. doi: 10.1074/mcp.M111.007948. http://dx.doi.org/10.1074/mcp.M111.007948. M111 007948. [DOI] [PMC free article] [PubMed]
- 8.Labaied M, Harupa A, Dumpit RF, Coppens I, Mikolajczak SA, Kappe SH. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infection and Immunity. 2007;75:3758–68. doi: 10.1128/IAI.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spring M, Murphy J, Nielsen R, Dowler M, Bennet JW, Zarling S, et al. First-in-human evaluation of genet. Vaccine. 2013;31:4975–83. doi: 10.1016/j.vaccine.2013.08.007. http://dx.doi.org/10.1016/j.vaccine.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 10.VanBuskirk KM, O’Neill MT, De La Vega P, Maier AG, Krzych U, Williams J, et al. Preerythrocytic, liveatte. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13004–9. doi: 10.1073/pnas.0906387106. http://dx.doi.org/10.1073/pnas.0906387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy SC, Prentice JL, Williamson K, Wallis CK, Fang FC, Fried M, et al. Real-time quantitative reverse tran. American Journal of Tropical Medicine and Hygiene. 2012;86:383–94. doi: 10.4269/ajtmh.2012.10-0658. http://dx.doi.org/10.4269/ajtmh.2012.10-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy M, Fishbaugher ME, Vaughan AM, Patrapuvich R, Boonhok R, Yimamnuaychok N, et al. A rapid and scalable density gradient purification method for Plasmodium sporozoites. Malaria Journal. 2012;11:421. doi: 10.1186/1475-2875-11-421. http://dx.doi.org/10.1186/1475-2875-11-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaushansky A, Rezakhani N, Mann H, Kappe SH. Development of a quantitative flow cytometry-based assay to assess infection by Plasmodium falciparum sporozoites. Molecular and Biochemical Parasitology. 2012;183:100–3. doi: 10.1016/j.molbiopara.2012.01.006. http://dx.doi.org/10.1016/j.molbiopara.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. Journal of Infectious Diseases. 2002;185:1155–64. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 15.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216:160–2. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 16.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, et al. Randomized, double-blind. Journal of Infectious Diseases. 2009;200:337–46. doi: 10.1086/600120. http://dx.doi.org/10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 17.Munier CM, Andersen CR, Kelleher AD. HIV vaccines: progress to date. Drugs. 2011;71:387–414. doi: 10.2165/11585400-000000000-00000. http://dx.doi.org/10.2165/11585400-000000000-00000. [DOI] [PubMed] [Google Scholar]