Abstract
Introduction
Identifying clinically relevant comorbid conditions might lead to effective control of prostate cancer–specific risk factors and provide opportunities to improve patient care and outcomes. There are challenges in assessing comorbidity using linked databases such as statewide hospital administrative data and state cancer registry. The objective was to compile a comprehensive list of clinically relevant comorbid conditions for patients with prostate cancer using registry and statewide diagnosis databases.
Methods
Florida Cancer Data System cases were linked with the inpatient/outpatient diagnosis information. The Elixhauser Comorbidity Index was used as a reference. Conditions not captured by Elixhauser were identified, and grouped into clinically meaningful categories. Descriptive analysis was performed on comorbidity conditions and major study population variables. Associations of comorbidity with selected demographic and disease characteristics were examined.
Results
Twenty-nine Elixhauser and 16 additional categories were examined within the 1 record per patient data set. Statistically significant association was found between comorbidity with race, stage, and age. Blacks had a higher mean number of conditions compared to whites. A higher proportion of blacks had at least 1 comorbid condition compared to whites. Additional conditions identified by this research capture more comorbidities for white men. Distinct trends towards larger number of comorbidities with older age at diagnosis and advanced disease stage were observed.
Conclusions
The Elixhauser Comorbidity Index captured the majority of comorbidities in the study population while the additional conditions identified by this research add more information. This study offers important insights into the challenges and process to identify relevant comorbidities for prostate cancer patients.
Keywords: administrative data, cancer registry, comorbidity, Elixhauser, prostate cancer
Introduction
Different approaches have been used to capture comorbidity, depending on the outcome measure, clinical setting, and source of data. Comorbidity has been defined as the co-occurrence of 1 or more diseases or disorders in an individual.1,2 Comorbidity reflects the aggregate effect of all clinical conditions a patient might have, excluding the disease of primary interest.3
The elderly population (those aged 65 years or older) in the United States is expected to double from approximately 35 million in 2013 to more than 70 million by 2030.4 The increased prevalence of cancer and comorbid conditions is associated with aging; however, there are unanswered questions on the relationship between comorbidity and cancer screening in the elderly.5,6 This aging American population, with a concomitant rise in the number of people living with chronic diseases, has major implications for health care services.
Effective management of chronic diseases such as prostate cancer often presents enormous challenges.7 Studies have shown that prostate cancer patients with high comorbidity and short life expectancy are less likely to receive aggressive therapy.8–10 These patients are also more likely to participate in active surveillance initiatives.8–10 Clinicians and patients alike can be overwhelmed by the need to address comorbid chronic conditions in addition to patients’ prostate cancer–specific treatment goals. Suboptimal management of concurrent disease, however, can lead to ineffective control of prostate cancer–specific risk factors and may miss opportunities to improve patients’ functioning and quality of life, and to decrease mortality risk.11
The complexity of comorbidity data and its potential for creating unwieldy analyses has led to the development of several summary comorbidity measures over the years. Examples of validated, database-derived comorbidity indices are the Charlson Comorbidity Index (CCI), the Elixhauser Comorbidity Index, The Cumulative Illness Rating Scale (CIRS), the Index of Coexistent Disease (ICED), and the Kaplan Index.12–16 These measures have significant differences in the method of development, type of data used, and number and type of diseases included. The CCI and the Elixhauser Index are the most extensively used in health research.16,17 The Elixhauser Index identifies 30 major coexisting conditions based on inpatient administrative data using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and diagnoses-related group (DRG) codes.13 The CCI, developed using a medical record review data set, includes 19 diseases weighted according to their association with mortality.12 The CCI score is constructed by assigning a weight to each comorbidity depending on the magnitude of the relative risk associated with each condition. The CCI was derived using a population of medical patients, and has been shown to be reliable and a valid predictor of mortality in a number of populations, including hospital inpatients and the critically ill.12,16 These 2 methods also differ from each other in terms of attribution of weights related to prognostic effect of individual conditions. This weighting is present in the 19 clinical conditions comprising the CCI. The Elixhauser methodology does not assign any weight to the 30 comorbidities it defines, and instead focuses exclusively on the number of pathologies present.
The broad goal of this project was to compile a comprehensive list of clinically relevant comorbid conditions applied to the context of prostate cancer diagnosis, and to assess whether the list of conditions could be expanded or reduced based on an existing comorbidity index (CCI and Elixhauser Index). The objectives of the current study were to 1) identify the type and prevalence of comorbid conditions in administrative data sets; and 2) examine the need to reduce the number of comorbidity conditions or expand it beyond an established comorbidity index based on statewide inpatient/outpatient and cancer registry data sets.
Methods
Data Sources
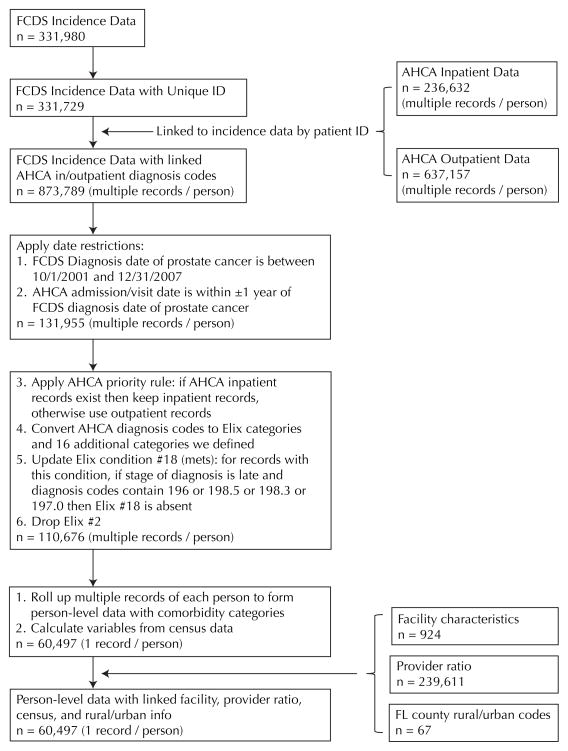
The Florida Cancer Data System (FCDS), Florida’s population-based statewide cancer registry, was used for this project. FCDS cases were linked with the inpatient and outpatient diagnosis information collected by Florida Agency for Health Care Administration (AHCA). Figure 1 shows the linkage process. AHCA’s hospital inpatient data program collects 3 types of discharge data from 269 inpatient health care facilities: acute care hospitals, long-term psychiatric facilities, short-term psychiatric facilities, and comprehensive rehabilitation facilities. Reportable events include all acute, intensive care, and psychiatric live discharges including newborn live discharges and deaths. The inpatient data comprises 52 data elements. AHCA’s ambulatory data set consists of 59 data elements, including patient demographic information, hospital identification information, payer information, charges, procedures, and diagnosis information.
Figure 1.
Flowchart of the Linkage Process
Time Window to Capture Comorbidity
The first step in creating comorbidity groups was to determine the preexisting and/or coexisting comorbid conditions at the time of prostate cancer diagnosis. To be inclusive, a time window was set to include relevant coexisting conditions that were not related to (or an outcome of) the prostate cancer diagnosis. Time intervals were calculated between the time of diagnosis of prostate cancer and the time of initial treatment of prostate cancer, between prostate cancer diagnosis and inpatient admission, and between prostate cancer diagnosis and outpatient visit. The computations were carried out by stage of prostate cancer at diagnosis. It was noted that for the majority of records (50%–75%), initial treatment, inpatient admission, or outpatient visit happened within 1 year before or after diagnosis of prostate cancer. Analyses also showed that 75% of inpatient admissions for any diseases occurred within 1 year of prostate cancer diagnosis, and more than 75% of outpatient visits for any reasons occurred within 1 year (+/−365 days) of prostate cancer diagnosis. Therefore a time window of 1 year before and 1 year after prostate cancer diagnosis date was used to capture inpatient admissions and outpatient visits for any diseases other than prostate cancer or its complications. All diagnoses of diseases independent of prostate cancer and its possible complications during this 2-year time window were included for comorbidity computation.
Comorbidity Measures
ICD-9-CM diagnosis code 185 was used to identify the prostate cancer patient group. Prostate cancer, the outcome disease, was excluded from the comorbidity groups in this study. The Elixhauser Index was used because it has been extensively validated and allows more disease states to be considered as comorbid conditions when compared to the CCI. This decision was based on the fact that prostate cancer patients present a wide range of coexisting disease states.18 This choice is further supported by several studies which have suggested that the Elixhauser method outperforms the CCI method as a predictor of mortality.19,20 Another study also suggested that if the model contains individual diagnosis information, the Elixhauser method performs better for in-hospital and 6-month mortality predictions.21
The Elixhauser Index used both DRGs and ICD-9-CM diagnosis codes from inpatient databases to identify 30 unweighted comorbidity indicators that are entered as separate indicator variables in a regression model. This project used ICD-9-CM diagnosis codes for both inpatient and outpatient within the 2-year time window centered on the prostate cancer diagnosis date.
Healthcare Cost and Utilization Project (HCUP) Comorbidity Software (versions 1.0–3.4, developed between the year 2001 and 2009), based on the Elixhauser Index, was used. Figure 1 shows the steps to combine data from multiple sources and to develop a final data file. Patient identification was included in both the incidence file received from FCDS and the AHCA inpatient/outpatient data files, and it was used to match merge diagnosis information to the incidence file. After examining the frequencies of all 30 Elixhauser Index categories based on 1 record per patient data, it was concluded that the current Elixhauser listing was adequately inclusive, with exception of the category of cancer. Cancer is complex and this category includes solid tumors vs blood-related tumors, metastatic (secondary) vs primary malignancies, unknown primaries, etc, which makes it quite cumbersome to verify. The following rule was adopted to distinguish if a metastatic cancer should or not be considered as a comorbidity: If prostate stage is localized, then any metastatic cancer in AHCA data is a comorbidity; if prostate stage is late, then metastatic cancers of the lymph nodes (ICD-9 code: 196), bone (spine and ribs, 198.5), brain (198.3), and lung (197.0) in AHCA data should not be considered as a morbidity.
Once patient comorbidities were grouped by the Elixhauser Index, conditions not captured by the Elixhauser Index were identified and categorized using the following procedure. First, grossly related conditions were grouped and given a common numeric code and a comprehensive clinically meaningful descriptor. Second, if a condition corresponded to either an Elixauser Index or previously defined category, it was assigned to that category. Third, the remaining conditions were discarded due to lack of clinical basis or scientific evidence to suggest that they are associated with or would impact late stage diagnosis or mortality. Upon review of the nature of these additional categories, 16 were found to be relevant to this analysis, as the conditions could potentially influence early diagnosis or mortality. These 16 additional categories were retained as a potential expansion of the Elixhauser Index. An expanded Elixhauser Index consisting of the original 30 plus 16 additionally identified categories was then computed to create a data set comprising 1 record per patient.
Cardiac arrhythmia posed a challenge because this condition was excluded from the HCUP Comorbidity Software after the year 2001. However, conditions from cardiac arrhythmias were not spread out nor combined into any other categories within the Elixhauser Index. Conditions related to cardiac arrhythmias were largely symptoms of bigger problems captured in heart failure. Since cardiac arrhythmias are largely risk factors or symptoms leading to congestive heart failure (CHF), those conditions will likely be covered by capturing CHF for patients in earlier and recent years. Furthermore, cardiac arrhythmias may also be associated with other categories such as valvular disease; thus, dropping cardiac arrhythmias eliminates inherent duplication. Based on this assessment, cardiac arrhythmia was removed from the list of 46 comorbidity categories, resulting in a definitive list of 29 conditions from the Elixhauser Index plus 16 additional categories, for a total of 45 comorbidity categories in the single record per patient data (Table 1).
Table 1.
List of Comorbidity Conditions (N = 60,497)
| Numbers | Comorbidity | Prevalence | % |
|---|---|---|---|
| 1 | Congestive heart failure | 1,465 | 2.42 |
| 2 | Valvular disease | 1,752 | 2.90 |
| 3 | Pulmonary circulation disorders | 215 | 0.36 |
| 4 | Peripheral vascular disorders | 1,304 | 2.16 |
| 5 | Hypertension, uncomplicated Hypertension, complicated Hypertension, complicated |
25,589 | 42.30 |
| 6 | Paralysis | 279 | 0.46 |
| 7 | Other neurological disorders | 924 | 1.53 |
| 8 | Chronic pulmonary disease | 5,528 | 9.14 |
| 9 | Diabetes, uncomplicated | 6,331 | 10.46 |
| 10 | Diabetes, complicated | 412 | 0.68 |
| 11 | Hypothyroidism | 1,572 | 2.60 |
| 12 | Renal failure | 1,350 | 2.23 |
| 13 | Liver disease | 314 | 0.52 |
| 14 | Peptic ulcer disease excluding bleeding | 188 | 0.31 |
| 15 | AIDS | 40 | 0.07 |
| 16 | Lymphomia | 357 | 0.59 |
| 17 | Metastatic cancer | 2,706 | 4.47 |
| 18 | Solid tumor without metastasis | 3,410 | 5.64 |
| 19 | Rheumatoid arthritis/collagen vascular diseases | 300 | 0.50 |
| 20 | Coagulopathy | 851 | 1.41 |
| 21 | Obesity | 1,230 | 2.03 |
| 22 | Weight loss | 527 | 0.87 |
| 23 | Fluid and electrolyte disorders | 3,692 | 6.10 |
| 24 | Chronic blood loss anemia | 516 | 85 |
| 25 | Deficiency anemias | 3,304 | 5.46 |
| 26 | Alcohol abuse | 545 | 0.90 |
| 27 | Drug abuse | 63 | 0.10 |
| 28 | Psychosis | 390 | 0.64 |
| 29 | Depression | 1,240 | 2.05 |
| 30* | Endocrine disorders, nutritional and metabolic, immunity | 11,667 | 19.29 |
| 31* | Ischemic heart disease | 8,385 | 13.86 |
| 32* | Digestive system disease | 9,211 | 15.23 |
| 33* | Genitourinary system disease | 14,761 | 24.40 |
| 34* | Injury and poisoning | 4,937 | 8.16 |
| 35* | Respiratory disorders | 2,887 | 4.77 |
| 36* | Infection | 2,502 | 4.14 |
| 37* | Other circulatory disease | 2,027 | 3.35 |
| 38* | Benign neoplasm and in-situ cancer | 2,211 | 3.65 |
| 39* | Other nervous system and sense organs disorders | 1,916 | 3.17 |
| 40* | Skin and subcutaneous tissue disease | 958 | 1.58 |
| 41* | Muscularskeletal and connective tissue disease | 7,109 | 11.75 |
| 42* | Other mental disorders | 873 | 1.44 |
| 43* | Other anemias | 825 | 1.36 |
| 44* | Congenital anomalies | 276 | 0.46 |
| 45* | Brain and other neurological disorders | 525 | 0.87 |
Additional comorbidity not in Elixhauser that were identified from the dataset.
Data Analysis
The final 1 record per patient data described at bottom of Figure 1 was used for analysis. Descriptive statistics (eg, frequencies, proportions, means, and standard deviations) were performed for all comorbidity conditions and other major study variables. Bivariate analyses including chi-square and analysis of variance (ANOVA) tests were conducted to examine associations of comorbidity with age, race, and stage at diagnosis. Type I error was set to 0.05 and all tests were 2-sided. SAS/STAT software, Version 9.3 of the SAS System for Windows, was used for data linkage and statistical analyses.
Results
The prevalence of the 29 conditions included in the Elixhauser Index was examined using data that combined information from multiple sources. The distribution of the 16 additional categories displayed both low and high frequencies. Relatively low frequencies were observed for congenital anomalies (0.46%), brain and other neurological disorders (0.87%), and other anemias (1.36%). The highest frequencies were found for genitourinary system disease (24.40%), endocrine, nutritional/metabolic and immunity disorders (19.29%), and digestive system disease (15.23%).
The distribution of comorbidities by age, race, and stage at diagnosis was further examined. Results are summarized in Table 2. Several associations between comorbidity and prostate cancer diagnosis were evident. Race and number of comorbid conditions were related in that blacks have a significantly higher mean number of comorbidity conditions compared to whites (2.53 vs 2.25). In addition, a higher proportion of blacks (77.04%) had at least 1 comorbid condition compared to 75.13% for whites. There was also a distinct trend towards increased number of comorbidities with later stage at diagnosis. Seventy-three percent of men with localized disease had no comorbidities, compared to 83.01% among men with regional disease, and 94.44% among those with distant disease. As expected, a positive association exists between number of comorbidities and age at diagnosis. Table 2 also shows that, compared to using Elixhauser categories only, the additional 16 categories enabled us to capture at least 1 more comorbidity per patient for various race, stage, and age subgroups. In addition, we were able to depict a disease profile for a much higher percentage of patients (percent with any vs percent with any Elixhauser category) for different patient subgroups using the additional comorbidity categories.
Table 2.
Comorbidity Summary by Race, Stage, and Age (N = 60,497)
| Number of Comorbidities | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Mean (SD) | Elix Mean (SD)* | Total Median | Elix Median* | % with any | % with any Elix* | % with 1 | % with 2 | % with 3 | ||
| Race | Black | 2.53(2.61) | 1.36(1.50) | 2 | 1 | 77.04 | 66.42 | 20.8 | 17.12 | 12.64 |
| White | 2.25(2.34) | 1.07(1.29) | 2 | 1 | 75.13 | 58.39 | 22.28 | 17.51 | 12.7 | |
| P-value | <.0001† | <.0001† | 0.0004† | <.0001† | 0.0045† | 0.4115 | 0.8823 | |||
| Stage | Localized | 2.02(2.14) | 0.96(1.186) | 1 | 1 | 72.82 | 56.05 | 23.31 | 17.88 | 12.42 |
| Regional | 2.51(2.28) | 1.26(1.29) | 2 | 1 | 83.01 | 68.47 | 22.25 | 20.08 | 15.83 | |
| Distant | 5.13(3.40) | 2.58(2.00) | 5 | 2 | 94.44 | 85.78 | 8.51 | 10.09 | 11.27 | |
| P-value | <.0001† | <.0001† | <.0001† | <.0001† | <.0001† | <.0001† | <.0001† | |||
| Age | [40, 65) | 1.89(2.04) | 0.91(1.14) | 1 | 1 | 72.25 | 54.96 | 25.03 | 18.51 | 11.92 |
| [65, 70) | 2.21(2.23) | 1.07(1.23) | 2 | 1 | 76.19 | 60.92 | 21.98 | 19.11 | 13.67 | |
| ≥70 | 2.66(2.65) | 1.29(1.49) | 2 | 1 | 77.56 | 62.22 | 19.69 | 15.6 | 12.8 | |
| P-value | <.0001† | <.0001† | <.0001† | <.0001† | <.0001† | <.0001† | <.0001† | |||
Calculated based on Elixhauser Index comorbidity categories only.
Significant at 5% level.
Discussion
The role of comorbidity on early diagnosis of cancer and mortality has been extensively examined.6,7,11,22–24 The literature is replete with examples demonstrating the complexity and challenges of comorbidity analysis using a variety of data sources.17,19–21 However, little has been reported for comorbidity, early diagnosis, or mortality of prostate cancer using state cancer registry data. The current study sought to identify the type and frequency of comorbid conditions in a prostate cancer registry and to assess the performance of the existing Elixhauser Index in such a database. The methodology employed in this study supports the utility of the Elixhauser Index in this context and yields an informative picture of the distribution of comorbid conditions as summarized in Tables 1 and 2. More importantly, this parsimonious approach reduces a rather unwieldy data set consisting of multiple ICD-9-CM codes per patient into broad, clinically meaningful categories. This provides a pragmatic data set which facilitates meaningful, descriptive, correlational, and inferential (planned) analyses on the impact of comorbidity. Bivariate analyses found that comorbidity was significantly associated with race, stage, and age at diagnosis. This finding corroborates the current literature.25,26 There was a distinct trend toward an increased number of comorbidities with later stage at diagnosis. As expected, a positive association between age and comorbidity was found.
Although these bivariate analyses are exploratory, the noted relationships will have to be evaluated further in multivariate analysis. The current study demonstrated that the Elixhauser Index is an adequate measure for capturing the most commonly occurring comorbid conditions among prostate cancer patients, and helps identify a disproportionate burden of common comorbidities among black men. The additional comorbidities uniquely identified in this research, however, tend to be more common among whites compared to blacks. This sets the stage to evaluate the relative contributions of these conditions to both early stage diagnosis and mortality in prostate cancer. This study provides a novel methodology that could be applied to other specific data sets.
Despite the challenges, the study was a necessary step to account for case mix and sickness profile when studying patient outcomes. It is better to include patient characteristics and their coexisting disease states than to assume 1 primary disease is the only thing that matters. In conclusion, our results suggest that it is important to examine the relevance and performance of established comorbidity measures in each disease-specific application. This study tested whether an expanded disease-specific comorbidity index based on the Elixhauser Index is needed for patients with prostate cancer. The Elixhauser Index was found capable to capture the majority of comorbid conditions in this population. However, this study offers important new insights on the challenges and methodology for working with cancer registry data and statewide inpatient and outpatient databases. Specifically, a 1-year time window before and after prostate cancer diagnosis yielded the most meaningful parameter for extraction and computation of comorbidity. Supplementing existing comorbidity information with the additional comorbidities identified in this study may contribute to the improvement of the value of cancer research and the care of cancer patients.
Acknowledgments
This study was funded by Grant #RSGT-10-082-01-CPHPS from the American Cancer Society. The authors thank the Florida Department of Health and Florida Cancer Data System for providing the prostate cancer data.
References
- 1.Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. J Chronic Dis. 1970;23(7):455–468. doi: 10.1016/0021-9681(70)90054-8. [DOI] [PubMed] [Google Scholar]
- 2.Iezzoni L, Foley S, Daley J, Hughes J, Fisher E, Heeren T. Comorbidities, complications, and coding bias. Does the number of diagnosis codes matter in predicting in-hospital mortality? JAMA. 1992;267(16):2197. doi: 10.1001/jama.267.16.2197. [DOI] [PubMed] [Google Scholar]
- 3.Greenfield S, Nelson EC. Recent developments and future issues in the use of health status assessment measures in clinical settings. Med Care. 1992 doi: 10.1097/00005650-199205001-00003. [DOI] [PubMed] [Google Scholar]
- 4.Federal Interagency Forum on Aging-Related Statistics. Older Americans 2008: Key indicators of well-being. US Independent Agencies and Commissions; 2008. [Google Scholar]
- 5.Ogle KS, Swanson GM, Woods N, Azzouz F. Cancer and comorbidity. Cancer. 2000;88(3):653–663. doi: 10.1002/(sici)1097-0142(20000201)88:3<653::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Terret C, Castel-Kremer E, Albrand G, Droz JP. Effects of comorbidity on screening and early diagnosis of cancer in elderly people. Lancet Oncol. 2009;10(1):80–87. doi: 10.1016/S1470-2045(08)70336-X. [DOI] [PubMed] [Google Scholar]
- 7.Groome PA, Rohland SL, Siemens DR, Brundage MD, Heaton J, Mackillop WJ. Assessing the impact of comorbid illnesses on death within 10 years in prostate cancer treatment candidates. Cancer. 2011;117(17):3943–3952. doi: 10.1002/cncr.25984. [DOI] [PubMed] [Google Scholar]
- 8.Bennett CL, Aronow H, Greenfield S, Ganz P, Vogelzang NJ, Elashoff RM. Patterns of care related to age of men with prostate cancer. Cancer. 1991;67(10):2633–2641. doi: 10.1002/1097-0142(19910515)67:10<2633::aid-cncr2820671039>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen PL, Chen MH, Renshaw AA, Loffredo M, Kantoff PW, D’Amico AV. Survival following radiation and androgen suppression therapy for prostate cancer in healthy older men: Implications for screening recommendations. Int J Radiat Oncol Biol Phys. 2010;76(2):337–341. doi: 10.1016/j.ijrobp.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 10.Droz JP, Balducci L, Bolla M, et al. Management of prostate cancer in older men: Recommendations of a working group of the international society of geriatric oncology. BJU Int. 2010;106(4):462–469. doi: 10.1111/j.1464-410X.2010.09334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall W, Jani A, Ryu J, Narayan S, Vijayakumar S. The impact of age and comorbidity on survival outcomes and treatment patterns in prostate cancer. Prostate Cancer and Prostatic Diseases. 2005;8(1):22–30. doi: 10.1038/sj.pcan.4500772. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Waldman E, Potter JF. A prospective evaluation of the cumulative illness rating scale. Aging (Milano) 1992;4(2):171–178. doi: 10.1007/BF03324087. [DOI] [PubMed] [Google Scholar]
- 15.Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of coexistent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31(2):141. doi: 10.1097/00005650-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 16.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity: A critical review of available methods. J Clin Epidemiol. 2003;56(3):221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 17.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 18.Stukenborg GJ, Wagner DP, Connors AF., Jr Comparison of the performance of two comorbidity measures, with and without information from prior hospitalizations. Med Care. 2001;39(7):727. doi: 10.1097/00005650-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Mnatzaganian G, Ryan P, Norman PE, Hiller JE. Accuracy of hospital morbidity data and the performance of comorbidity scores as predictors of mortality. J Clin Epidemiol. 2011 doi: 10.1016/j.jclinepi.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42(4):355. doi: 10.1097/01.mlr.0000118861.56848.ee. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS hierarchical condition category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Services Research. 2010;10(1):245. doi: 10.1186/1472-6963-10-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teppo H, Alho O. Comorbidity and diagnostic delay in cancer of the larynx, tongue and pharynx. Oral Oncol. 2009;45(8):692–695. doi: 10.1016/j.oraloncology.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Reid BC, Warren JL, Rozier G. Comorbidity and early diagnosis of head and neck cancer in a medicare population. Am J Prev Med. 2004;27(5):373–378. doi: 10.1016/j.amepre.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Alho O, Teppo H, Mäntyselkä P, Kantola S. Head and neck cancer in primary care: Presenting symptoms and the effect of delayed diagnosis of cancer cases. Can Med Assoc J. 2006;174(6):779–784. doi: 10.1503/cmaj.050623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daskivich TJ, Chamie K, Kwan L, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117(10):2058–2066. doi: 10.1002/cncr.25751. [DOI] [PubMed] [Google Scholar]
- 26.Evans S, Metcalfe C, Ibrahim F, Persad R, Ben-Shlomo Y. Investigating Black-White differences-in prostate cancer prognosis: A systematic review and meta analysis. Int J Cancer. 2008;123(2):430–435. doi: 10.1002/ijc.23500. [DOI] [PubMed] [Google Scholar]