Abstract
Nicotine regulates respiratory pattern by modulating excitatory neuro-transmission affecting inspiratory neurons within the preBötzinger Complex (preBötC). The nicotinic acetylcholine receptor (nAChR) subtypes mediating these effects are unknown. Using a medullary slice preparation from neonatal rat, we recorded spontaneous respiratory-related rhythm from the hypoglossal nerve (XIIn) and patch-clamped inspiratory neurons in the preBötC simultaneously. The α7 nAChR antagonists α-bungarotoxin or methyllycaconitine (MLA) had little effect on the actions of low concentrations of nicotine (0.5 μM), which included an increase in respiratory frequency; a decrease in amplitude of XIIn inspiratory bursts; a tonic inward current associated with an increase in membrane noise; an increase in the frequency and amplitude of spontaneous excitatory postsynaptic currents (sEPSCs), and; a decrease in the amplitude of inspiratory drive current in voltage-clamped preBötC inspiratory neurons. These nicotinic actions were completely reversed by dihydro-β-erythroi-dine (DH-β-E) or hexamethonium and reduced by d-tubocurarine. Comparable concentrations of RJR-2403 (0.5–1 μM), an agonist selective for α4β2 nAChRs, increased respiratory frequency to 186% and decreased the amplitude of XIIn inspiratory bursts to 83% of baseline. In voltage-clamped preBötC inspiratory (including pacemaker) neurons, RJR-2403 induced a tonic inward current of −15.2 pA associated with an increase in membrane noise, increased the frequency to 157% and amplitude to 106% of spontaneous EPSCs, and decreased the amplitude of inspiratory drive current to 80% of baseline. MLA had little effect on RJR-2403 actions, while DH-β-E completely reversed them. These results suggest that the predominant subtype of nAChRs in preBötC in neonatal rats that mediates the modulation of respiratory pattern by low concentrations of nicotine is an α4β2 combination and not an α7 subunit homomer. We do not exclude the possibility that co-assembly of α4β2 with other subunits or other nAChR subtypes are also expressed in preBötC neurons. The parallel changes in the cellular and systems level responses induced by different nicotinic agonists and antagonists support the idea that modulation of excitatory neurotransmission affecting preBötC inspiratory neurons is a mechanism underlying the cholinergic regulation of respiratory pattern (Shao and Feldman 2001). This study provides a useful model system for evaluating potential therapeutic cholinergic agents for their respiratory effects and side effects.
INTRODUCTION
Nicotinic receptors are targets for the neurotransmitter acetylcholine (ACh) and exogenous cholinergic ligands such as nicotine from cigarette smoke. Nicotonic ACh receptors (nAChRs) are expressed in many brain stem areas involved in cardiorespiratory control (Dominguez del Toro et al. 1994; Wada et al. 1989), and ACh plays a role in cardiovascular and respiratory regulation (Burton et al. 1994; Shao and Feldman 2001; Wang et al. 2001; Weinstock et al. 1981). Nicotine is implicated in various cardiorespiratory disorders. Maternal smoking, a potent source of nicotine in fetal brain, is a major risk factor for sudden infant death syndrome (SIDS) (Klonoff-Cohen et al. 1995; Taylor and Sanderson 1995). Smoking is also a risk factor for sleep-disordered breathing, i.e., sleep apnea (Wetter et al. 1994). Prenatal nicotine exposure delays early postnatal changes in breathing pattern and increases the frequency of apnea in mice (Robinson et al. 2002). Low concentrations of nicotine affect respiratory pattern in vivo (Howell 1995; Stepans and Wilkerson 1998). We have shown that activation of nAChRs in the preBötzinger Complex (pre-BötC), the hypothesized site for respiratory rhythm generation (Gray et al. 1999; Smith et al. 1991), affects respiratory frequency and pattern in vitro. Activation of nAChRs modulates excitatory neurotransmission by potentiating tonic excitatory input to, and inhibiting excitatory coupling between, preBötC inspiratory neurons (Shao and Feldman 2001). The nicotinic receptor subtypes that mediate these effects are unknown.
nAChRs are ligand-gated ion channels formed as pentameric assemblies of subunits. Ten α (α1–10), four β (β1–4), and γ, δ, and ε subunits have been identified (Lukas et al. 1999; Lustig et al. 2001). Different combinations of these subunits can form functional nAChRs often with distinct pharmacological profiles (Chavez-Noriega et al. 1997; Luetje and Patrick 1991; McGehee and Role 1995). Different subtypes of nAChRs formed from a variety of nicotinic subunit combinations are found in the mammalian CNS (Jones et al. 1999; Klink et al. 2001; le Novere et al. 1999; Zoli et al. 1998). Are these diverse nAChR subtypes physiologically significant? Are different nAChRs linked to different functions? Resolving these questions is an ongoing challenge for the field of nicotinic pharmacology with obvious clinical implications for neurological diseases related to deficit of cholinergic functions (Clementi et al. 2000; Picciotto et al. 2000).
In the ventrolateral medulla (which includes the preBötC), the nAChR subunits α4, α7, and β2 are present (Dominguez del Toro et al. 1994; Wada et al. 1989). The purpose of this study was to identify the subtypes of nAChRs mediating the effects of nicotine/ACh on central control of respiration. We examined the differential effects of subtype selective nicotinic agonists and antagonists. Pharmacological characterization of these nAChRs may provide a basis for treatment strategies for SIDS and sleep apnea (Gothe et al. 1985) as well as central respiratory failure during organophosphate poisoning due to pesticides or nerve gases, e.g., sarin (Lotti 1991; Rickett et al. 1986). Such characterization may also provide a basis for understanding and ultimately reducing the respiratory side effects of therapeutic use of cholinergic agents for Parkinson's disease, Alzheimer's disease, schizophrenia, and analgesia (Jones et al. 1999; Rezvani and Levin 2001; Rusted et al. 2000).
METHODS
Slice preparation
Experiments were performed on a medullary slice preparation that retains functional respiratory networks and generates respiratory rhythm in vitro (Smith et al. 1991). Briefly, Sprague-Dawley neonatal rats (0–3 days old) were anesthetized by hypothermia (incubated on ice for 3–4 min) and then promptly decerebrated. The cerebellum was removed and the brain stem-spinal cord was isolated. The brain stem-spinal cord was mounted in the specimen vise of a Vibratome (VT 100, Technical Products International) oriented vertically with rostral end upward. The brain stem was sectioned serially in the coronal plane under a dissection microscope until the landmarks at rostral boundary of preBötC were visible. One transverse slice (500– 650 μm thick) was cut. The slice was transferred to a recording chamber of 3-ml volume and stabilized with a threaded frame. The dissection and slicing were performed in an artificial cerebrospinal fluid (ACSF) bubbled with 95% O2-5% CO2 at room temperature. The ACSF contained (in mM) 128 NaCl, 3.0 KCl, 1.5 CaCl2, 1.0 MgSO4, 23.5 NaHCO3, 0.5 NaH2PO4, and 30 glucose. During electrophysio-logical recording, the slice was continuously superfused (2.5–3.5 ml/min) with ACSF with increased KCl (9 mM) that was recycled into a reservoir equilibrated with 95% O2-5%CO2. The ACSF in the recording chamber was maintained at 27 ± 1°C. All slices studied had rhythmic activities from XIIn that were similar in frequency and in temporal pattern to the respiratory activities recorded from en bloc brain-stem-spinal cord preparations (Smith et al. 1991).
Electrophysiological recording
Neurons within 100 μm of the slice surface were visualized with an infrared-differential interference contrast (IR-DIC) microscope (Axioskop, Zeiss). The respiratory neurons we recorded in this study fired in phase with the inspiratory bursts from XIIn and were located ventral to the nucleus ambiguus. Patch electrodes were pulled from thick-wall (0.32 mm) borosilicate glass with tip size of 1–1.5 μm (resistance: 4–6.5 MΩ). The electrode-filling solution contained (in mM) 140 K-gluconate, 5.0 NaCl, 0.1 CaCl2, 1.1 EGTA, 10 HEPES, and 2.0 ATP (Mg2+ salt), pH adjusted to 7.3 with KOH. Intracellular signals were amplified with a patch-clamp amplifier (AXOPATCH 200A, Axon Instruments, Foster City, CA). A −10-mV junction potential was determined experimentally; reported values of potential are corrected values.
Respiratory-related rhythmic motor activity was recorded from the cut ends of XIIn roots with a suction electrode, amplified 10,000– 20,000 times and band-pass filtered (3–3,000 Hz) with an amplifier (GRASS Instruments). Both signals from intracellular recording and from XIIn roots were recorded on video cassettes via pulse code modulation (A. R. Vetter,). Selected segments of intracellular signals were low-pass filtered at 1 kHz (except otherwise stated) with a 8-pole Bessel filter (Frequency Devices) and XIIn nerve activity were rectified and integrated (Paynter filter, τ = 15 ms), then both were digitized at 4,000 Hz sampling frequency with DIGIDATA 1200 and software CLAMPEX 8 (AXON Instruments) on a Pentium-based computer. For measuring the phasic inward current of inspiratory neurons, membrane current signals were filtered at 20 Hz and digitized at a sampling frequency of 100 Hz.
Drug application
Nicotinic agonists or antagonists were applied to the perfusate. For agonists, the baseline was measured immediately prior to the application and the effects were measured 3–5 min after adding them. For antagonists, the effects were measured 4–6 min after adding them.
(−)-Nicotine (hydrogen tartrate salt), RJR-2403 (hemigalactarate salt), α-bungarotoxin (α-BgTx), d-tubocurarine chloride (d-TC), Methyllycaconitine citrate, dihydro-β-erythroidine hydrobromide, hexamethonium chloride (HMT), were obtained from SIGMA/RBI (Sigma-Aldrich).
Data analysis
Respiratory periods were averaged from 10 consecutive periods in the baseline or drug application condition for each preparation and were used in statistical tests. Respiratory frequency was taken as reciprocal of period. The inspiratory amplitude of XIIn activity and the phasic inward current amplitude of inspiratory neurons were measured from averaged envelope of 5 consecutive inspiratory periods triggered by the up-stroke of the integrated inspiratory XIIn bursts (CLAMPEX 8). Then they were averaged across neurons or preparations and presented as mean ± SD, n = number of cells (for whole cell recording) or preparations (for XIIn motor output recording) is indicated. Usually, we measured electrophysiological parameters before (predrug baseline), during application of agonist and during agonist + antagonist for one neuron or one slice. Repeated-measures ANOVA (Neter et al. 1990) was used to test the statistical significance of the responses. Post hoc multiple comparison analyses in different situations, when necessary, are described in the Figure Legends. The procedure MIXED in the data analysis software package SAS (V8.2, SAS Institute,) was used for these analyses. P < 0.05 was the criterion for statistical significance.
Spontaneous EPSC (sEPSC) data were analyzed with a program written in AXOBASIC (AXON Instruments). This program read the Axon Binary Files (ABF) containing two channels of digitized data: the whole cell patch-clamp signal and the integrated XIIn activity. The program detected sEPSCs during expiratory periods by setting a threshold for the derivative of the neuronal signal and then measured the time as well as the peak amplitude of sEPSCs. The program ignored the neuronal activity and the time during inspiratory periods, when neurons receive substantial endogenous currents. Statistical significance for difference in rates, i.e., frequency, of sEPSCs was analyzed with a method detailed in Shao and Feldman (2001). Because the amplitude of sEPSCs is not normally distributed, statistical significance for difference in sEPSC amplitude was analyzed with Kolmogorov-Smirnov test (Mini Analysis Program V5, Synaptosoft, GA). Rates and amplitudes of sEPSCs were tested during application of cholinergic agents versus baseline conditions for each neuron.
RESULTS
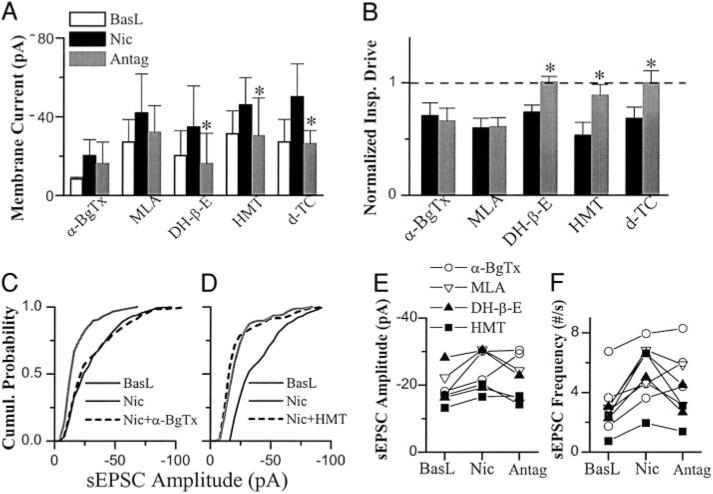
We examined the effects of the nAChR antagonists on the responses of respiratory-related rhythmic XIIn activity pattern and inspiratory neurons induced by low concentrations of nicotine [0.5 μM, equivalent to the arterial blood nicotine concentration after a cigarette has been smoked (Henningfield et al. 1993)]. The various nicotinic antagonists α-BgTx (0.2 μM, Fig. 1A), MLA (1 μM, Fig. 1B), DH-β-E (0.2 μM, Fig. 1C), HMT (10 μM, Fig. 1D) ,or d-TC (10 μM, Fig. 1E) were applied immediately after the maximum effects of nicotine were observed which was usually at 3–5 min (Fig. 2A). To examine the possible confounding effects of nAChR desensitization, we analyzed the time course of the nicotine-induced increase in respiratory frequency. Under baseline conditions, the XIIn burst frequency was 6.64 ± 1.9/min. Within 3–5 min after bath application of nicotine, the frequency increased to 276 ± 56% (n = 18) of baseline; this effect slightly desensitized in the continuous presence of nicotine (Fig. 2A). At about 10 min after application, the frequency decreased to 254 ± 36% of baseline and did not return to the baseline level in the presence of nicotine for as long as 30 min (n = 3). α-BgTx or MLA, potent antagonists for α7 nAChR, had no significant effect on the frequency compared with control group (application of nicotine alone; frequency was measured at the time points equivalent to time points when the antagonist effects were measured following application of antagonists; Fig. 2B). In contrast, either DH-β-E or HMT reduced the frequency close to baseline levels, whereas d-TC induced a partial reduction. The decrease in frequency with DH-β-E, HMT, and d-TC were significant compared with the control desensitization while the decrease with α-BgTx or MLA was not (analyzed by 2-way repeated-measures ANOVA and the post hoc analysis based on Dunnett (SAS Institute, 1999). When we switched the order by adding α-BgTx before adding nicotine in the bath, we got the similar results (n = 2, data not shown).
FIG. 1.
Nicotinic antagonists (Antag) α-bungarotoxin (α-BgTx, 0.2 μM, A) and methyllycaconitine (MLA, 1 μM, B) had little effect on the responses in inspiratory neurons and pattern of respiratory-related rhythmic activity from hypoglossal nerve (XIIn) induced by nicotine (Nic, 0.5 μM) while dihydro-β[notdef]erythroidine (DH-β-E, 0.2 μM, C), hexamethonium (HMT, 10 μM, D) and d-tubocurarine (d-TC, 10 μM, E) reversed these responses. In each panel, top traces are Im: membrane current of preBötzinger Complex (preBötC) neuron voltage-clamped at −60 mV; bottom traces are ∫XIIn: integrated XIIn activity. Right panels: low-pass filtered (10 Hz) traces of averages of 5 consecutive inspiratory drive currents and integrated inspiratory bursts of XIIn at extended scales at baseline, Nic and Nic plus Antag conditions and were superimposed. Averages were triggered by the onset of the integrated inspiratory bursts from XIIn. All vertical scales represent 40 pA.
FIG. 2.
Systems level effects of Nic and Antag. A: Nic (0.5 μM) increased respiratory frequency; this effect slightly desensitized. Nic was bath applied at time 0 min and was continuously present for 30 min (n = 3). B: the effects of nicotinic antagonists α-BgTx (n = 4) or MLA (n = 6) on Nic-induced increase in respiratory frequency are not significant compared with control (Ctrl). DH-β-E (n = 4), HMT (n = 5) and d-TC (n = 6) reversed these effects. Control value was taken at about 10 min during bath application of nicotine in the group of control experiment as A without adding antagonist. Ten minutes was equivalent to the time the effects of antagonists were measured (4–6 min after Antag application and Antag was applied 3–5 min after Nic application) in the groups of antagonist experiments. Two-way repeated-measures ANOVA and the post hoc analysis based on Dunnett were used to analyze the change in frequency with antagonists compared with the control desensitization. C: the effects of α-BgTx or MLA on nicotine-induced decrease in amplitude of inspiratory bursts were not significant, while DH-β-E, HMT and d-TC reversed these nicotinic effects. One-way repeated-measures ANOVA for each group and post hoc analysis based on Tukey (SAS Institute 1999) were used. *, statistically significant. The frequency and amplitude data were normalized to baseline values prior to Nic application for each preparation. Raw data (nonnormalized) were used in statistical tests.
Under baseline conditions, the amplitude of integrated XIIn inspiratory bursts was 128 ± 82 μV. Nicotine (0.5 μM) decreased the amplitude to 78 ± 11% of baseline; DH-β-E, HMT, or d-TC reversed this effect while α-BgTx and MLA did not (Fig. 2C).
In preBötC inspiratory neurons voltage-clamped at −60 mV [n = 15 including 3 pacemaker neurons, a subset of inspiratory neurons which fire ectopic bursts of action potentials during the normally silent expiratory periods if depolarized to −45 to −55 mV, (Smith et al. 1991)], nicotine induced a tonic inward current of −17 ± 13.6 pA associated with an increase in membrane noise (random current fluctuations) and decreased the phasic inspiratory drive current to 64 ± 12% from a baseline level of −63 ± 40 pA. DH-β-E, HMT and d-TC reversed these effects while α-BgTx and MLA did not (Fig. 3, A and B). The nicotine-induced tonic inward current appeared to partially recover during MLA application, but this was not statistically significant (Figs. 1 and 3A).
FIG. 3.
Cellular level effects of Nic and Nic Antag in preBötC inspiratory neurons voltage-clamped at −60 mV. The effects of α-BgTx (n = 4) or MLA (n = 4) on Nic-induced tonic inward current (A) and decrease in amplitude of phasic inspiratory drive current (B) were not significant; DH-β-E (n = 5), HMT (n = 4), and d-TC (n = 4) reversed these nicotine-induced responses. The amplitude of phasic inspiratory drive current was normalized by the values prior to application of nicotine (baseline: BasL). One-way repeated-measures ANOVA for each group and posthoc analysis based on Tukey were used for A and B. *, statistically significant. C: α-BgTx did not reduce nicotine-induced increase in amplitude of spontaneous EPSCs (sEPSCs). Cumulative (Cumul) histogram from 1 representative neuron. D: HMT reversed the Nic-induced increase in amplitude of sEPSCs. Summaries of the effects of α-BgTx, MLA, DH-β-E, and HMT on the Nic-induced changes in amplitude (E) and frequency (F) of sEPSCs. sEPSCs were recorded for 1–2 min, and 100–600 events were collected in each condition for each neurons. Each symbol indicates 1 neuron. Refer to results for statistical analyses for C–F.
Bath application of nicotine increased the frequency of sEPSC to 158 ± 76.5% from a baseline of 3.12 ± 1.6/s (n = 14) and increased the amplitude of sEPSC to 115 ± 31.6% from a baseline of −20.2 ± 5.4 pA in voltage-clamped inspiratory neurons during expiratory periods. The increase in frequency of sEPSCs was statistically significant in 9 of 14 neurons. Figure 3F summarizes of the effects of α-BgTx, MLA, DH-β-E, or HMT on sEPSC frequency for these nine neurons. The sEPSC frequency of all four neurons in the group that antagonist DH-β-E or HMT was applied (filled symbols in Fig. 3F) was significantly decreased by the antagonist; while the frequency of four of the five of neurons that antagonist α-BgTx or MLA was applied (open symbols in Fig. 3F) was not significantly decreased by the antagonist. Figure 3D shows a representative neuron in which 10 μM HMT reversed the nicotine-induced increase in spontaneous EPSC amplitude while α-BgTx did not (Fig. 3C). We analyzed the sEPSC amplitude with Kolmogorov-Smirnov test for each neuron. Most neurons (7 of 9) in which the sEPSC frequency was increased by nicotine also exhibited an increase in amplitude. Figure 3E summarizes the effects of α-BgTx, MLA, DH-β-E, or HMT on sEPSC amplitude of the seven neurons with which sEPSC amplitude was increased by nicotine. The sEPSC amplitude of three of four neurons was significantly decreased by the antagonist DH-β-E or HMT (filled symbols in Fig. 3E), while the amplitude of two of three neurons was not significantly decreased by α-BgTx or MLA (open symbols in Fig. 3E).
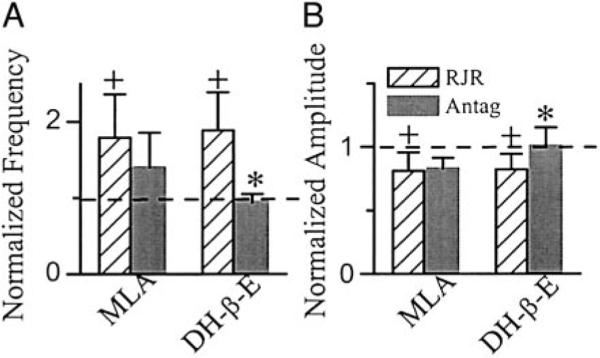
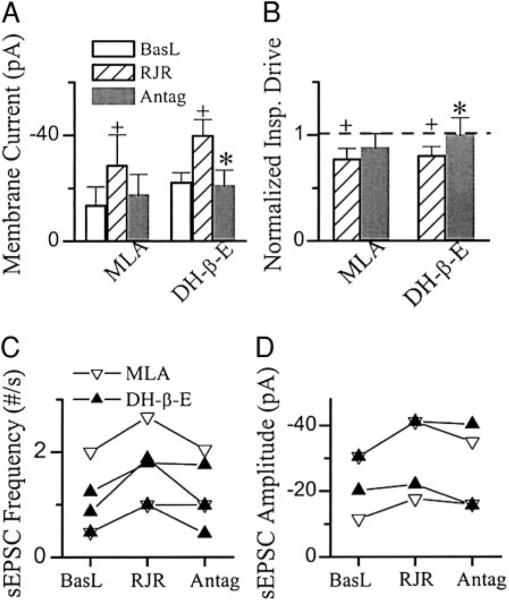
Bath application of RJR-2403 (0.5–1 μM, comparable to the concentrations of nicotine we used), an agonist selective to α4β2 nAChR (Bencherif et al. 1996), increased respiratory frequency to 186 ± 48% of baseline of 9.3 ± 3.2/min and decreased the amplitude of inspiratory bursts in XIIn to 83 ± 13% of baseline level of 107 ± 36 μV (n = 9; Figs. 4, A and B, and 5, A and B). In preBötC inspiratory neurons voltage-clamped at −60 mV (n = 9 including 3 pacemaker neurons), RJR-2403 induced a tonic inward current of −15.2 ± 10.1 pA associated with an increase in membrane noise and decreased the amplitude of phasic inspiratory drive current to 80 ± 9% of baseline −65 ± 42 pA (Figs. 4, A and B, and 6, A and B). The frequency of sEPSCs during expiratory periods was 4.5 ± 4.5/s and the amplitude was −27 ± 12 pA in baseline conditions (n = 8). RJR-2403 increased the frequency of these sEPSCs to 157 ± 54% and the amplitude to 106 ± 25% of baseline (Figs. 4, A and B, and 6C). Statistical analyses were done for frequency and amplitude of sEPSCs (refer to methods) for each neuron. The increase in frequency was significant in five of eight neurons and in four of these five neurons; the amplitude of sEPSC was also increased by RJR-2403 (Fig. 6D). The effects of RJR-2403 at both systems and cellular levels were similar to the effects of nicotine, while the changes induced by RJR-2403 were smaller than those induced by nicotine. These effects were reversed by DH-β-E (0.2 μM) but only minimally affected by MLA (1 μM; Figs. 4, A and B, 5, A and B, and 6, A and B). Figure 6, C and D, summarizes the effects of MLA or DH-β-E on RJR-2403-induced changes in sEPSCs. In the five neurons with RJR-2403-induced increase in sEPSC frequency, the frequency was decreased significantly by DH-β-E in two of three neurons; the frequency was not decreased by MLA in one of two neurons. RJR-2403 induced increase in sEPSC amplitude in four neurons, the amplitude was decreased by MLA (n = 2) or by DH-β-E (n = 2).
FIG. 4.
Nic agonist RJR-2403 (RJR, 0.5–1 μM) induced responses in inspiratory neurons and pattern of respiratory-related rhythmic activity from XIIn. Nic Antag MLA (1 μM; A) did not, but DH-β-E (0.2 μM; B) did, reverse the RJR-induced responses. Right: low-pass filtered (10 Hz) traces of average of 5 consecutive inspiratory drives of the neurons and integrated inspiratory bursts of XIIn at extended scales at baseline, RJR and RJR plus Antag conditions and were superimposed. Averages were triggered by the onset of integrated inspiratory bursts from XIIn. The vertical scales represent 40 pA.
FIG. 5.
Systems level effects of Nic agonists RJR-2403 (RJR) and Antags MLA and DH-β-E. RJR increased respiratory frequency (A) and decreased the amplitude of inspiratory bursts (B). Effects of MLA (n = 5) were not significant, whereas DH-β-E (n = 7) reversed these responses. One-way repeated-measures ANOVA for each group and post hoc analysis based on Tukey were used. +, statistically significant difference between RJR application and baseline conditions. *, statistically significant difference between Antag and RJR conditions. The frequency and amplitude data were normalized by dividing the numbers by that in the baseline condition prior to RJR application for each preparation. Raw data (nonnormalized) were used in the statistical tests.
FIG. 6.
Cellular effects of Nic agonist RJR-2403 (RJR) and Antags MLA and DH-β-E. RJR induced a tonic inward current (A) and decreased the amplitude of inspiratory drive currents (B) in preBötC inspiratory neurons voltage-clamped at −60 mV. The effects of MLA (n = 5) were not significant, whereas DH-β-E (n = 6) reversed the RJR-induced responses. Effects on amplitude of phasic inspiratory drive current presented were normalized by the values prior to application of RJR, whereas the nonnormalized data were used for statistical tests. One-way repeated-measures ANOVA for each group and post hoc analysis based on Tukey were used. +, statistically significant difference between RJR and basline (BasL) conditions. *, statistically significant difference between Antag and RJR conditions. Summary of the effects of RJR, MLA, and DH-β-E on frequency (C) and amplitude (D) of spontaneous EPSCs (sEPSCs). sEPSCs were recorded for 1–2 min and 100–600 events were collected in each condition for each neurons. Each symbol indicates 1 neuron. Refer to results for statistical analyses for C and D.
We observed parallel changes in preBötC inspiratory neurons and in the respiratory-related motor output induced by various nicotinic agonists and antagonists. Whenever a nicotinic agonist induced a tonic inward current associated with an increase of membrane noise, decreased the phasic inspiratory drive current, and increased the frequency and amplitude of sEPSCs during expiratory periods, we concurrently observed an increase in frequency and a decrease in amplitude of respiratory-related rhythmic motor activity in the XIIn. When the nicotinic agonist-induced responses at the cellular level were reduced by an antagonist, the increase in frequency and decrease in amplitude of the respiratory-related motor activity in the XIIn were concurrently reduced.
DISCUSSION
We demonstrated that the pharmacological properties of the nAChR subtypes in the neonatal rat preBötC mediating the modulatory effects of low concentrations of nicotine on respiratory pattern were different from those of α7 subunit-containing receptors. The nicotinic receptor antagonists α-BgTx or MLA had little effect on the nicotinic actions, which included increasing respiratory frequency, decreasing the amplitude of inspiratory bursts of respiratory-related motor activity from XIIn, inducing a tonic inward current associated with an increase in membrane noise, increasing the frequency and amplitude of sEPSCs during the expiratory period, and decreasing the amplitude of phasic inspiratory drive inward current in preBötC inspiratory neurons. These nicotinic effects were completely reversed by DH-β-E or HMT and reduced by d-TC. The nicotinic agonist RJR-2403 had effects similar to those of nicotine at both the systems level, i.e., respiratory frequency and pattern of XIIn, and the cellular level. MLA had little effect on the actions of RJR-2403 while DH-β-E completely reversed these actions. These results suggest that the subtypes of nAChR that mediate the modulatory effects of low concentration of nicotine on respiratory frequency and pattern are primarily an α4β2 subunit combination. On the basis of these results, we do not exclude the possibility that co-assembly with other subunits or other nAChR subtypes are expressed in preBötC neurons. We observed that RJR-2403 was apparently less potent than nicotine at the concentrations of 0.5–1 μM in modulating respiratory frequency. The parallel changes in cellular events in preBötC inspiratory neurons and in the respiratory-related motor output pattern induced by different nicotinic agonists and antagonists support the hypothesis that nicotine/ ACh regulate respiratory frequency and pattern by modulating excitatory neurotransmission via an enhancement of the tonic excitatory input to, and an inhibition of the phasic excitatory coupling between, preBötC inspiratory neurons (Shao and Feldman 2001).
Antagonist profile of nAChRs that mediate the modulatory effects of nicotine
The predominant forms of functional nAChRs in the brain are pentameric heteromeric assemblies of α4 and β2 subunits and homomeric assemblies of α7 subunits. The α7 homomeric nAChR is α-BgTx sensitive and is rapidly desensitized. The α4β2 heteromer is insensitive to α-BgTx and MLA but sensitive to DH-β-E (Jones et al. 1999). We showed that α-BgTx and MLA had minimal blocking effects on the nicotine-induced responses at both the cellular and systems levels, but these responses were completely blocked by DH-β-E or HMT and partially blocked by d-TC. This antagonist profile resembles that seen in rat retinal ganglion cells (Lipton et al. 1987) and that of α4β2 nAChRs expressed in mouse fibroblasts (Whiting et al. 1991). Thus our data suggest that the predominant preBötC nAChR involved in respiratory modulation by low concentrations of nicotine is an assembly of α4 β2 subunits and not a homomeric assembly of α7. Our data are consistent with the observations that the ACh-induced increase in respiratory frequency in the en bloc brain stem-spinal cord preparation can be completely abolished by a combination of DH-β-E and atropine (Murakoshi et al. 1985); the excitatory effects of iontophoretically applied nicotine on respiratory-related and nonrespiratory neurons can be blocked by DH-β-E (Bradley and Lucy 1983); and nAChR subunits α4 and β2 are present in the reticular formation in ventrolateral medulla including the preBötC (Wada et al. 1989).
Effects of RJR-2403
The newly developed nicotinic agonist RJR-2403 is selective for α4β2 nAChR subtypes (Bencherif et al. 1996; Lippiello et al. 1996; Papke et al. 2000). Here, RJR-2403 evoked changes in respiratory-related rhythmic activity pattern and in preBötC inspiratory neurons similar to those induced by nicotine, and MLA had little effect while DH-β-E completely blocked these responses. These data suggest the nAChR sub-type mediating the modulatory effects of nicotine/ACh on respiratory pattern is α4β2. Bencherif et al. (1996) showed that the potency and efficacy of RJR-2403 in activating rat thalamic synaptosomes was comparable to nicotine. Papke et al. (2000) showed that RJR-2403 was more potent and more efficacious than nicotine in activating human α4β2 nAChR expressed in Xenopus oocytes. In this study, the effects induced by RJR-2403 were smaller than those induced by nicotine (e.g., 186 vs. 276% change in frequency) in a similar concentration range. There could be two possible explanations for the smaller effects of RJR-2403: RJR-2403 is a less potent agonist for α4β2 nAChRs in neonatal rat preBötC that are somehow different from those in adult thalamus and from human α4β2 nAChRs expressed in oocytes or RJR-2403 is selective for α4β2 nAChR compared with nicotine, but nicotine acts on wider range of nAChR subtypes in preBötC that may also be involved in modulation of respiratory pattern. We are not able to exclude the latter possibility because, besides α4β2 and α7, pairwise combinations of α2-α4 with β2 or β4 as well as some triple subunit combinations such as α3β4β2 and α3β2α5 can form functional nAChRs when expressed in Xenopus oocytes or other expression systems (Chavez-Noriega et al. 1997; Colquhoun and Patrick 1997; Gopalakrishnan et al. 1996; Luetje and Patrick 1991; McGehee and Role 1995; Papke et al. 2000). Native functional nAChRs containing subunits other than α4, β2, and α7 are found in a limited number of brain regions (Klink et al. 2001; le Novere et al. 1999; Zoli et al. 1998) and are much less well characterized.
Functional implications
nAChR subtypes are linked to a wide variety of brain functions such as cognition, addiction, locomotion, and pain sensitivity as well as pathological conditions such as Parkinson's disease, Alzheimer's disease, epilepsy, and schizophrenia (Jones et al. 1999; Nomikos et al. 2000; Picciotto et al. 2000). In anesthetized animals in vivo, ACh enhances ventilation; these effects are potentiated by the cholinesterase inhibitor physostigmine (Gesell et al. 1943). Iontophoretic administration of ACh excites some medullary respiratory neurons, while it inhibits or has no effect on others (Böhmer et al. 1987, 1989; Bradley and Lucy 1983; Haji et al. 1996; Jordan and Spyer 1981; Kirsten et al. 1978; Salmoiraghi and Steiner 1963). These studies are difficult to interpret due to the confounding effects of anesthesia, the lack of precise anatomical, and/or physiological characterization of neurons. Using the more reduced medullary slice preparation combined with IR-DIC microscopy, we determined the differential effects of nicotine on the preBötC and the hypoglossal nucleus as well as the underlying cellular mechanisms (Shao and Feldman 2001). In the current study, we demonstrated that α4β2 nAChRs in the preBötC played a role in respiratory modulation. In contrast, Neff et al. (1998) showed that, in a medullary area adjacent to preBötC, nicotine modulated presynaptic glutamate release onto cardiac vagal neurons by actions at α7 containing nAChRs. These results are physiologically significant as two different nAChR subtypes are linked to distinct respiratory and cardiac modulatory functions (although probably not exclusively) in the medulla. Given that many brain stem respiratory-related areas are excluded in our slices, ACh/nicotine could also affect ventilation by acting on neurons in these other respiratory-related areas.
nAChRs are classified by their molecular composition (Lukas et al. 1999). To identify the subunit combinations of native functional nAChRs in the brain has been difficult because of lack of specific agonists or antagonists for most nAChR sub-types. One approach to gain insight into the molecular composition of native nAChRs is to compare their functional and pharmacological profiles with those obtained with recombinant receptors. Although with the available pharmacological tools the precise molecular composition of the preBötC nAChRs mediating the modulatory effects of nicotine on respiratory pattern cannot be unambiguously identified, our pharmacological characterization of these receptors provides useful information relevant for the therapeutic use of nicotinic agents (Gothe et al. 1985; Levin et al. 1999; Rezvani and Levin 2001; Rusted et al. 2000). With the preparation described in this study, the effects of various drugs on respiratory neurons, on neurotransmitter systems in the preBötC, and on respiratory-related motor activity can be easily examined, where the respiratory rhythm generation circuits are highly accessible for pharmacological manipulation. These results provide a basis for the investigation of nicotinic agonists in the treatment of obstructive sleep apnea (Gothe et al. 1985; discussion in Shao and Feldman 2001) and of antagonists for central respiratory failure resulting from nerve gas exposure (Rickett et al. 1986). This preparation can be used to screen for potential respiratory side effects of nicotinic agents developed to treat nonrespira-tory neurological disorders, e.g., for Parkinson's disease or Alzheimer's disease.
Low concentrations of nicotine enhance tonic excitatory input to and inhibits excitatory coupling between preBötC inspiratory neurons. Based on computational models of respiratory rhythm generation (Butera et al. 1999), these cellular effects of nicotine can account for the cholinergic modulation of respiratory frequency and pattern (Shao and Feldman 2001). Here, we observed that whenever a nicotinic agonist induced a tonic inward current associated with an increase of membrane noise, increased the frequency and amplitude of sEPSCs in the preBötC inspiratory neurons (indicating an enhancement of tonic excitatory input to these neurons) as well as decreased the phasic inspiratory drive current (indicating an inhibition of excitatory coupling between these neurons), we concurrently observed an increase in frequency and a decrease in amplitude of the respiratory-related rhythmic activity in the XIIn (Figs. 4–6). When the nicotinic agonist-induced responses at the cellular level were blocked by an antagonist, the increase in frequency and decrease in amplitude of the respiratory-related motor activity in XIIn were blocked and vice versa (Figs. 1–3). These parallel changes in preBötC inspiratory neurons and in the respiratory motor output induced by various nicotinic agonists and antagonists suggest that the modulation of excitatory neurotransmission affecting preBötC inspiratory neurons is a mechanism underlying the regulation of respiratory frequency and pattern by nicotine/ACh.
Acknowledgments
This research was supported by Tobacco-Related Disease Research Program Grant 10RT-0241 and National Heart, Lung, and Blood Institute Grant HL-40959.
REFERENCES
- Bencherif M, Lovette ME, Fowler KW, Arrington S, Reeves L, Caldwell WS, Lippiello PM. RJR-2403: a nicotinic agonist with CNS selectivity. I. In vitro characterization. J Pharmacol Exp Ther. 1996;279:1413–1421. [PubMed] [Google Scholar]
- Böhmer G, Schmid K, Baumann M. Evidence for a respiration-modulated cholinergic action on the activity of medullary respiration-related neurons in the rabbit. An iontophoretic study. Pflügers Arch. 1989;415:72–80. doi: 10.1007/BF00373143. [DOI] [PubMed] [Google Scholar]
- Böhmer G, Schmid K, Schmidt P, Stehle J. Cholinergic effects on spike density and burst duration of medullary respiration-related neurons in the rabbit: an iontophoretic study. Neuropharmacology. 1987;26:1561–1572. doi: 10.1016/0028-3908(87)90002-5. [DOI] [PubMed] [Google Scholar]
- Bradley PB, Lucy AP. Cholinoceptive properties of respiratory neurons in the rat medulla. Neuropharmacology. 1983;22:853–858. doi: 10.1016/0028-3908(83)90131-4. [DOI] [PubMed] [Google Scholar]
- Burton MD, Nouri K, Baichoo S, Samuels-Toyloy N, Kazemi H. Ventilatory output and acetylcholine: perturbations in release and muscarinic receptor activation. J Appl Physiol. 1994;77:2275–2284. doi: 10.1152/jappl.1994.77.5.2275. [DOI] [PubMed] [Google Scholar]
- Butera R, Rinzel J, Smith J. Models of respiratory rhythm generation in the pre-Bötzinger complex. II. Populations of coupled pacemaker neurons. J Neurophysiol. 1999;82:398–451. doi: 10.1152/jn.1999.82.1.398. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4, and h alpha 7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–356. [PubMed] [Google Scholar]
- Clementi F, Fornasari D, Gotti C. Neuronal nicotinic receptors, important new players in brain function. Eur J Pharmacol. 2000;393:3–10. doi: 10.1016/s0014-2999(00)00066-2. [DOI] [PubMed] [Google Scholar]
- Colquhoun LM, Patrick JW. Alpha3, beta2, and beta4 form heterotrimeric neuronal nicotinic acetylcholine receptors in Xenopus oocytes. J Neurochem. 1997;69:2355–2362. [PubMed] [Google Scholar]
- Dominguez Del Toro E, Juiz JM, Peng X, Lindstrom J, Criado M. Immunocytochemical localization of the alpha 7 subunit of the nicotinic acetylcholine receptor in the rat central nervous system. J Comp Neurol. 1994;349:325–342. doi: 10.1002/cne.903490302. [DOI] [PubMed] [Google Scholar]
- Gesell R, Hansen E, Worzniak J. Humoral intermediation of nerve cell activation in the central nervous system. Am J Physiol. 1943;138:776–791. [Google Scholar]
- Gopalakrishnan M, Monteggia LM, Anderson DJ, Molinari EJ, Piattoni-Kaplan M, Donnelly-Roberts D, Arneric SP, Sullivan JP. Stable expression, pharmacologic properties and regulation of the human neuronal nicotinic acetylcholine alpha 4 beta 2 receptor. J Pharmacol Exp Ther. 1996;276:289–297. [PubMed] [Google Scholar]
- Gothe B, Strohl KP, Levin S, Cherniack NS. Nicotine: a different approach to treatment of obstructive sleep apnea. Chest. 1985;87:11–17. doi: 10.1378/chest.87.1.11. [DOI] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji A, Furuichi S, Takeda R. Effects on iontophoretically applied acetylcholine on membrane potential and synaptic activity of bulbar respiratory neurones in decerebrate cats. Neuropharmacology. 1996;35:195–203. doi: 10.1016/0028-3908(95)00159-x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend. 1993;33:23–29. doi: 10.1016/0376-8716(93)90030-t. [DOI] [PubMed] [Google Scholar]
- Howell LL. Effects of caffeine on ventilation during acute and chronic nicotine administration in rhesus monkeys. J Pharmacol Exp Ther. 1995;273:1085–1094. [PubMed] [Google Scholar]
- Jones S, Sudweeks S, Yakel JL. Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci. 1999;22:555–561. doi: 10.1016/s0166-2236(99)01471-x. [DOI] [PubMed] [Google Scholar]
- Jordan D, Spyer KM. Effects of acetylcholine on respiratory neurons in the nucleus ambiguus-retroambigualis complex of the cat. J Physiol (Lond) 1981;320:103–111. doi: 10.1113/jphysiol.1981.sp013937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsten EB, Satayavivad J, St John WM, Wang SC. Alteration of medullary respiratory unit discharge by iontophoretic application of putative neurotransmitters. Br J Pharmacol. 1978;63:275–281. doi: 10.1111/j.1476-5381.1978.tb09757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, De Kerchove D'exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the mid-brain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonoff-Cohen HS, Edelstein SL, Lefkowitz ES, Srinivasan IP, Kaegi D, Chang JC, Wiley KJ. The effect of passive smoking and tobacco exposure through breast milk on sudden infant death syndrome. J Am Med Ass. 1995;273:795–798. doi: 10.1001/jama.1995.03520340051035. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Zoli M, Lena C, Ferrari R, Picciotto MR, Merlo-Pich E, Changeux JP. Involvement of alpha6 nicotinic receptor subunit in nicotine-elicited locomotion demonstrated by in vivo antisense oligonucleotide infusion. Neuroreport. 1999;10:2497–2501. doi: 10.1097/00001756-199908200-00012. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bettegowda C, Blosser J, Gordon J. AR-R17779, an alpha7 nicotinic agonist, improves learning and memory in rats. Behav Pharmacol. 1999;10:675–680. doi: 10.1097/00008877-199911000-00014. [DOI] [PubMed] [Google Scholar]
- Lippiello PM, Bencherif M, Gray JA, Peters S, Grigoryan G, Hodges H, Collins AC. RJR-2403: a nicotinic agonist with CNS selectivity. II. In vivo characterization. J Pharmacol Exp Ther. 1996;279:1422–1429. [PubMed] [Google Scholar]
- Lipton SA, Aizenman E, Loring RH. Neural nicotinic acetylcholine responses in solitary mammalian retinal ganglion cells. Pflügers Arch. 1987;410:37–43. doi: 10.1007/BF00581893. [DOI] [PubMed] [Google Scholar]
- Lotti M. Treatment of acute organophosphate poisoning. Med J Aust. 1991;154:51–55. doi: 10.5694/j.1326-5377.1991.tb112852.x. [DOI] [PubMed] [Google Scholar]
- Luetje CW, Patrick J. Both alpha- and beta-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J Neurosci. 1991;11:837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novère N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, Dani JA, Grady SR, Kellar KJ, Lindstrom JM, Marks MJ, Quik M, Taylor PW, Wonnacott S. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- Lustig LR, Peng H, Hiel H, Yamamoto T, Fuchs PA. Molecular cloning and mapping of the human nicotinic acetylcholine receptor alpha10 (CHRNA10). Genomics. 2001;73:272–283. doi: 10.1006/geno.2000.6503. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- Murakoshi T, Suzue T, Tamai S. A pharmacological study on respiratory rhythm in the isolated brain stem-spinal cord preparation of the newborn rat. Br J Pharmacol. 1985;86:95–104. doi: 10.1111/j.1476-5381.1985.tb09439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff RA, Humphrey J, Mihalevich M, Mendelowitz D. Nicotine enhances presynaptic and postsynaptic glutamatergic neurotransmission to activate cardiac parasympathetic neurons. Circ Res. 1998;83:1241–1247. doi: 10.1161/01.res.83.12.1241. [DOI] [PubMed] [Google Scholar]
- Neter J, Wasserman W, Kutner MH. Applied Linear Statistical Models. 3rd ed. Richard D Irwin; Homewood, IL: 1990. Repeated measures and related designs. pp. 1035–1073. [Google Scholar]
- Nomikos GG, Schilstrom B, Hildebrand BE, Panagis G, Grenhoff J, Svensson TH. Role of alpha7 nicotinic receptors in nicotine dependence and implications for psychiatric illness. Behav Brain Res. 2000;113:97–103. doi: 10.1016/s0166-4328(00)00204-7. [DOI] [PubMed] [Google Scholar]
- Papke RL, Webster JC, Lippiello PM, Bencherif M, Francis MM. The activation and inhibition of human nicotinic acetylcholine receptor by RJR-2403 indicate a selectivity for the alpha4beta2 receptor subtype. J Neurochem. 2000;75:204–216. doi: 10.1046/j.1471-4159.2000.0750204.x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, King SL, Zachariou V. Nicotinic receptors in the brain. Links between molecular biology and behavior. Neuropsychopharmacology. 2000;22:451–465. doi: 10.1016/S0893-133X(99)00146-3. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Rickett DL, Glenn JF, Beers ET. Central respiratory effects versus neuromuscular actions of nerve agents. Neurotoxicology. 1986;7:225–236. [PubMed] [Google Scholar]
- Robinson DM, Peebles KC, Kwok H, Adams BM, Clarke LL, Woollard GA, Funk GD. Prenatal nicotine exposure increases apnoea and reduces nicotinic potentiation of hypoglossal inspiratory output in mice. J Physiol (Lond) 2002;538:957–973. doi: 10.1113/jphysiol.2001.012705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusted JM, Newhouse PA, Levin ED. Nicotinic treatment for degenerative neuropsychiatric disorders such as Alzheimer's disease and Parkinson's disease. Behav Brain Res. 2000;113:121–129. doi: 10.1016/s0166-4328(00)00207-2. [DOI] [PubMed] [Google Scholar]
- Salmoiraghi GC, Steiner FA. Acetylcholine sensitivity of Cat’s medullary neurons. J Neurophysiol. 1963;26:581–597. doi: 10.1152/jn.1963.26.4.581. [DOI] [PubMed] [Google Scholar]
- Sas Institute Inc. SAS/STAT User's Guide. 8 ed. SAS Institute; Cary, NC: 1999. The MIXED procedure. pp. 2083–2226. [Google Scholar]
- Shao XM, Feldman JL. Mechanisms underlying regulation of respiratory pattern by nicotine in preBötzinger Complex. J Neurophysiol. 2001;85:2461–2467. doi: 10.1152/jn.2001.85.6.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brain stem region that may generate respiratory rhythm in mammals. Science. 1991;254:716–719. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepans MBF, Wilkerson N. Physiologic effects of maternal smoking on breast-feeding infants. J Am Acad Nurse Practitioners. 1998;5:105–113. doi: 10.1111/j.1745-7599.1993.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Sanderson M. A reexamination of the risk factors for the sudden infant death syndrome. J Pediatr. 1995;126:887–891. doi: 10.1016/s0022-3476(95)70202-4. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, Mettenleiter TC, Mendelowitz D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Ann NY Acad Sci. 2001;940:237–246. doi: 10.1111/j.1749-6632.2001.tb03680.x. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Roll D, Zilberman Y. An analysis of the respiratory stimulant effect of physostigmine and neostigmine in the conscious rabbit. Clin Exp Pharmacol Physiol. 1981;8:151–158. doi: 10.1111/j.1440-1681.1981.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994;154:2219–2224. [PubMed] [Google Scholar]
- Whiting P, Schoepfer R, Lindstrom J, Priestley T. Structural and pharmacological characterization of the major brain nicotinic acetylcholine receptor subtype stably expressed in mouse fibroblasts. Mol Pharmacol. 1991;40:463–472. [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]