Abstract
Disparities in prostate cancer diagnosis among racial/ethnic groups and across Florida were mapped for the period 1996–2002 and their relationship with putative factors (individual, census tract and county level) was investigated using multilevel modeling and contingency analysis. More counties had higher rates of late-stage diagnosis for Black men than for White men and the location of these racial disparities changed with time. An important finding was the substantially larger correlation between county-level rates for Black and White men in 2002 relatively to 1996, which suggests a convergence in their spatial patterns. Major significant factors for late-stage diagnosis included lack of insurance, low household income, smoking, not being married and presence of farm house. These findings should help the design of intervention programs to target counties with the greatest racial disparities in health outcomes. Additional analysis is needed to disentangle the observed racial/ethnic and geographic differences.
Keywords: Racial disparities, spatial-temporal pattern, prostate cancer, late-stage diagnosis, multilevel modeling, GIS
Prostate cancer is the most common solid malignancy and the second leading cause of cancer-related death for American men. It has been estimated that there will be 240,890 new cases and 33,720 deaths from this disease in the United States in 2011.1 The State of Florida ranks second behind California for both incidence (16,780 estimated new cases) and mortality (2,160 estimated deaths) from prostate cancer in 2011.1
Striking racial and ethnic differences in incidence and mortality persist in the U.S. and Florida. Racial differences are greater for prostate cancer than for any other major cancer site (e.g., colorectal and lung). During 2003–2007, incidence was 60% higher and mortality was 2.4 times greater for Blacks than Whites in the U.S.,1,2 and the rates for Blacks exceeded all other racial and ethnic minorities. Despite declines in incidence and mortality, and even after adjusting for individual-level risk factors, the risk for Blacks was found to be slightly higher than for Whites.1-6
Previous research has attempted to explain racial differences in prostate cancer incidence and mortality. Tumor grade, late stage of disease at diagnosis, and differences in access to definitive and adjuvant treatment contribute to mortality.7-10 Diet and cooking practices, selenium intake, exposure to pesticides and fertilizers, physical activity, use of health care services, genetic susceptibility and both individual-level and area-level socioeconomic status (SES) are linked to racial difference in incidence.11-15 A growing body of evidence supports the association of prostate cancer risk to farming due to exposure to toxic chemicals, especially pesticides.13,14,16-19 Disparities in late-stage diagnosis by geographical location have been associated with poor access to primary health care, lack of health insurance and difference in coverage.20-23
Interpretation of cancer incidence and mortality rates in a defined population requires an understanding of multiple factors that vary across time and space.24 These factors include changes in medical practices related to screening and treatment, and changes in how data are collected and reported. Analyzing temporal trends in prostate cancer incidence and mortality rates can provide a clearer picture of the burden of the disease and generate insight into the effect of various interventions.25 In addition, since it has long been recognized that the incidence of cancer varies significantly by county and state, the analysis of temporal trends requires a spatial dimension.26 Visualizing, analyzing, and interpreting racial disparities from a geographic perspective can improve cancer epidemiology, control, and surveillance.27
Although some studies have applied spatial analyses to the study of health disparities, the spatial and temporal scales used by these studies are inconsistent, and the statistical methods and visualization techniques are inadequate.12,28-32 What is needed is geostatistical software that can handle both individual-level and aggregated data to control adequately for behavioral and environmental factors associated with health outcomes at the various levels.
Given the gaps in the literature, the objectives of this study are 1) to map and examine patterns of late-stage prostate cancer over time across the state of Florida using innovative geostatistical methods; and 2) to evaluate the association of prostate cancer late-stage diagnosis to individual and area-level characteristics using multilevel modeling.
Methods
Population studied
Men aged 40 or older who were diagnosed with prostate cancer in the State of Florida during 1996–2002 were included in this study.
Data sources
The study used data from three sources. First, prostate cancer incidence data for years 1996–2002 were obtained from the State of Florida Department of Health, which contracts with Florida Cancer Data System (FCDS) housed at the University of Miami. The FCDS is the largest population-based, cancer incidence registry in the nation. It contains information on patient demographics, residence, prostate tumor characteristics and other information such as tobacco use and primary payer of health insurance. The data were geocoded based on patient residential address by an independent geocoding firm contracted by the Florida Department of Health.
Second, data on socio-demographic and environmental characteristics were extracted at the census tract level from the U.S. Census Bureau (Census 2000, Summary File-3) public use files for the State of Florida.
Third, health provider information by county was obtained from the Florida Department of Health Division of Medical Quality Assurance to calculate provider to case ratios. Specifically, the number of primary health providers and urologists was divided by the number of diagnosed prostate cancer cases for each county during 1996–2002. This measure was used to capture provider accessibility.
Data obtained from the three sources were merged into a single dataset. Cancer registry records were excluded from the analyses if they could not be linked to census data or provider information.
The following tasks were undertaken to address the first study objective: mapping and examining patterns of late-stage prostate cancer over time across the state of Florida using innovative geostatistical methods.
County-level mapping
A technique known as binomial kriging33 was used to filter the noise attached to incidence rates computed from small population sizes (small number problem). This geostatistical method accounts for the size and shape of each county, as well as the pattern of spatial autocorrelation of incidence rates as modeled by a population-weighted semivariogram. Disparity statistics34 were used to detect significant absolute disparities (rate difference) between two ethnic groups.
Contingency analysis
Three-way contingency tables35 were used to examine in greater detail the influence of two important contextual variables: census tract-level median household income and county-level provider to cases ratio. Contingency tables are useful because they graphically display potential interactions and non-linear impact of putative covariates on the proportion of late-stage diagnosis. The significance of this impact was tested using a Monte-Carlo simulation procedure that accounts for the pattern of spatial correlation of prostate cancer data while correcting for multiple testing.
All mapping analyses above were conducted using the SpaceStat software developed by BioMedware, Inc.
Multivariate multilevel analysis
A multilevel logistic model was applied to address the second study objective, to evaluate the association of prostate cancer late-stage diagnosis to individual and area-level characteristics. The dependent variable in the logistic regression was stage of diagnosis (late vs. early). Independent variables were measured at three levels: 1) individual patient characteristics (age, race, marital status, tobacco use history, and health insurance), 2) census tract information (median household income, percentage African American, and presence of a farmhouse*), and 3) county-level provider accessibility (ratio of primary health providers and urologists to prostate cancer cases). In the multilevel logistic model, individuals from the same census tract shared the same census tract-level random effect. In addition people in the same county shared another common county random term. Specifically, let Yijk be the binary diagnosis outcome of the kth individual living in the jth census tract that belongs to the ith county. The probability that this person’s diagnosis is late stage, pijk=P(Yijk=1), is modeled using
where is the vector of observed covariates at three levels, β is the vector of regression coefficients to be estimated, vi is the county-level random effect, and uij is the census tract-level random effect. The distributional assumptions are , ), and the two random effects are independent.
Odds ratios for independent variables measured at different levels were estimated. Due to their highly skewed distributions, the following covariates were log transformed: median household income, African American percentage, and provider-to-case ratio. The regression analyses for this paper were conducted using SAS/STAT software, Version 9.2 of the SAS System for Windows (Copyright © 2002–2008 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA).
Results
Population characteristics
60,289 men diagnosed with prostate cancer in Florida during 1996–2002 were included in the analyses after merging the three datasets. Seventy-nine percent were non-Hispanic White (NHW), 10% were non-Hispanic Black (NHB) and 11% were Hispanic. The average age at diagnosis was 69 years and 80% of the study subjects were married. The characteristics of the study population are summarized in Table 1.
Table 1.
CHARACTERISTICS OF THE STUDY POPULATION (N=60,289)
| Variable | Mean (SD)/ Percentage |
Variable | Percentage |
|---|---|---|---|
| Age | 68.52 (8.58) | Race/Ethnicity | |
| Year of Diagnosis | NH White | 78.81 | |
| 1996 | 11.09 | NH Black | 10.37 |
| 1997 | 13.45 | Hispanic | 10.82 |
| 1998 | 14.69 | Smoking | |
| 1999 | 15.64 | Non-Current Smokers | 83.05 |
| 2000 | 15.11 | Current Smokers | 16.95 |
| 2001 | 16.14 | Insurance | |
| 2002 | 13.88 | Insured | 98.50 |
| Marital Status | Not Insured | 1.50 | |
| Unmarried | 20.25 | ||
| Married | 79.75 |
County-level mapping
The proportions of prostate cancer cases with late-stage diagnosis averaged over the period 1996–2002 and noise-filtered using binomial kriging are mapped by race/ethnicity in Figure 1. Because of the small Hispanic population in the North, a few counties have missing values (i.e., no case diagnosed) while other counties display extreme low or high rates even after noise filtering. In particular, none of the high rates in the North (i.e., rates. 0.4) are based on more than five cases. Proportions for NHB and NHW men were moderately correlated (r=0.57) with higher values observed in North Florida. For NHW men, higher rates are clearly confined to the Big Bend region (nine counties delineated with thick borders in Figure 1, which is one of the most rural regions in Florida. On the other hand, late-stage diagnosis was more spread out for NHB and the highest rate of 0.42 was recorded in Dixie County (southeast end of the Big Bend region), yet it was based on only four cases and lacks reliability. Large reliable rates are observed for Escambia County (northwest end of Florida, rate=0.31, population=241 cases) and Gadsden County (northwest end of the Big Bend region, rate=0.32, population=81 cases).
Figure 1.
Proportion of late-stage diagnosis for prostate cancer in Florida, 1996–2002. The nine counties with thick borders (left map) form the Big Bend region. The map for NHB names the counties with the largest proportions of late-stage diagnosis, see text for detailed statistics.
The three-dimensional graphs in Figure 2 allow one to visualize simultaneously how proportions of late-stage diagnosis have changed over time and across Florida counties. The space-time pattern was the clearest for NHW men: an initial decline was followed by a slight rise the last two years, particularly in the Big Bend region. On the other hand, the proportion of late-stage diagnosis for NHB men first increased before declining steadily since 1998. This decline occurred mostly in Central and South Florida, and the higher proportions since 2000 were confined to the Big Bend region like for NHW men. It is noteworthy that the correlation between county-level rates of these two ethnic groups experienced a twofold increase from 1996 (r=0.28) to 2002 (r=0.67), suggesting a convergence in their spatial patterns. Both spatial and temporal fluctuations were much more erratic for the Hispanic population.
Figure 2.
Proportion of late-stage diagnosis for prostate cancer estimated by binomial kriging at the county-level annually over the period 1996–2002.
To examine potential disparities across the state, counties where NHB and NHW men had significantly different annual proportions of late-stage diagnosis were mapped (Figure 3). Except in 2002, significant differences were always associated with poorer health outcomes for the minority group. The two counties with negative differences in 2002 (Madison and Hendry), each had no more than 10 cases for the two races combined. The frequency and location of significant disparities has changed over time.
Figure 3.
Counties displaying significant absolute disparities in proportion of late-stage prostate cancer diagnosis between non-Hispanic White (NHW) and non-Hispanic Black (NHB) for the period 1996–2002. (The two named counties in the 2002 map are anomalies where the proportion is significantly larger for NHW than NHB males.)
In the most recent years, fewer counties had significant differences, and they tended to be confined to South Florida and in the Big Bend region.
Contingency analysis
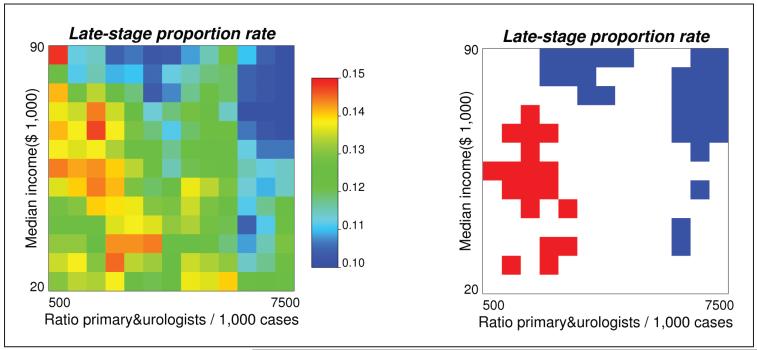
The combined impact of provider accessibility and median household income on late-stage diagnosis for all races is presented in Figure 4. Late-stage prostate cancer diagnosis rates were computed for 169 (13×13) classes of income and density of primary health providers and urologists (Figure 4, left graph). The choice of 13 classes represents a balance between too few classes that provide a coarse discretization of the two axes and too many classes that lead to too few cases within each class and unreliable rate estimates. The observed rates were then compared with expected values to test the hypothesis that the two covariates would not impact late-stage diagnosis. Classes with significantly higher proportions of late-stage diagnosis are colored red while classes with lower proportions are in blue. Late-stage cancer diagnosis is significantly lower than expected with higher income or greater provider accessibility. In addition, the impact of lower provider accessibility seems mitigated by higher income, a fact that was not detected during the multilevel analysis.
Figure 4.
Late-stage prostate cancer incidence rates (all races) computed for 169 classes of income × density of primary providers and urologists. In the right graph, blue (red) pixels represent incidence rates that are significantly lower (higher) than the incidence rate expected under the assumption of no impact of covariates on late diagnosis (a=0.05).
Multivariate multilevel analysis
Table 2 presents the stepwise multilevel analysis results. In step 1 only the individual-level variables were included. Controlling for the individual-level variables, step 2 added census tract-level covariates, and in step 3 the county-level variable was entered into the model. Variances of county-level and census tract-level random effects were significant under all three models displayed in Table 2. The addition of variables in each step did not dramatically change the impact of variables already in the model. The multilevel logistic regression indicated individuals, who were non-Hispanic Black, not married, current smokers, and not insured were more likely to be diagnosed with late-stage prostate cancer. Although significant, the odds ratio for age was close to 1, reflecting almost no age effect. Being Hispanic was not significantly associated with diagnosis. At the census tract-level, higher likelihood of late-stage diagnosis was associated with areas characterized by lower median household income and the presence of farm houses. The primary physician and urologist ratio at the county-level was not significantly related to prostate cancer diagnosis. It is useful to note that interactions between race/ethnicity and other independent variables was tested, but not included in the final model since none of them was significant.
Table 2.
MULTIVARIATE MULTILEVEL ANALYSIS (DEPENDENT VARIABLE: STAGE OF DIAGNOSIS)
| Step 1 | Step 2 | Step 3 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Independent Variables |
Odds Ratio |
95% CI | Odds Ratio |
95% CI | Odds Ratio |
95% CI |
| Individual-level | ||||||
| Age 40 | 0.99 | 0.99–1.00 | 0.99 | 0.99–1.00 | 0.99 | 0.99–1.00 |
| Year 1996 (reference) | 1 | 1 | 1 | |||
| Year 1997 | 0.78 | 0.71–0.85 | 0.78 | 0.71–0.85 | 0.78 | 0.71–0.85 |
| Year 1998 | 0.75 | 0.68–0.82 | 0.75 | 0.68–0.82 | 0.75 | 0.68–0.82 |
| Year 1999 | 0.67 | 0.62–0.74 | 0.68 | 0.62–0.74 | 0.68 | 0.62–0.74 |
| Year 2000 | 0.61 | 0.56–0.67 | 0.61 | 0.56–0.67 | 0.61 | 0.56–0.67 |
| Year 2001 | 0.62 | 0.57–0.68 | 0.62 | 0.57–0.68 | 0.59 | 0.50–0.71 |
| Year 2002 | 0.61 | 0.55–0.67 | 0.61 | 0.56–0.67 | 0.58 | 0.49–0.70 |
| NH White (reference) | 1 | 1 | 1 | |||
| NH Black | 1.30 | 1.20–1.40 | 1.27 | 1.17–1.38 | 1.27 | 1.17–1.38 |
| Hispanic | 1.01 | 0.91–1.12 | 1.00 | 0.91–1.11 | 1.00 | 0.91–1.11 |
| Married (reference) | 1 | 1 | 1 | |||
| Unmarried | 1.30 | 1.23–1.38 | 1.29 | 1.22–1.37 | 1.29 | 1.22–1.37 |
| Non-current smoker (reference) |
1 | 1 | 1 | |||
| Current smoker | 1.21 | 1.13–1.28 | 1.20 | 1.13–1.28 | 1.20 | 1.13–1.28 |
| Insured (reference) | 1 | 1 | 1 | |||
| Not insured | 2.76 | 2.38–3.21 | 2.75 | 2.37–3.20 | 2.75 | 2.37–3.20 |
| Census Tract-Level | ||||||
| LogMedianIncome | 0.81 | 0.67–0.98 | 0.80 | 0.66–0.97 | ||
| LogAApercent | 1.02 | 0.96–1.09 | 1.02 | 0.96–1.08 | ||
| Without farm house (reference) |
1 | 1 | ||||
| With farm house | 1.14 | 1.05–1.23 | 1.14 | 1.05–1.24 | ||
| County-Level | ||||||
| LogPriUroRatio | 1.54 | 0.40–5.99 | ||||
CI = Confidence Interval
Discussion
This study mapped the proportions of late-stage prostate cancer diagnosis by county and year using innovative geo-statistical methods to illustrate their temporal and spatial trends (Objective #1). In addition, the study examined the association between prostate cancer diagnosis and factors at three levels, namely individual patient, census tract and county-levels over a seven-year period in the State of Florida (Objective #2). Main contributions of this study are three-fold. First, the mapping techniques were significantly improved from the traditional methods. Specifically, these innovative methods of binomial kriging, 3-D mapping of racial disparities and contingency analysis maps took into consideration the impact of time and place on the health outcome of interest among ethnic groups. The contingency analysis supplemented the multilevel analysis by examining the interaction between the two variables without assuming linear relationships. In this case, the impact of less provider accessibility seems mitigated by higher income, a fact that was not detected during the multilevel analysis. These new methods allow the reader to visualize, analyze, and interpret geographical and racial disparities in late-stage diagnosis in a context of multiple and complex factors that change across time and space. The findings provide a clearer picture of the burden of prostate cancer and bring important knowledge that will greatly benefit cancer epidemiology, control, and surveillance. Second, the observed geographic and racial/ethnic variation in diagnosis and their temporal patterns are of great clinical importance. An important finding of the present study is the substantially larger correlation between county-level rates for NHB and NHW men found in 2002 relatively to 1996, suggesting a convergence in their spatial patterns which might be caused by urbanization. Finally, to our knowledge, this is the first study in Florida to investigate potential risk factors of late-stage diagnosis using the statewide cancer registry data linked with census and county-level data. These findings add local community perspective, which is important for planning community outreach programs to reduce geographic and racial/ethnic disparities.
During the period 1996–2002, counties across Florida exhibited different temporal trends in late-stage prostate cancer incidence and more counties had higher rates of late-stage prostate cancer for NHB men than for NHW men. The location of racial disparities in late-stage diagnosis changed over time and in 2002 racial disparities were the greatest in South Florida and the Big Bend region. These spatial patterns inform and may facilitate the design of intervention programs to target counties with the greatest racial disparities in health outcomes. The value of this approach has also been illustrated elsewhere.11,12,15,36,37 Additional analyses (such as boundary and cluster analysis) are needed to disentangle the observed geographic differences to plan targeted interventions.
With the widespread use of PSA and digital rectal examination screening techniques, more prostate cancer has been diagnosed at an early stage for men of all races, a fact apparent in the temporal trend detected by both the statistical analysis and mapping at the county-level. However, NHB were still more likely than NHW and Hispanic men to be diagnosed with late-stage cancer, which is consistent with the literature.23,38 In this study, age was statistically significant, indicating younger men were more likely to be diagnosed at late-stage cancer. However the difference is minimal. This result agrees with a previous study of area-level influence on prostate cancer grade and stage at diagnosis.11,12
Married men were more likely than single, divorced, or widowed men to be diagnosed with early-stage disease. A plausible explanation for the observed effects of marital status is unavailable; yet our findings suggest a possible role of the spouse in the early detection of prostate cancer. This observation is consistent with studies showing that wives have a proscreening attitude and want to be involved in decisions about prostate cancer screening.39,40 Additional studies are needed to explore the effect of familial support. Possible interventions should involve spouses.
Our study showed that men without insurance coverage have worse diagnoses than those with coverage, consistent with existing studies.22,23 This finding may have health policy implications. Moreover, our findings on smoking confirm the detrimental effect of tobacco use on prostate health.41,42 Although evidence of direct benefit of smoking cessation is lacking, it appears that it could help reduce late-stage prostate cancer. Targeted public health interventions can be implemented in those counties with high proportion of late-stage prostate cancer, especially among men who are unmarried, have no insurance and have a history of smoking.
At the area (census tract) level, median household income was found to be a protective factor against late-stage diagnosis, which confirms findings of other studies.10,12 The presence of a farm house was a risk factor in this study which agrees with the literature on the effect of environmental exposure on health outcomes.16-19
The provider-to-case ratio was not significant in the multivariate analyses, although higher ratios were a significant protective factor in the bivariate relationship not presented here. This suggests that the provider effect may have been accounted for by other variables in the model. For instance, physicians generally prefer to locate in populous areas hence lower provider ratios in rural areas. This might explain the presence of a farm house as a risk factor in the model.
The current study has a number of limitations. First, because individual screening data were not available, it is possible that our findings showing higher late-stage proportion of prostate cancer diagnosis for non-Hispanic Black men could be a reflection of lower screening rates in this population. Second, census tract and county-level data on SES were used due to lack of individual-level or block group-level information which would have provided richer information for the analyses. Finally, comorbidity information, which is unavailable in the cancer registry, might be related to stage of diagnosis. An effort to link comorbidity data with cancer registry data is underway in the State of Florida.
Acknowledgments
This study was funded by Grant #RSGT-10-082-01-CPHPS from the American Cancer Society. The authors thank the Florida Department of Health and Florida Cancer Data System for providing the prostate cancer incidence data. The valuable assistance of Askal Ali, M.A. and Georges Adunlin, M.A. is acknowledged. The authors also appreciate the review and feedback from Dr. Janet Barber and Dr. Ellen Campbell.
Footnotes
“Farmhouse presence” was created based on “number of farmhouses” from census data as an indicator of environmental exposure to farming.” The census collects this info as part of their housing characteristics. We included this in our model because literature show prostate cancer is associated with farming.
Contributor Information
Hong Xiao, A&M University in the College of Pharmacy and Pharmaceutical Sciences.
Fei Tan, Department of Mathematical Sciences at Indiana University in Indianapolis.
Pierre Goovaerts, BioMedware Inc. in Ann Arbor, Michigan and Courtesy Associate Professor at the University of Florida.
Notes
- 1.American Cancer Society . Cancer facts and figures, 2011. American Cancer Society; Atlanta, GA: 2011. Available at: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-029771.pdf. [Google Scholar]
- 2.American Cancer Society . Cancer facts and figures for African American, 2011–2012. American Cancer Society; Atlanta, GA: 2011. Available at: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-027765.pdf. [Google Scholar]
- 3.Mettlin CJ, Murphy GP. Why is the prostate cancer death rate declining in the United States? Cancer. 1998 Jan;82(2):249–51. doi: 10.1002/(sici)1097-0142(19980115)82:2<249::aid-cncr1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Platz EA, Rimm EB, Willett WC, et al. Racial variation in prostate cancer incidence and in hormonal system markers among male health professionals. J Natl Cancer Inst. 2000 Dec;92(24):2009–17. doi: 10.1093/jnci/92.24.2009. [DOI] [PubMed] [Google Scholar]
- 5.Park Y, Mitrou PN, Kipnis V, et al. Calcium, dairy foods, and risk of incident and fatal prostate cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2007 Dec;166(11):1270–9. doi: 10.1093/aje/kwm268. [DOI] [PubMed] [Google Scholar]
- 6.Koh KA, Sesso HD, Paffenbarger RS, et al. Dairy products, calcium and prostate cancer risk. Br J Cancer. 2006 Dec;95(11):1582–5. doi: 10.1038/sj.bjc.6603475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tewari A, Horninger W, Pelzer AE, et al. Factors contributing to the racial differences in prostate cancer mortality. BJU Int. 2005 Dec;96(9):1247–52. doi: 10.1111/j.1464-410X.2005.05824.x. [DOI] [PubMed] [Google Scholar]
- 8.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002 Mar;94(5):334–57. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 9.Shavers VL, Brown ML, Potosky AL, et al. Race/ethnicity and the receipt of watchful waiting for the initial management of prostate cancer. J Gen Intern Med. 2004 Feb;19(2):146–55. doi: 10.1111/j.1525-1497.2004.30209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marlow NM, Halpern MT, Pavluck AL, et al. Disparities associated with advanced prostate cancer stage at diagnosis. J Health Care Poor Underserved. 2010 Feb;21(1):112–31. doi: 10.1353/hpu.0.0253. [DOI] [PubMed] [Google Scholar]
- 11.Klassen AC, Platz EA. What can geography tell us about prostate cancer? Am J Prev Med. 2006 Feb;30(2 Suppl 1):S7–15. doi: 10.1016/j.amepre.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Klassen AC, Curriero FC, Hong JH, et al. The role of area-level influences on prostate cancer grade and stage at diagnosis. Prev Med. 2004 Sep;39(3):441–8. doi: 10.1016/j.ypmed.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 13.Brawley OW, Knopf K, Thompson I. The epidemiology of prostate cancer part II: the risk factors. Semin Urol Oncol. 1998 Nov;16(4):193–201. [PubMed] [Google Scholar]
- 14.Wu K, Hu FB, Willett WC, et al. Dietary patterns and risk of prostate cancer in U.S. men. Cancer Epidemiol Biomarkers Prev. 2006 Jan;15(1):167–71. doi: 10.1158/1055-9965.EPI-05-0100. [DOI] [PubMed] [Google Scholar]
- 15.Oliver MN, Smith E, Siadaty M, et al. Spatial analysis of prostate cancer incidence and race in Virginia, 1990-1999. Am J Prev Med. 2006 Feb;30(2 Suppl):S67–76. doi: 10.1016/j.amepre.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Alavanja MC, Samanic C, Dosemeci M, et al. Use of agricultural pesticides and prostate cancer risk in the agricultural health study cohort. Am J Epidemiol. 2003 May;157(9):800–14. doi: 10.1093/aje/kwg040. [DOI] [PubMed] [Google Scholar]
- 17.Settimi L, Masina A, Andrion A, et al. Prostate cancer and exposure to pesticides in agricultural settings. Int J Cancer. 2003 Apr;104(4):458–61. doi: 10.1002/ijc.10955. [DOI] [PubMed] [Google Scholar]
- 18.Parker AS, Cerhan JR, Putnam SD, et al. A cohort study of farming and risk of prostate cancer in Iowa. Epidemiology. 1999 Jul;10:452–5. doi: 10.1097/00001648-199907000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Meyer TE, Coker AL, Sanderson M, et al. A case-control study of farming and prostate cancer in African-American and Caucasian men. Occup Environ Med. 2007 Mar;64(3):155–60. doi: 10.1136/oem.2006.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandelblatt JS, Yabroff KR, Kerner JF. Equitable access to cancer services: a review of barriers to quality care. Cancer. 1999 Dec;86(11):2378–90. [PubMed] [Google Scholar]
- 21.Mullins CD, Blatt L, Gbarayor CM, et al. Health disparities: a barrier to high-quality care. Am J Health Syst Pharm. 2005 Sep;62(18):1873–82. doi: 10.2146/ajhp050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roetzheim RG, Pal N, Tennant C, et al. Effects of health insurance and race on early detection of cancer. J Natl Cancer Inst. 1999 Aug;91(16):1409–15. doi: 10.1093/jnci/91.16.1409. [DOI] [PubMed] [Google Scholar]
- 23.Talcott JA, Spain P, Clark JA, et al. Hidden barriers between knowledge and behavior: the North Carolina prostate cancer screening and treatment experience. Cancer. 2007 Apr;109(8):1599–606. doi: 10.1002/cncr.22583. [DOI] [PubMed] [Google Scholar]
- 24.Tunstall HV, Shaw M, Dorling D. Places and health. J Epidemiol Community Health. 2004 Jan;58(1):6–10. doi: 10.1136/jech.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potosky AL, Feuer EJ, Levin DL. Impact of screening on incidence and mortality of prostate cancer in the United States. Epidemiol Rev. 2001;23(1):181–6. doi: 10.1093/oxfordjournals.epirev.a000787. [DOI] [PubMed] [Google Scholar]
- 26.Cooper GS, Yuan Z, Jethva RN, et al. Determination of county-level prostate carcinoma incidence and detection rates with Medicare claims data. Cancer. 2001 Jul;92(1):102–9. doi: 10.1002/1097-0142(20010701)92:1<102::aid-cncr1297>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 27.Goovaerts P. Three-dimensional visualization, interactive analysis and contextual mapping of space-time cancer data; Proceedings of 13th Agile International conference on geographic information science; Guimarães, Portugal. 2010; May, Available at: http://agile2010.dsi.uminho.pt/pen/ShortPapers_PDF%5C89_DOC.pdf. [Google Scholar]
- 28.Hsu CE, Mas FS, Miller JA, et al. A spatial-temporal approach to surveillance of prostate cancer disparities in population subgroups. J Natl Med Assoc. 2007 Jan;99(1):72–80. 85–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Mather FJ, Chen VW, Morgan LH, et al. Hierarchical modeling and other spatial analyses in prostate cancer incidence data. Am J Prev Med. 2006 Feb;30(2 Suppl):S88–100. doi: 10.1016/j.amepre.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Johnson GD. Small area mapping of prostate cancer incidence in New York State (USA) using fully Bayesian hierarchical modeling. Int J Health Geogr. 2004 Dec;3(1):29. doi: 10.1186/1476-072X-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seidman CS. An introduction to prostate cancer and geographic information systems. Am J Prev Med. 2006 Feb;30(2 Suppl 1):S1–2. doi: 10.1016/j.amepre.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Kulldorff M, Song C, Gregorio D, et al. Cancer map patterns: are they random or not? Am J Prev Med. 2006 Feb;30(2 Suppl):S37–49. doi: 10.1016/j.amepre.2005.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goovaerts P. Combining area-based and individual-level data in the geostatistical mapping of late-stage cancer incidence. Spattemporal Epidemiol. 2009 Oct;1(1):61–71. doi: 10.1016/j.sste.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goovaerts P, Meliker JR, Jacquez GM. A comparative analysis of aspatial statistics for detecting racial disparities in cancer mortality rates. Int J Health Geogr. 2007;6:32. doi: 10.1186/1476-072X-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goovaerts P. Visualizing and testing the impact of place on late-stage breast cancer incidence: a non-parametric geostatistical approach. Health Place. 2010 Mar;16(2):321–30. doi: 10.1016/j.healthplace.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schootman M, Jeffe DB, Lian M, et al. The role of poverty rate and racial distribution in the geographic clustering of breast cancer survival among older women: a geographic and multilevel analysis. Am J Epidemiol. 2009 Mar;169(5):554–61. doi: 10.1093/aje/kwn369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lian M, Schootman M, Yun S. Geographic variation and effect of area-level poverty rate on colorectal cancer screening. BMC Public Health. 2008;8:358. doi: 10.1186/1471-2458-8-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffman RM, Gilliland FD, Eley JW, et al. Racial and ethnic differences in advanced-stage prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst. 2001 Mar;93(5):388–95. doi: 10.1093/jnci/93.5.388. [DOI] [PubMed] [Google Scholar]
- 39.Jones RA, Steeves R, Williams I. How African American men decide whether or not to get prostate cancer screening. Cancer Nurs. 2009 Mar-Apr;32(2):166–72. doi: 10.1097/NCC.0b013e3181982c6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Jaarsveld CH, Miles A, Edwards R, et al. Marriage and cancer prevention: does marital status and inviting both spouses together influence colorectal cancer screening participation? J Med Screen. 2006;13(4):172–6. doi: 10.1177/096914130601300403. [DOI] [PubMed] [Google Scholar]
- 41.Xiao H, Gwede CK, Kiros G, et al. Analysis of prostate cancer incidence using geographic information system and multilevel modeling. J Natl Med Assoc. 2007 Mar;99(3):218–25. [PMC free article] [PubMed] [Google Scholar]
- 42.Plaskon LA, Penson DF, Vaughan TL, et al. Cigarette smoking and risk of prostate cancer in middle-aged men. Cancer Epidemiol Biomarkers Prev. 2003 Jul;12(7):604–9. [PubMed] [Google Scholar]