Summary
Growth hormone (GH) affects somatic growth, sexual maturation, body composition and metabolism, as well as aging and longevity. Mice lacking GH or GH receptor outlive their normal siblings and exhibit symptoms of delayed aging associated with improved insulin signaling and increased stress resistance. Beneficial effects of eliminating the actions of GH are counterintuitive but conform to the concept of antagonistic pleiotropy. Evolutionary selection for traits promoting early life fitness and reproductive success may account for detrimental effects post-reproductively. Reciprocal relationships between GH signaling and longevity discovered in mutant mice apply also to normal mice, other mammalian species, and perhaps humans. This review summarizes the present understanding of the multifaceted relationship between somatotropic signaling and mammalian aging.
Physiological actions of GH
Growth hormone (GH) is a protein hormone secreted by the anterior pituitary in vertebrate animals. Named after its major stimulatory role in the control of somatic growth and adult body size, it exerts a multitude of other effects. Growth hormone influences most and likely all tissues and organs either directly or via growth factors. In mammals, insulin-like growth factor-1 (IGF-1) is a major mediator of its actions. Moreover, effects of GH on carbohydrate, fat and protein metabolism, on nutrient sensing pathways and on secretory activity of adipose tissue are important determinants of the internal milieu of an organism and influence virtually every physiological function.
Studies conducted during the past 15 years firmly established that in addition to its various growth-promoting and metabolic actions, GH also affects aging and longevity. Surprisingly, the best defined effects of the somatotropic axis (which consists of the hypothalamic GH Releasing Hormone (GHRH), GH and IGF-1) on longevity are negative. Mutant mice with GH deficiency or resistance outlive their normal siblings by as much as 50% and occasionally even more (1–3). Genetic disruption of IGF-1 signaling can also extend murine lifespan, while transgenic mice with supraphysiological excess of GH and IGF-1 are short-lived (reviewed in 4, 5). One is forced to conclude that the physiological actions of GH involve significant costs in terms of longevity, which is not entirely surprising when viewed in the light of the present understanding of evolutionary and genetic control of aging. The concept of antagonistic pleiotropy implies that genes and phenotypes detrimental to post-reproductive survival are not eliminated by natural selection if they confer evolutionary advantage earlier in life. Thus in terms of evolutionary fitness, the apparent “pro-aging” effects of GH may be more or less irrelevant, or else are trumped by the stimulatory effects of the somatotropic axis on growth, sexual maturation and fecundity (Figure 1). Importantly, the reciprocal relationship between GH actions and longevity demonstrated in mutant, gene knock-out and transgenic mice applies also to genetically normal mice and to other mammalian species including rats, dogs, horses and perhaps also humans (4, 6, 7).
Figure 1. Effects of growth hormone (GH) during different life stages.
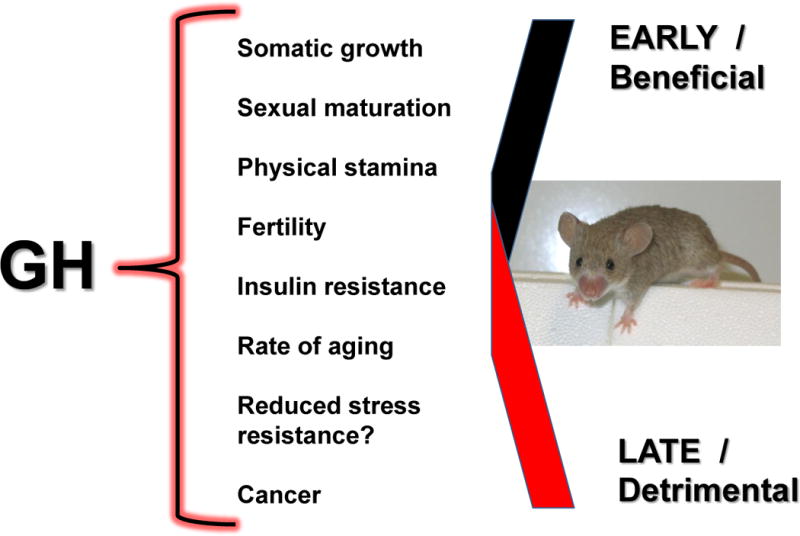
Stimulation of growth, maturation and fertility by growth hormone (GH) apparently involves ‘costs’ of accelerated aging and increased susceptibility to age-related disease. Current understanding of the evolution of aging suggests that the detrimental GH actions late in life have not been eliminated because of beneficial effects of GH on early reproductive fitness.
In this article, we will discuss recent progress in identifying mechanisms linking GH actions with aging and longevity in mice and present findings concerning the relationships of GH to aging in other species. This will include information on the phenotypic characterization of humans with GH deficiency or resistance.
Long-lived GH- and IGF-1-related mutants
Studies reported during the last four years expanded the list of GH-related mutations that extend longevity in mice. Long-lived mice were produced by gene deletions that affect tissue levels of bio-available (free) IGF-1 (8) or levels of insulin receptor substrates (IRS) 1 or 2, mediators of IGF-1 and insulin signals (9, 10). Furthermore, mouse longevity was extended by deleting S6 kinase, which is related to GH actions via mammalian target of rapamycin (mTOR) (11). Importance of the mTOR pathway in the control of mammalian aging was shown by demonstrating that rapamycin, an inhibitor of mTOR, extends longevity of mice (12). Our ongoing studies indicate that both sexes of the recently produced mice with isolated GH deficiency due to genetic deletion of hypothalamic GHRH are also long-lived (Spong et al., unpublished).
Comparison of the impact of various somatotropic axis-related mutations on longevity reveals some interesting and not readily anticipated trends. In general, the impact on longevity from mutations affecting IGF-1 levels, IGF-1 receptors (IGF1R) or signaling events downstream from IGF1R is smaller than the effects of disrupting GH biosynthesis or actions (13). Moreover, GH resistance or deficiency extends longevity in both sexes, while several of the mutations directly affecting IGF-1 signaling increase lifespan only in females. Some of these differences may be due to the effects of genetic background, diet or other differences between studies conducted at different laboratories or to the fact that complete deletion of IGF-1 signaling causes severe developmental problems, including lethality (14). Consequently, IGF-1-related mutants available for study have partial suppression of IGF-1 levels or actions, as opposed to the complete absence of GH signals seen in the hypopituitary, GH-resistant or GH-deficient mutants. Nevertheless, the available data suggest existence of some differences between the effects of GH and IGF-1 on aging and longevity. Since IGF-1 expression in many organs is partially or completely GH-independent, eliminating GH action does not interfere with the beneficial actions of locally expressed IGF-1 in the brain, heart or other organs, while it reduces circulating (primarily liver-derived) IGF-1 levels thus protecting the animals from cancer (13, 14). In contrast, genetic suppression of IGF-1, IGF1R or IRS interferes with IGF-1 actions everywhere, thus presumably producing a combination of detrimental and protective effects. Finally, the available data suggest that GH resistance or deficiency may influence aging by mechanisms unrelated to the regulation of IGF-1 expression. Prominent among the likely “GH-specific” mechanisms affecting longevity is regulation of insulin sensitivity, an action of GH that is not mediated by IGF-1 and is, in fact opposite to the IGF-1 effects. While GH is anti-insulinemic and promotes insulin resistance, IGF-1 is an insulin sensitizer and mimics some of insulin’s actions. Direct actions of GH on adipose tissue are likely to contribute to its impact on insulin signaling via alterations in the levels of circulating adipokines and fatty acids.
The evidence that IGF-1 is importantly involved in the control of longevity was strengthened by a recent demonstration which compared 31 inbred mouse strains, and found that circulating IGF-1 levels were negatively related to the median lifespan of females (15). These findings extend previous reports showing that serum IGF-1 levels measured during young adulthood in individual mice were negatively correlated with their survival (16) and that similar relationships were detected in comparisons of different breeds of domestic dogs (17). Interestingly, the expected positive correlation of circulating IGF-1 levels and adult body size which was evident in these and many other studies may not apply to comparisons between different species. Stuart and Page (18) recently reported that IGF-1 levels are, surprisingly, lower in larger species. This was based on analysis of published data derived from 36 mammalian species ranging from mice to cattle and polar bears. These data are intriguing and potentially very important for explaining why longevity is generally negatively correlated with body size within a species but, with a few exceptions, positively correlated with body size in comparisons involving different species (e.g., mouse, dog, horse, elephant, whale). The role of IGF-1 in promoting cancer growth together with the apparent “pro-aging” actions of the somatotropic axis could explain why low IGF-1 levels are associated with increased life expectancy both in large species and in small individuals within a species.
Mechanisms linking reduced GH signaling with extended longevity
Although association of GH deficiency (isolated or combined with deficiency of thyrotropin and prolactin) or GH resistance with extended longevity in laboratory mice is strong, consistent and reproducible, the underlying mechanisms remain to be clearly identified. We have previously suggested that multiple mechanisms are almost certainly involved and that interactions between these mechanisms may amplify their individual effects (4, 13) (Figure 2). Based on concomitant reduction of circulating insulin and glucose levels and on the correlation between the effects of different “longevity genes” and calorie restriction (CR) on longevity and on whole-animal insulin sensitivity, we have further suggested that improved insulin signaling is important in linking reduction in GH actions with increased lifespan (19, 20). Because 30% CR initiated at 2 months of age had little if any effect on whole-animal insulin sensitivity or longevity in Ghr−/− (growth hormone receptor “knockout” or GHRKO) mice while it readily increased both in normal animals from the same strain, we examined the interactive effects of CR and Ghr gene deletion on individual steps of insulin signaling in the liver and in the skeletal muscle (21). In the liver, insulin receptor (IR) levels and its activating phosphorylation in response to acute insulin stimulation were greater in GHRKO than in normal mice and were increased by CR in normal mice but not in the mutants. Activation of PI3Kinase (PI3K) measured by Y99 phosphorylation of its 85 subunit was also increased by CR only in normal animals, but it did not differ between the normal and mutant mice and thus did not reflect the differences in insulin sensitivity detected at the whole-animal level. In hind limb muscles, IR levels were higher in GHRKO than in normal mice, while their acute activation by a large dose of insulin did not differ between genotypes. However, the downstream signaling events—PI3K activation, Akt 1 and 2 levels and activation, and GLUT 4 levels—corresponded to whole-animal insulin sensitivity, i.e., they were elevated in GHRKO vs. normal mice but increased by CR only in the latter group. Phosphorylation of IRS 1 at Serine 307 which is known to be associated with reduced association with PI3K, increased IRS 1 degradation and insulin resistance (22, 23) was markedly reduced in GHRKO as compared to normal mice (21). This could be related to reduced JNK 1 activation and mTOR signaling and increased adiponectin levels in GHRKO animals (21, 24). Surprisingly, CR did not alter inhibitory (S 307) IRS 1 phosphorylation in either genotype. Recent studies comparing the effects of surgical removal of intra-abdominal fat in GHRKO and normal mice suggest that the remarkable insulin sensitivity of GH-resistant mutants is importantly related to altered secretory profile of adipose tissue (Masternak et al., unpublished). These studies implicate fat depot-specific adiponectin secretion as one of the factors responsible for differences in the control of glucose levels (19, 25) and lipid oxidation (26) in GH-resistant as compared to normal (wild-type) mice.
Figure 2. Mechanisms linking reduced GH signaling with extended longevity in GH-resistant or GH-deficient mice.
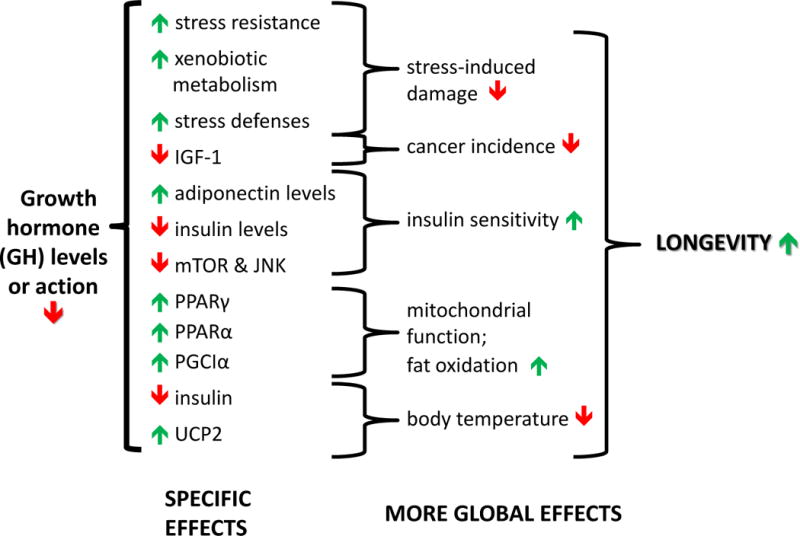
Simplified outline of mechanisms of GH effects on longevity. Interactions and reciprocal relationships omitted for clarity.
 = increase;
= increase;
 = decrease
= decrease
This diagram lists consequences of GH deficiency or resistance that constitute likely mechanisms of extended longevity. The Specific Effects listed in the first column include those detected in long-lived mutants, and most have been linked to GH actions by evidence from other experimental systems. The More Global Effects listed in the second column represent broader functional consequences of these changes known to be involved in the control of aging and longevity. The diagram is not intended to be comprehensive or all-inclusive. For example, plausible mechanisms of extended longevity that have been demonstrated in only one mutant have not been included.
Enhanced stress resistance has been identified as another likely mechanism linking reduced GH signals with extended longevity. Several reports have documented increased resistance of dermal fibroblasts derived from hypopituitary (GH-deficient) or GH-resistant mice to a variety of cytotoxic stresses in vitro (27, 28). Importantly, in one of these long-lived mutants, increased fibroblast resistance to the toxic effects of Paraquat in vitro was associated with increased resistance to the lethal effects of the same toxin in vivo (29). Amador-Noguez and his colleagues reported that long-lived Ghrhr −/− (Little) mice with isolated GH deficiency are more resistant than normal animals to hepatotoxins and other xenobiotics in vivo. They related these findings to increased activity of hepatic enzymes involved in detoxification (30). Expression of xenobiotic detoxification genes in the liver is increased in these mutants as well as in Ames dwarf mice that lack GH and other pituitary hormones and this increase can be ascribed to augmented levels of bile acids and their actions via farnesoid X receptors (30, 31). Evidence for a cause-effect relationship between reduced GH signaling and enhanced stress resistance was provided by recent studies of cultured fibroblasts derived from Ames dwarfs that had been injected with GH for several weeks (32). These cells no longer displayed resistance to multiple cytotoxic stresses and their responses to paraquat, cadmium, an alkylating agent or a mitochondrial poison resembled those measured in cells derived from genetically normal, control mice (32). These findings suggest that increased resistance to various stressors is an important mechanism linking reduced somatotropic signaling to delayed aging in the examined mutant mice. Moreover, they imply that association of enhanced stress resistance and extended longevity demonstrated previously in invertebrates, applies also to mammals.
Studies of passive avoidance and spatial memory in hypopituitary and GH-resistant mutants as compared to normal mice provided evidence that the long-lived mutant are remarkably resistant to the age-related loss of cognitive function (33–35). L. Sun and his colleagues related maintenance of learning and memory in Ames dwarfs into advanced chronological age to normal or elevated expression of IGF-1 in the hippocampus (36, 37). Using organotypic slice preparation, Schrag et al. (38) have shown that the hippocampus of Ames dwarf mice is resistant to beta amyloid toxicity as measured by tau protein phosphorylation and levels of apoptosis-related proteins. More recently, S. Sharma et al. (39) reported increased resistance of the hippocampus of Ames dwarf mice to oxidative stress induced by kainic acid. Their conclusions were supported by disparate alterations in the glutathione:glutathione disulfide ratio and glutathione peroxidase activity in dwarf as compared to normal mice and by assessment of spatial memory after kainic acid exposure. Thus, the remarkable maintenance of cognitive function in the long-lived mouse mutants may be due to neuroprotective effects of local expression of IGF-1 in the brain and enhanced stress resistance of neuronal circuits involved in learning and memory.
Intriguingly, treatment of senescence accelerated SAM98 mice with an antagonist of GH-releasing hormone was recently reported to ameliorate various aspects of aging including oxidative stress in the brain and cognition (40). Moreover, this treatment increased telomerase activity and mean (but not maximal) longevity in the short-lived SAMP8 mice (40).
Several recent studies sought to identify cellular mechanisms that might explain enhanced stress resistance of GH-deficient and GH-resistant mice. The levels of mRNAs for multiple heat-shock proteins were reduced in the liver, kidney and heart of Snell dwarfs (GH-deficient mutants with endocrine phenotype identical to Ames dwarfs) and in GHRKO mice, while expression of other heat-shock proteins was elevated in tissues from Snell dwarfs only (41). In both Snell and Ames dwarf mice, the expression of Nrf-2-sensitive genes was elevated in various organs (42). Moreover, the responses of Ames dwarf and normal mice to treatment with diquat, a known hepatotoxin, differed in terms of activation of stress responsive pathways including ERK, JNK, p38MAPK, ERK-dependent and Nrf2-dependent genes, as well as Nrf2 protein (43). In fibroblasts derived from Snell dwarf mice, exposure to oxidative stress in vitro led to reduced activation of ERK but, surprisingly, increased expression of ERK-dependent genes in comparison to the corresponding values measured in fibroblasts derived from normal mice (44). Ongoing studies aim to identify functional alterations in stress-responsive pathways that are associated with extension of longevity by various genetic and dietary interventions. Further work should also allow relating these findings to the considerable evidence that Ames dwarf mice have enhanced activity of anti-oxidant enzymes and reduced oxidative damage to macromolecules including mitochondrial and nuclear DNA, proteins and lipids (45) and that enhanced resistance of these mutants to oxidative stress is related to alterations in metabolism of methionine and glutathione (46).
Involvement of other mechanisms in mediating the effects of reduced GH signaling on aging and longevity is suggested by the recent reports that GH-deficient, long-lived Ames dwarf mice differ from normal animals by reduced mutation rate (47), altered expression of multiple microRNA species (48) and genes related to purine metabolism (48), and reductions in the content of extra-cellular collagen in the heart (49) and in the size of cardiomyocytes (49). Reduced cell size likely reflects reduced levels of translation due to diminished levels and activation of mTOR in these animals (21). Moreover, improved maintenance of bone marrow stem cells was recently reported in GH-resistant mice (Ratajczak et al. 2011; 50).
Increased levels of adiponectin (24), diminished expression of inflammatory markers (51) and delayed aging of the immune system (3) in various GH-deficient and GH-resistant mutants suggest that thorough exploration of immune function and inflammatory processes in these animals and relating the findings to the established anti-inflammatory and anti-atherogenic effects of adiponectin represents another potentially exciting area for future studies.
Role of GH in mediating the effects of calorie restriction
Reduction in food intake generally reduces GH release, and the impact of reduced GH levels on circulating IGF-1 under conditions of diminished energy intake is amplified by reduced responsiveness of the liver to GH signals. Reduced levels of GH and IGF-1 are believed to be one of the mechanisms by which CR delays aging and extends longevity. The impact of CR on the somatotropic axis is complex and time-dependent. In rats, short term CR reduces GH levels while long term CR maintains pulsatile GH release (52), presumably by postponing age-related alterations in the hypothalamic control of GH secretion. In humans, short-term CR has little or no effect on circulating GH and IGF-1 levels unless protein intake is substantially reduced (53). In hypopituitary Ames dwarf mice, 30% CR initiated at approximately 2 months of age produces an additional increase in longevity, while the identical CR regimen has no effect on the longevity of GH-resistant GHRKO males and a very small effect on the longevity of GHRKO females (19, 54). These findings together with the analysis of gene expression in Ames dwarf, GHRKO and CR-exposed mice indicate that GH and CR influence longevity by different although overlapping pathways.
Recent findings reopened the issue of the role of GH in mediating the effects of CR (55). Based on studies in mice with brain-specific deletion of Sirt1 gene coding for one of mammalian sirtuins, this group suggested that CR-induced changes in Sirt1 expression lead to suppression of the somatotropic axis, including reduced GH release and that these hormonal changes play a key role in the shift from growth and reproduction to maintenance and repair. This shift is generally believed to represent activation of the evolutionarily conserved survival program and provides an attractive explanation for extended longevity under nutritionally challenging or adverse conditions.
Interaction of GH and nutrient availability was addressed in a recent study of domestic cattle. Comparison of the GH gene in seven breeds of Bos indicus and Bos taurus revealed relatively low frequencies of a wild-type (G1) and preponderance of a “domestic” (G2) allele (56). The frequency of the G2 allele increased further under selection for post-weaning weight gain under ad-libitum feeding conditions. Interestingly, under CR conditions, G1 promoted better growth while G2 was detrimental. These findings support a suggestion that domestication and selection of domestic, companion and laboratory animals lead to enrichment of genetic variants (primarily single nucleotide polymorphisms) that favor growth and reproduction under conditions of unrestricted or optimized feeding but may reduce chances of thriving under more challenging conditions.
Growth hormone and human aging
In contrast to the major and well documented impact of GH resistance and GH deficiency on aging and longevity in mice, the impact of the corresponding endocrine syndromes on human longevity is unclear and certainly not major. Association of genetic defects in GH signaling with both a reduction and an increase in life expectancy have been reported (57, 58), along with the evidence that some hypopituitary and GH-resistant individuals can reach very advanced, although not extraordinarily old age (58, 59). Within the last few years, multiple aging-related traits were examined in large cohorts of individuals with isolated GH deficiency or GH resistance who had not been given hormone replacement therapy. M.H. Aguiar-Oliveira, R. Salvatori and their colleagues reported that subjects with isolated GH deficiency due to Ghrhr gene mutations were characterized by proportional dwarfism, delayed menarche, reduced parity, indications that menopause may be advanced, and obesity which, unexpectedly was associated with increased levels of adiponectin but no changes in the levels of leptin. These individuals were partially protected from atherosclerosis and exhibited no differences in various parameters used to assess the quality of life with exception of reduced physical endurance. Although mortality of young women was increased, there were no differences from matched normal controls in the overall risk of death or lifespan of those that reached the age of 20 years (60, 61).
The Laron dwarfism syndrome of GH resistance is associated with protection from cancer and preservation of normal endothelial function (62, 63). Long-term follow-up of an unrelated cohort of 99 Ecuadorian individuals with mutations leading to GH receptor deficiency revealed the expected reduction in circulating IGF-1 levels, dwarfism and reduced insulin levels. Insulin sensitivity was enhanced in spite of increased incidence of obesity. Diabetes was absent and there was only one case of malignancy. Analysis of age distribution compared to the general population suggested increased early mortality and comparable rates, but not causes, of mortality afterwards. In particular, GH receptor-deficient individuals appeared less likely to die of stroke but more likely to die of accidents, alcohol-related events and perhaps also cardiac disease (64). Thus drastic reduction or absence of GH signaling in humans appears to have no major or consistent effect on life expectancy but is associated with protection from some age-related diseases.
Concluding remarks
Recent studies of the role of the somatotropic axis and specifically, GH in the control of aging and longevity, added new evidence for the anti-aging and life-extending effects of GH resistance and GH deficiency in laboratory mice, began to identify endocrine and molecular mechanisms involved in these effects and provided new evidence for linking extended longevity of GH-related mutants to enhancement of their stress resistance. Continuation of studies of stress resistance and stress-induced damage in mice with global or organ-specific Ghr gene deletion should identify pathways related to enhanced stress resistance of cells that had not been exposed to GH signals. The recent report of apparently IGF-1-independent effects of muscle-specific GH resistance on body composition and metabolic characteristics likely related to aging and longevity (65) indicates that this approach should be very fruitful. Studies of GH-deficient and GH-resistant humans provided evidence that some, although definitely not all, of the findings in mutant mice with similar endocrine syndromes apply to our own species.
In contrast to the effects of reduced GH signaling, expression of transgenes that increase GH levels considerably above the physiological range causes many changes resembling aging in mice and also in fish (66, 67) and hypersecretion of GH by pituitary tumors in patients with acromegaly increases risk for various age-related diseases and reduces life expectancy (68, 69). Perhaps pathological features of acromegaly and gigantism could be interpreted as accelerated aging.
There is accumulating evidence for the anti-aging and protective effects of GH deficiency and resistance and the apparent “pro-aging” effects of pathological GH excess. This raises an important question: what is the relationship of variations in GH signaling within the normal (physiological) range to aging and longevity? A likelihood that such a relationship may in fact exist in the human advises extreme caution in the widely promoted use of GH as an anti-aging agent.
Acknowledgments
Our studies on this topic were supported by NIA, The Ellison Medical Foundation, The Glenn Foundation for Medical Research and the institutional Geriatrics Research Initiative. The author apologizes to those whose work pertinent to this topic was not discussed or cited due to strict format limitations or inadvertent omissions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the aging process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 2.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 3.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci USA. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartke A. Growth hormone and aging: a challenging controversy. Clin Interv Aging. 2008;3:659–665. doi: 10.2147/cia.s3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- 6.Miller RA. Kleemeier Award Lecture: Are there genes for aging? J Gerontol A Biol Med Sci. 1999;54A:B297–B307. doi: 10.1093/gerona/54.7.b297. [DOI] [PubMed] [Google Scholar]
- 7.Samaras TT. Human body size and the laws of scaling: physiological, performance, growth, longevity and ecological ramifications. New York: Nova Science Publishers Inc; 2007. [Google Scholar]
- 8.Conover CA, Bale LK. Loss of pregnancy-associated plasma protein A extends lifespan in mice. Aging Cell. 2007;6:727–729. doi: 10.1111/j.1474-9726.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- 9.Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- 10.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor I in mouse postnatal growth. Dev Biol. 2001;229:141–162. doi: 10.1006/dbio.2000.9975. [DOI] [PubMed] [Google Scholar]
- 14.Bartke A, Bonkowski MS, Masternak MM. Growth hormone, IGF-1, insulin and long-lived mutant mice. In: Pamplona R, Barja G, editors. Longevity, Mitochondria and Oxygen Free Radicals. Kerala, India: Research Signpost; 2010. pp. 137–156. [Google Scholar]
- 15.Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, et al. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8:277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller RA, Harper JM, Galecki A, Burke DT. Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell. 2002;1:22–29. doi: 10.1046/j.1474-9728.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- 17.Eigenmann JE, Amador A, Patterson DF. Insulin-like growth factor I levels in proportionate dogs, chondrodystrophic dogs and in giant dogs. Acta Endocrinol (Copenh) 1988;118:105–108. doi: 10.1530/acta.0.1180105. [DOI] [PubMed] [Google Scholar]
- 18.Stuart JA, Page MM. Plasma IGF-1 is negatively correlated with body mass in a comparison of 36 mammalian species. Mech Ageing Dev. 2010;131:591–598. doi: 10.1016/j.mad.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Bonkowski MS, Rocha JS, Masternak MM, Al-Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci. 2009;64:516–521. doi: 10.1093/gerona/glp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonkowski MS, Dominici FP, Arum O, Rocha JS, Al Regaiey KA, Westbrook R, Spong A, Panici J, Masternak MM, Kopchick JJ, et al. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS ONE. 2009;4:e4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Dominici FP, Argentino DP, Munoz MC, Miquet JG, Sotelo AI, Turyn D. Influence of the crosstalk between growth hormone and insulin signalling on the modulation of insulin sensitivity. Growth Horm IGF Res. 2005;15:324–336. doi: 10.1016/j.ghir.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Adochio R, Leitner JW, Hedlund R, Draznin B. Rescuing 3T3-L1 adipocyes from insulin resistance induced by stimulation of AKT-mTor-S6K1 pathway and serine phosphoylation of IRS-1: Effect of reduced p85a subunit of phosphatidylinositol 3 kinase and S6K1 kinase. Endocrinology. 2008 doi: 10.1210/en.2008-0437. [DOI] [PubMed] [Google Scholar]
- 24.Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor 1/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–860. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Wagner TE, Cataldo LA, Coschigano K, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Nat Acad Sci USA. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J Gerontol A Biol Sci Med Sci. 2009;64A:443–451. doi: 10.1093/gerona/gln075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289:E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- 28.Miller RA. Cell stress and aging: new emphasis on multiplex resistance mechanisms. J Gerontol A Biol Sci Med Sci. 2009;64:179–182. doi: 10.1093/gerona/gln072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived ames dwarf mice are resistant to chemical stressors. J Gerontol A Biol Sci Med Sci. 2009;64:819–827. doi: 10.1093/gerona/glp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amador-Noguez D, Yagi K, Venable S, Darlington G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell. 2004;3:423–441. doi: 10.1111/j.1474-9728.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- 31.Amador-Noguez D, Zimmerman J, Venable S, Darlington G. Gender-specific alterations in gene expression and loss of liver sexual dimorphism in the long-lived Ames dwarf mice. Biochem Biophys Res Comm. 2005;332:1086–1100. doi: 10.1016/j.bbrc.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 32.Panici JA, Wang F, Bonkowski MS, Spong A, Bartke A, Pawlikowska L, Kwok PY, Masternak MM. Is altered expression of hepatic insulin-related genes in growth hormone receptor knockout mice due to GH resistance or a difference in biological life spans? J Gerontol, A BiolSci Med Sci. 2009;64:1126–1133. doi: 10.1093/gerona/glp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav. 2001;39:277–284. doi: 10.1006/hbeh.2001.1654. [DOI] [PubMed] [Google Scholar]
- 34.Kinney BA, Coschigano KT, Kopchick JJ, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav. 2001;72:653–660. doi: 10.1016/s0031-9384(01)00423-1. [DOI] [PubMed] [Google Scholar]
- 35.Kinney-Forshee B, Kinney N, Steger R, Bartke A. Could a deficiency in growth hormone signaling be beneficial to the aging brain? Physiol Behav. 2004;80:589–594. doi: 10.1016/j.physbeh.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26:929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Sun LY, Bartke A. Adult neurogenesis in the hippocampus of long-lived mice during aging. J Gerontol A Biol Sci Med Sci. 2007;62:117–125. doi: 10.1093/gerona/62.2.117. [DOI] [PubMed] [Google Scholar]
- 38.Schrag M, Sharma S, Brown-Borg H, Ghribi O. Hippocampus of Ames dwarf mice is resistant to beta-amyloid-induced tau hyperphosphorylation and changes in apoptosis-regulatory protein levels. Hippocampus. 2008;18:239–244. doi: 10.1002/hipo.20387. [DOI] [PubMed] [Google Scholar]
- 39.Sharma S, Rakoczy S, Dahlheimer K, Brown-Borg H. The hippocampus of Ames dwarf mice exhibits enhanced antioxidative defenses following kainic acid-induced oxidative stress. Exp Gerontol. 2010;45:936–949. doi: 10.1016/j.exger.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun LY, Steinbaugh MJ, Masternak MM, Bartke A, Miller RA. Fibroblasts from long-lived mutant mice show diminished ERK1/2 phosphorylation but exaggerated induction of immediate early genes. Free Radic Biol Med. 2009;47:1753–1761. doi: 10.1016/j.freeradbiomed.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banks WA, Morley JE, Farr SA, Price TO, Ercal N, Vidaurre I, Schally AV. Effects of a growth hormone-releasing hormone antagonist on telomerase activity, oxidative stress, longevity, and aging in mice. Proc Natl Acad Sci USA. 2010;107:22272–22277. doi: 10.1073/pnas.1016369107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swindell WR, Masternak MM, Kopchick JJ, Conover CA, Bartke A, Miller RA. Endocrine regulation of heat shock protein mRNA levels in long-lived dwarf mice. Mech Ageing Dev. 2009;130:393–400. doi: 10.1016/j.mad.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leiser SF, Miller RA. Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol Cell Biol. 2010;30:871–884. doi: 10.1128/MCB.01145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun LY, Bokov AF, Richardson A, Miller RA. Hepatic response to oxidative injury: qualitative differences in long-lived Ames dwarf mice. Faseb J. 2011;25:398–408. doi: 10.1096/fj.10-164376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown-Borg H, Johnson W, Rakoczy S, Romanick M. Mitochondrial oxidant generation and oxidative damage in Ames dwarf and GH transgenic mice. J Amer Aging Assoc. 2001;24:85–96. doi: 10.1007/s11357-001-0012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown-Borg HM, Rakoczy SG, Uthus EO. Growth hormone alters methionine and glutathione metabolism in Ames dwarf mice. Mech Ageing Dev. 2005;126:389–398. doi: 10.1016/j.mad.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Garcia AM, Busuttil RA, Calder RB, Dollé MET, Diaz V, McMahan CA, Bartke A, Nelson J, Reddick R, Vijg J. Effect of Ames dwarfism and caloric restriction on spontaneous DNA mutation frequency in different mouse tissues. Mech Ageing Dev. 2008;129:528–533. doi: 10.1016/j.mad.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bates DJ, Li N, Liang R, Sarojini H, An J, Masternak MM, Bartke A, Wang E. MicroRNA regulation in Ames dwarf mouse liver may contribute to delayed aging. Aging Cell. 2010;9:1–18. doi: 10.1111/j.1474-9726.2009.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helms SA, Azhar G, Zuo C, Theus SA, Bartke A, Wei JY. Smaller cardiac cell size and reduced extra-cellular collagen might be beneficial for hearts of Ames dwarf mice. Int J Biol Sci. 2010;6:475–490. doi: 10.7150/ijbs.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ratajczak J, Shin DM, Wan W, Liu R, Masternak MM, Piotrowska K, Wiszniewska B, Kucia M, Bartke A, Ratajczak MZ. Higher number of stem cells in the bone marrow of circulating low Igf-1 level Laron dwarf mice-novel view on Igf-1, stem cells and aging. Leukemia. 2011;25:729–733. doi: 10.1038/leu.2010.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z, Al-Regaiey KA, Masternak MM, Bartke A. Adipocytokines and lipid levels in Ames dwarf and caloric restricted mice. J Gerontol A Biol Sci Med Sci. 2006;61A:323–331. doi: 10.1093/gerona/61.4.323. [DOI] [PubMed] [Google Scholar]
- 52.Sonntag WE, Xu X, Ingram RL, D’Costa A. Moderate caloric restriction alters the subcellular distribution of somatostatin mRNA and increases growth hormone pulse amplitude in aged animals. Neuroendocrinology. 1995;61:601–608. doi: 10.1159/000126885. [DOI] [PubMed] [Google Scholar]
- 53.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Longevity: Extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- 55.Cohen DE, Supinski AM, Bonkowski MS, Donmez G, Guarente LP. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009;23:2812–2817. doi: 10.1101/gad.1839209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dani SU, Dani MA, Freire IL, Gouvea SP, Knackfuss FB, Lima FP, Mercadante ME, Monteiro E, Paggiaro SM, Razook AG, et al. Survival of the thriftiest: restricted nurture reveals the thrifty nature of a growth gene in Bos indicus. Genet Mol Res. 2010;9:1032–1044. doi: 10.4238/vol9-2gmr844. [DOI] [PubMed] [Google Scholar]
- 57.Mullis PE, Robinson IC, Salemi S, Eble A, Besson A, Vuissoz JM, Deladoey J, Simon D, Czernichow P, Binder G. Isolated autosomal dominant growth hormone deficiency (IGHD II): An evolving pituitary deficit? A multi-center follow-up study. J Clin Endocrinol Metab. 2005;10:1210. doi: 10.1210/jc.2004-1280. [DOI] [PubMed] [Google Scholar]
- 58.Krzisnik C, Kolacio Z, Battelino T, Brown M, Parks JS, Laron Z. The “Little People” of the island of Krk – revisited. Etiology of hypopituitarism revealed. J Endocrine Genet. 1999;1:9–19. [Google Scholar]
- 59.Laron Z. Do deficiencies in growth hormone and insulin-like growth factor-1 (IGF-1) shorten or prolong longevity? Mech Ageing Devel. 2005;126:305–307. doi: 10.1016/j.mad.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 60.Oliveira CR, Salvatori R, Meneguz-Moreno RA, Aguiar-Oliveira MH, Pereira RM, Valenca EH, Araujo VP, Farias NT, Silveira DC, Vieira JG, et al. Adipokine profile and urinary albumin excretion in isolated growth hormone deficiency. J Clin Endocrinol Metab. 2010;95:693–698. doi: 10.1210/jc.2009-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aguiar-Oliveira MH, Oliveira FT, Pereira RM, Oliveira CR, Blackford A, Valenca EH, Santos EG, Gois-Junior MB, Meneguz-Moreno RA, Araujo VP, et al. Longevity in untreated congenital growth hormone deficiency due to a homozygous mutation in the GHRH receptor gene. J Clin Endocrinol Metab. 2010;95:714–721. doi: 10.1210/jc.2009-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res. 2007;17:54–57. doi: 10.1016/j.ghir.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Shechter M, Ginsberg S, Scheinowitz M, Feinberg MS, Laron Z. Obese adults with primary growth hormone resistance (Laron Syndrome) have normal endothelial function. Growth Horm IGF Res. 2007;17:165–170. doi: 10.1016/j.ghir.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 64.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mavalli MD, DiGirolamo DJ, Fan Y, Riddle RC, Campbell KS, van Groen T, Frank SJ, Sperling MA, Esser KA, Bamman MM. Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J Clin Invest. 2010;120:4007–4020. doi: 10.1172/JCI42447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartke A, Brown-Borg HM, Bode AM, Carlson J, Hunter WS, Bronson RT. Does growth hormone prevent or accelerate aging? Exp Gerontol. 1998;33:675–687. doi: 10.1016/s0531-5565(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 67.Rosa CE, Kuradomi RY, Almeida DV, Lannes CF, Figueiredo Mde A, Dytz AG, Fonseca DB, Marins LF. GH overexpression modifies muscle expression of anti-oxidant enzymes and increases spinal curvature of old zebrafish. Exp Gerontol. 2010;45:449–456. doi: 10.1016/j.exger.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 68.Jadresic A, Banks LM, Child DF, Diamant L, Doyle FH, Fraser TR, Joplin GF. The acromegaly syndrome. Quart J Med. 1982;202:189–204. [PubMed] [Google Scholar]
- 69.Orme SM, McNally RJQ, Cartwright RA, Belchetz PE. Mortality and cancer incidence in acromegaly: a retrospective cohort study. J Clin Endocrinol Metabol. 1998;83:2730–2734. doi: 10.1210/jcem.83.8.5007. [DOI] [PubMed] [Google Scholar]


