Abstract
Purpose
TGFβ signaling plays a key role in tumor progression, including malignant glioma. Small-molecule inhibitors such as LY2157299 monohydrate (LY2157299) block TGFβ signaling and reduce tumor progression in preclinical models. To use LY2157299 in the treatment of malignancies, we investigated its properties in a first-in-human dose (FHD) study in patients with cancer.
Experimental Design
Sixty-five patients (58 with glioma) with measurable and progressive malignancies were enrolled. Oral LY2157299 was given as a split dose morning and evening on an intermittent schedule of 14 days on and 14 days off (28-day cycle). LY2157299 monotherapy was studied in dose escalation (part A) first and then evaluated in combination with standard doses of lomustine (part B). Safety was assessed using Common Terminology Criteria for Adverse Events version 3.0, echocardiography/Doppler imaging, serum troponin I, and brain natriuretic peptide (BNP) levels. Antitumor activity was assessed by RECIST and Macdonald criteria.
Results
In part A, 16.6% (5/30) and in part B, 7.7% (2/26) of evaluable patients with glioma had either a complete (CR) or a partial response (PR). In both parts, 15 patients with glioma had stable disease (SD), 5 of whom had SD ≥6 cycles of treatment. Therefore, clinical benefit (CR+PR+SD ≥6 cycles) was observed in 12 of 56 patients with glioma (21.4%). LY2157299 was safe, with no cardiac adverse events.
Conclusions
On the basis of the safety, pharmacokinetics, and antitumor activity in patients with glioma, the intermittent administration of LY2157299 at 300 mg/day is safe for future clinical investigation.
Introduction
TGFβ ligands (TGFβ1, TGFβ2, TGFβ3), identified in 1980s, are shown to regulate diverse biologic functions (1, 2). All three ligands first engage the specific receptor TGFβRI (3), which then heterodimerizes with TGFβRII. This heterodimer complex phosphorylates the intracellular proteins SMAD2 and SMAD3 activating a signaling cascade to induce several nuclear transduction proteins. With the induction of such proteins the TGFβ signaling pathway influences cellular proliferation, differentiation, motility, survival, and apoptosis in tumor cells. This can promote epithelial–mesenchymal transition (EMT) of a tumor, such as malignant glioma (4). In the microenvironment, TGFβ signaling affects several cell types such as immune cells (5), cancer-initiating cells (6, 7), endothelial cells (8), and fibroblasts (9). The overall effect of these microenvironment changes results in tumor progression and metastasis (10). TGFβ signaling is present in most malignancies (11–14), such as hepatocellular carcinoma (15), pancreatic cancer (16), and myelodysplastic syndromes (17). Because of this prominent role, several small-molecule inhibitors (SMI) have been developed to block the TGFβ signaling pathway with the intention to reduce tumor growth.
SMIs blocking the TGFβ signaling are associated with unique cardiovascular toxicities in animals and these nonmonitorable toxicities have prevented the clinical development of TGFβ inhibitors (18). Like previous TGFβ SMI (18), LY2157299 monohydrate, hereafter referred to as LY2157299, also induces heart valve lesions and aneurysms of the ascending aorta at high doses in animals (19). To predict a safe therapeutic window for a first-in-human dose (FHD) study a preclinical pharmacokinetic/pharmacodynamic (PK/PD) model was developed (20, 21). After establishing the PK/PD model, LY2157299 was investigated in the FHD study with the objective to characterize its safety, PK, and document its antitumor activity. Because exposure was identified as a main driver for the cardiotoxicity in animals, doses were escalated to a predefined exposure level that was predicted to be safe and efficacious according to the PK/PD model. During the dose escalation, patients were continuously monitored for exposure and safety, which included an integrated cardiac safety evaluation. Using this PK/PD-based safety assessment, the predicted therapeutic window for a safe dose and dose schedule of LY2157299 was confirmed and thus LY2157299 was advanced into phase II clinical investigation.
Materials and Methods
Patients
Eligible patients must have progressed on prior effective therapies and had a histologic or cytologic diagnosis of a malignancy. Starting with cohort 3, only patients with relapsed and progressive glioma were eligible for this study. Before enrollment, investigators determined progression based on clinical symptoms or radiographic progression. All patients were assessed by the Response Evaluation Criteria in Solid Tumors (RECIST) and were required to have measurable tumor lesions. Starting with cohort 3 onward response was also assessed by Macdonald criteria (22). All patients had to have performance status (PS) of ≤2 on ECOG scale. Patients were required to have adequate hematologic, hepatic, and renal function, and discontinued all previous therapies, including radiotherapy, for cancer at least 4 weeks before study enrolment. Exclusion criteria included medically uncontrolled cardiovascular illness, electrocardiogram anomalies, and serious preexisting medical conditions. Pathologic diagnosis performed at time of first diagnosis was not reassessed by central pathology review in this safety study. Tumor tissue was submitted for assessment of pSMAD2 protein expression and remaining tissue was submitted for genetic mutation evaluation. The details of the biomarker evaluation will be reported elsewhere.
The study was conducted according to the principles of good clinical practice, applicable laws and regulations, and the Declaration of Helsinki. Each institution's review board approved the study and all patients signed an informed consent document before study participation.
IDH1/2 mutation
Where sufficient tumor tissue was available, 287 cancer-related genes were assessed for somatic variants using massive parallel sequencing methods to identify base substitutions, short insertions and deletions, and copy number changes (Foundation Medicine; refs. 23, 24).
Study drug and design
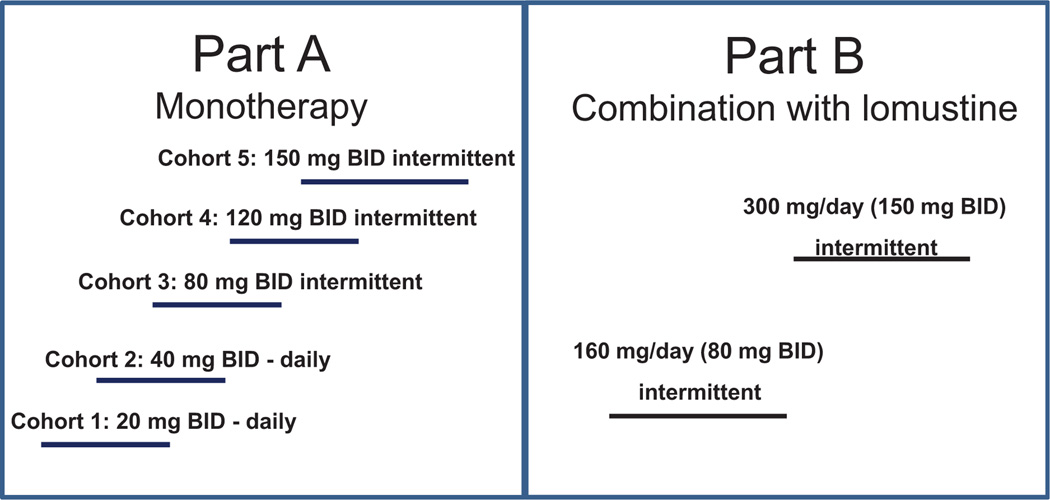
LY2157299 is a serine/threonine kinase inhibitor that blocks specifically the TGFβRI kinase (25). LY2157299 was evaluated in a multicenter open-label, nonrandomized, dose escalation first-in-human phase I study (Fig. 1).
Figure 1.
FHD study design: part A, monotherapy and dose escalation. Cohorts 1 and 2 continuous dosing (twice daily, BID) and cohorts 3 to 5 of intermittent dosing (twice daily) on 14-day on and 14-day off cycle (28-day cycle). Part B, combination therapy with lomustine (100 to 130 mg/m2) with >3 patients in each dose group. The combination therapy in two cohorts at 160 mg/day (80 mg twice daily) and 300 mg/day (150 mg twice daily) with LY2157299 given intermittently.
Part A was a dose escalation study. First, patients with advanced or metastatic cancer received daily continuous LY2157299 monotherapy. Then, starting with cohort 3 and for the remainder of the study, patients received LY2157299 on an intermittent dose regimen of 14 days on/14 days off (28-day cycle; ref. 21). The change in dose regimen was prespecified in the protocol depending on the outcome of a 6-month animal toxicology evaluation of the continuous dosing.
Part B was a safety study using LY2157299 on an intermittent dose regimen (14 days on/14 days off = 28-day cycle) at 160 mg/day (80 mg twice daily) and 300 mg/day (150 mg twice daily) in combination with lomustine given once every 6 weeks in patients with recurrent malignant glioma who progressed on effective treatments. Dose escalation was used to ascertain that the PK profile of LY2157299 remained unchanged in the combination with lomustine.
A part C was also conducted to assess different novel tablet formulation. The results of this relative bioavailability (RBA) phase will be reported elsewhere.
Treatment: dose and dose levels
In part A, LY2157299 was given orally twice daily at doses 20 mg (40 mg/day), 40 mg (80 mg/day), 80 mg (160 mg/day), 120 (240 mg/day), and 150 mg (300 mg/day) as a tablet in the morning and evening on an empty stomach. No dose adjustments or reductions were allowed and patients were to stop study treatment at any signs of medically significant cardiac toxicity. In part B, patients in cohort 1 received LY2157299 at 160 mg/day on intermittent dosing as defined in part A. Lomustine 100 to 130 mg/m2 was given orally once every 6 weeks beginning 1 week after initial LY2157299 dosing. In cohort 2, 300 mg/day LY2157299 was given on intermittent dosing schedule and lomustine as in cohort 1.
Maximum tolerated dose and dose-limiting toxicity assessment
The maximum-tolerated dose (MTD) was defined as the dose level below which ≥2 patients (of up to 6 patients) experienced a DLT during cycle 1. If DLT were to occur, the previous dose level was to be declared the MTD. Dose escalation to the next cohort proceeded only after: (i) 3 patients completed 1 treatment cycle without a DLT; (ii) after assessment of their PK profile and meeting the predefined PK profile (21); (iii) the observed exposure did not exceed the concentrations as defined by the PK/PD model for a safe therapeutic window; (iv) complete safety assessment, including cardiac safety information. Cohorts were also to be expanded if the PK variability was equivocal and required further exploration.
At each dose level hematologic or nonhematologic drug-associated toxicity with grade ≥3 was considered a DLT according to the National Cancer Institute and the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. Any signs of medically significant cardiotoxicity would have stopped the clinical trial.
Safety assessment
Safety was evaluated in patients who received at least one dose of LY2157299 and was based on summaries of adverse events (AE; CTCAE version 3.0), possible relatedness to study drug (LY2157299 or the combination of LY2157299 + lomustine), DLTs, laboratory changes (including monthly BNP and troponin I levels), changes in ECOG performance status, electrocardiogram (ECG), and echocardiography/Doppler (every 2 cycles and reviewed by a central cardiologist). In addition, standard chemistry, hematology, and urinalysis panels were performed. All concomitant medications were documented throughout the patient's participation in the study.
Efficacy assessment
Radiographic changes (CR, PR, SD, PD) were evaluated by investigators using RECIST 1.0 for all patients and Macdonald criteria (22) for patients with glioma. There was no central review of radiographic images. Investigators reported lesion measurements in case report forms for subsequent statistical analyses. Patients with SD and who started 6 or more cycles were considered as having a clinically important benefit or tumor growth control. Such patients were added to the responder group (CR+PR+SD ≥6 cycles).
Statistical analyses
Patient disposition, demographics, and disease characteristics, safety (including cardiac safety examinations), concomitant medication and response data were summarized by study part, using line plots to investigate trends over time, waterfall plots for change in lesion size based on investigator-reported measurements (using Macdonald criteria), summary statistics or frequencies as appropriate.
Results
FHD study design, patient disposition, and characteristics
Although the FHD study included a RBA part, we here report on the results of 65 patients from 4 investigational sites who participated in parts A and B (design in Fig. 1). Results from patients enrolled in the RBA part will be reported elsewhere. The entire study started in 2006 and was closed in 2012. During this period, the study was on clinical hold for approximately 2 years; the hold was lifted after intermittent dosing in animal toxicology supported the safety of the predicted therapeutic window.
In part A (LY2157299 monotherapy), 39 patients were enrolled; 32 of these patients had glioma. With cohort 5, the predefined maximum exposure level was reached without significant toxicities or DLT. In part B (LY2157299 combined with lomustine), 26 patients received LY2157299 in combination with standard dose of lomustine (15 patients at 160 mg/day and 11 patients at 300 mg/day).
At baseline, the mean age of the patients was 51.8 and 44.5 years in parts A and B, respectively (Table 1). Male patients comprised 76.9% and 73.1% of the population in parts A and B, respectively. Using baseline Eastern Cooperative Oncology Group (ECOG) PS, patients in part A had a better PS than patients in part B. The majority of patients had a primary WHO grade 4 glioma (15/30 or 50% in part A, 20/26 or 76.9% in part B; Table 1).
Table 1.
Patient baseline characteristics
| Characteristics | Part A N = 39 |
Part B N = 26 |
|---|---|---|
| Age, y | ||
| Mean (SD) | 51.8 (14.88) | 44.5 (10.35) |
| Median (range) | 54.0 (22–77) | 43.5 (25–61) |
| Sex, n (%) | ||
| Male | 30 (76.9) | 19 (73.1) |
| Female | 9 (23.1) | 7 (26.9) |
| Origin, n (%) | ||
| Caucasian | 39 (100) | 24 (92.3) |
| Hispanic | — | 1 (3.8) |
| West Asian | — | 1 (3.8) |
| ECOG, n (%) | ||
| 0 | 15 (38.5) | 3 (11.5) |
| 1 | 19 (48.7) | 17 (65.4) |
| 2 | 5 (12.8) | 6 (23.1) |
| Prior regimens, n (%) | ||
| 1 | 17 (43.6) | 7 (26.9) |
| 2 | 13 (33.3) | 11 (42.3) |
| 3 | 4 (10.3) | 7 (26.9) |
| >3 | 5 (12.8) | 1 (3.8) |
| Prior radiation, n (%) | 35 (89.7) | 26 (100) |
| Prior surgery, n (%) | 30 (76.9) | 25 (96.1) |
| Glioma patients only | N = 32 | N = 26 |
| Time from initial diagnosis to before first dose, median (range: earliest to most recent) in months | 22.1 (172.4–2.8) | 18.0 (154.6–7.0) |
| Prior surgery, n (%) | 32 (100) | 26 (100) |
| Prior bevacizumab, n (%) | 10 (31.3) | 7 (26.9) |
| Prior temozolomide and radiation, n (%) | 32 (100) | 26 (100) |
| Glioma WHO, n (%) at study entry | n = 30a | n = 26 |
| Grade 1 | 1 (3.3) | — |
| Grade 2 | 2 (6.7) | — |
| Grade 3 | 6 (20) | 4 (15.4) |
| Grade 4 | 21 (70) | 22 (84.6) |
| Secondary grade 4 | 6 (20) | 2 (7.7) |
| Primary grade 4 | 15 (50) | 20 (76.9) |
| Tissue samples for deep sequencing | 11 | 10 |
| IDH1/2 mutation/tissue examined (%) | 3/11 (27.3) | 2/10 (20) |
Data on grade not available for 2 patients in cohort 3.
Glioma patients in part A had a slightly longer length of disease before enrolling in the study compared with part B patients (~22 months compared to 18 months). All but 3 glioma patients (55/58 or 94.8%) had prior surgery, all patients were treated with temozolomide and radiation before their relapse but only approximately 30% were treated with bevacizumab before enrolling into the study. In part A, there were about 20% (6/30) of patients with secondary grade 4 glioma and 30% (9/30) had low-grade glioma (WHO grade 1–3). Tissue was available in 21 patients (11 in part A and 10 in part B), 5 of whom had IDH1/2 mutation (5/21; 23.8%).
At study closure in 2012 in part A, 33 patients (33/39; 84.6%) discontinued the study treatment due to progressive disease, one patient died during treatment as the tumor progressed, and 3 patients withdrew from the study (Table 2). In part B, 22 patients (22/26; 84.6%) discontinued due to progressive disease, one patient died during treatment due to his cancer, and one withdrew from the study. As of June 2014, 3 patients were still receiving LY2157299: 2 in part A (R19 and R33, treated for 73 and 55 cycles, respectively, or 5 and 4.2 years, respectively) and 1 in part B (R42, treated for 48 cycles or 3.7 years).
Table 2.
Patient discontinuation from treatment and treatment responses
| Reasons | Part A (N = 39) n (%) |
Part B (N = 26) n (%) |
|---|---|---|
| Adverse event | — | 1 (3.8) |
| Platelets | 1 (3.8) | |
| Other | 3 (7.7) | 1 (3.8) |
| Withdrawn consent | 1 (2.6) | 1 (3.8) |
| Patient/investigator decision | 2 (5.1) | — |
| Progressive disease | 33 (84.6) | 22 (84.6) |
| Death | 1 (2.6) | 1 (3.8) |
| Disease progression ("study disease") | 1 (2.6) | 1 (3.8) |
| Cycles on study treatment, median (range) | 2 (1–46) | 2 (1–22) |
| Treatment responsea | N = 30 | N = 26 |
| n (%) | n (%) | |
| CR/PR | 5 (16.6) | 2 (7.7) |
| SD ≥6 cycles | 1 (3.3) | 4 (15.4) |
| CR/PR/SD ≥6 cycles | 6 (20.0) | 6a (23.1) |
| SD | 10 (33.3) | 5 (19.2) |
| On study treatment at study closure in 2012 | 2 (6.6) | 1 (3.8) |
Macdonald criteria for all but 1 patient, in whom RECIST was used.
Safety measures (including integrated cardiac monitoring results)
Although some possible drug-related toxicities were observed in this study, there was no cluster of serious adverse events or (AE) as determined byCTCAEv.3 grading (Table 3). No definitive drug-related toxicity or DLT was reported in either of the two study parts.
Table 3.
Possible drug-related CTCAE: maximum grade 3 or 4
| Reasons | Part A Patients (N = 39) n (%) |
Part B Patients (N = 26) n (%) |
|---|---|---|
| Nonlaboratory | ||
| Diarrhea | — | — |
| Febrile neutropenia | — | 1 (3.8) |
| Fatigue | — | 1 (3.8) |
| CNS ischemia | 1 (2.6) | — |
| Thrombosis/embolism | 1 (2.6) | — |
| Dyspnea | 1 (2.6) | — |
| Laboratory | ||
| Leukopenia | — | 3 (11.5) |
| Neutropenia | — | 2 (7.7) |
| Thrombocytopenia | 1 (2.6) | 5 (19.2) |
| Lymphopenia | — | 4 (15.4) |
NOTE: Some events might have been observed in the same patient.
In part A, three patients (7.7%) had four grade 3/4 toxicities that were considered possibly drug related. One such patient experienced a grade 4 thrombocytopenia that was considered a possible DLT. This patient recovered from the serious AE (SAE) but later died due to tumor progression. The second patient discontinued the study due to ischemic stroke (grade 4) after surgical resection of his tumor during the off-period of LY2157299 treatment. The third patient experienced grade 4 pulmonary embolism and dyspnea, and after hospitalization died of tumor progression. Thirty-seven of 39 (94.9%) patients experienced at least 1 treatment-emergent AE (TEAE). No patient discontinued due to an AE.
In part B, there were no DLTs, including at the highest dose of LY2157299 (300 mg/day) combined with lomustine. A total of 23 patients (88.5%) had grade 3 or 4 events. In 8 patients (30.8%), there were 16 of the possibly drug-related SAEs (Table 3). All 26 patients of this study part experienced at least 1 TEAE; 15 (57.7%) of these patients experienced at least 1 study treatment–related TEAE. The TEAEs were likely due to lomustine, but relatedness could not be ascribed to only LY2157299 or lomustine. One patient discontinued because of a grade 2 thrombocytopenia associated with drug treatment.
The integrated cardiovascular safety monitoring detected no clinically significant cardiac safety findings in both parts of the study. There were no pathologic changes in troponin I and plasma BNP levels, or medically significant cardiac electrophysiological readings or functional changes as assessed by echocardiography/Doppler. Also, computer tomography scans of the ascending aorta and aortic arch did not detect any aneurysms.
Cumulative dose of LY2157299 and concomitant medications
Dose intensity (percentage of the actual cumulative dose over planned cumulative dose) was high in both parts of the study suggesting a low toxicity profile. The median dose intensity ranged from 89.1% in cohort 1 to 99.3% in cohort 5 of part A and 100% in both cohorts of part B. There was no cumulative toxicity observed in either parts of the study.
The concomitant medications were consistent with the expected comedications for patients with advanced or metastatic disease, such as pain medication. The most common concomitant drugs in both parts of the study were as follows: dexamethasone (60.0% part A, 84.6% part B), omeprazole (56.4% part A, 50.0% part B), levetiracetam (43.6% part A, 84.6% part B), and acetaminophen (41.0% part A, 34.6% part B).
Efficacy measures
Radiographic responses based on Macdonald criteria in parts A and B for patients with glioma
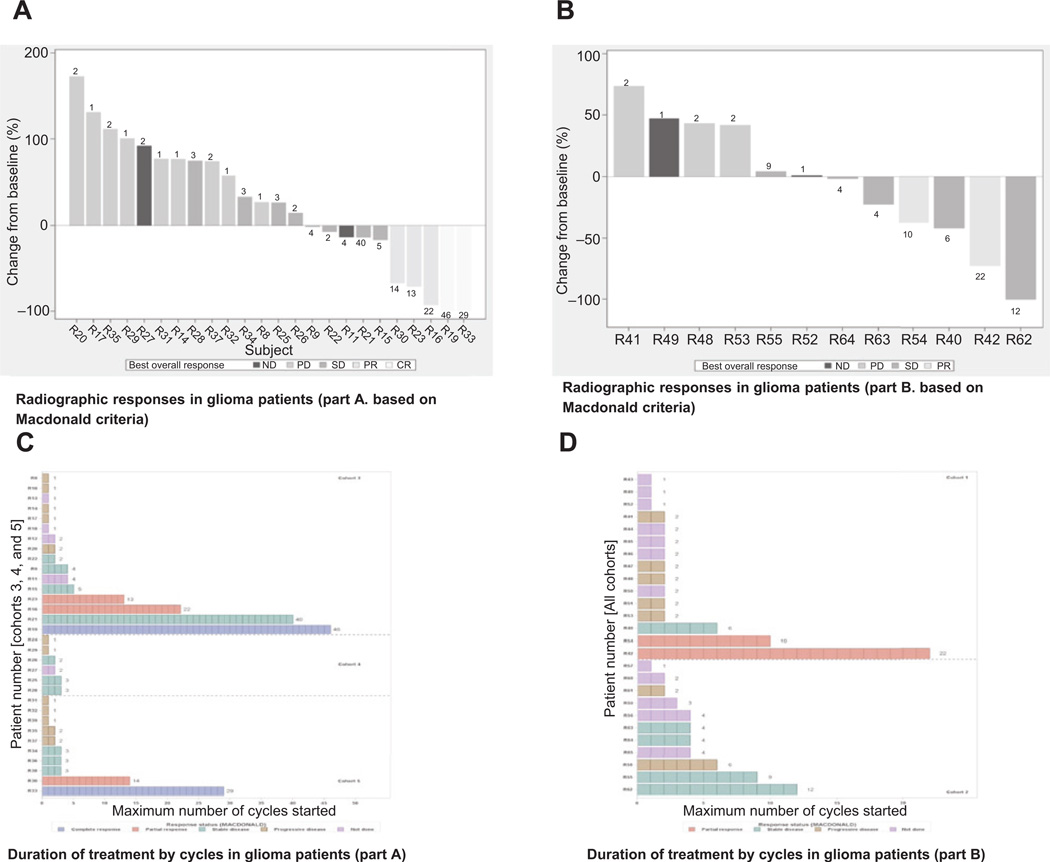
In part A, there were 33.3% (13/39) deaths that occurred on treatment or during follow-up and were related to tumor progression. Baseline measurements according to the Macdonald method were available for 26 of 30 patients and radiographic responses were assessed in 24 patients (Fig. 2A). Of the 30 patients with glioma, overall response rate was 16.6% (5/30), including 2 CRs (2/30, 6.6%) and 3 PRs (3/30, 10%; Table 2). These responses were assessed on the basis of measurable lesions. Ten patients (10/30; 33.3%) had stable disease (SD). The rate of clinical benefit (CR+PR+SD ≥6 cycles) was 20% (6/30). In part B, there were 57.7% (15/26) deaths on or during follow-up and all related to tumor progression. Baseline measurements according to Macdonald were available for 18 of 26 patients, of whom only 12 were evaluated for radiographic response (Fig. 2B).
Figure 2.
Radiographic responses in patients with glioma based on Macdonald criteria (waterfall plots, A and B) and number of cycles on treatment (C and D) in parts A and B, respectively. Waterfall plot of minimum percent change over baseline in sum of lesion measurements with best overall investigator-reported response. In parts A and B, 26 of the 30 patients and 18 of 26 patients were assessed at baseline using Macdonald criteria. A and B, waterfall plot for monotherapy (part A; 24 patients with pre- and postdose Macdonald assessments) and combination therapy (part B: 12 patients with pre- and postdose Macdonald assessments): numbers on the bars indicate the number of cycles on treatment. In part A, there were 5 patients with PR (R30, R23, and R16) and CR (R19 and R33), and in part B, there were 2 patients with PR (R42 and R54). C and D, at time of study closure, duration of treatment is shown, and each box shows a full cycle (28 days) for cohorts 3 to 5 in part A and for both cohorts in part B.
Duration of treatment by number of cycles is presented in Fig. 2C for part A. On average, patients received 2 cycles of treatment and a subgroup of patients was treated beyond cycle 6 (Table 2; Fig. 2C). The duration of treatment by number of cycles is presented in Fig. 2D. Two patients (2/26; 7.7%) had PR; one of these patients had a possible CR (patient R62). A clinical benefit response (CR+PR+SD ≥6 cycles) rate of 23.1% (6/26) was observed (Table 2).
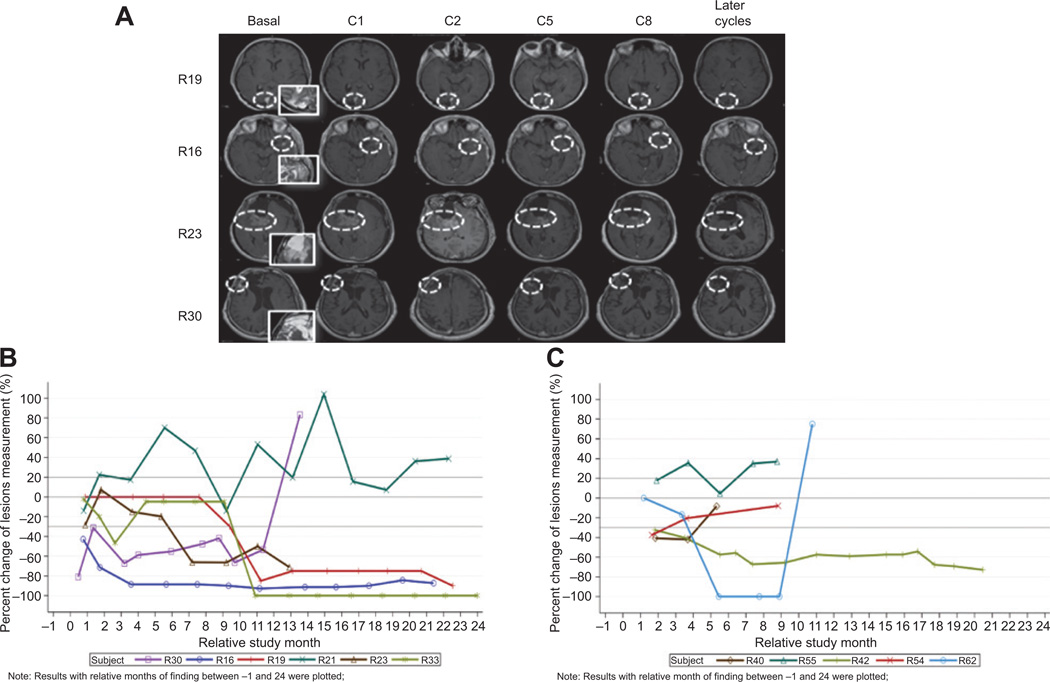
The clinical responders had an initial disease stabilization followed by radiographic tumor lesion reduction (Fig. 3A–C). The radiographic responses were not dependent on tumor size before initiation of treatment (Fig. 3A). One patient (R19) had a complete response in cycle 28, which was confirmed in cycle 32. Another patient had first a pseudoprogression with no clinical progression (part A, patient R21) and then had a marked radiographic response. The remaining patients had a gradual reduction of their tumor. In 2 of these patients, a CR developed and is still being observed after at least 3 years of treatment (Fig. 3B).
Figure 3.
A, examples of 4 patients with partial and complete responses after cycles 1, 2, 5, and 8, and later follow-up assessments. Insets show T2 images at baseline for target lesion. White circles show the target lesions over time of assessment. Lesion assessment was conducted at the level of the largest diameter. B and C, radiographic response over time based on Macdonald criteria in part A (B) and part B (C). Note: one patient (R19 in part A) had a complete response in cycle 28 and confirmed in cycle 32 (therefore not attaining 100% reduction in this plot). A second patient in part B (R58) was treated for 6 cycles, but assessment was not carried out using Macdonald criteria until progression.
Tumor characteristics and clinical response
In part A, 50% (15/30) of patients had either low-grade glioma (9/30) or secondary glioblastoma (6/30), and 50% (15/30) had primary glioblastoma (Table 1). In low-grade glioma or secondary glioblastoma, 20% (3/15) of patients showed tumor response (CR/PR) and a similar response rate (13.3%, 2/15) was observed in patients with primary glioblastoma (Data not shown). In part B, there were fewer patients with low-grade glioma or secondary glioblastoma (23.1%, 6/26) compared with part A, and more patients with primary glioblastoma (76.9%, 20/26; Table 1). Responses (CR/PR) were observed in 33.3% (2/6) of patients with low grade glioma or secondary glioblastoma and SD ≥ 6 cycles (3/20; 15%) were observed only in patients with primary glioblastoma (data not shown). There were 5 of 21 patients with IDH1/2 mutations (Table 1). Aclinical benefit (CR/PR/≥SD6 cycles) was observed in 4 of the 5 patients (80%; 4/5—two in each parts of the study; data not shown).
Discussion
In the current study, we show that the novel SMI LY2157299 targeting TGFβ signaling can be safely administered and has a clinical benefit in a tumor type where this pathway is considered a key driver of progression. The favorable toxicity profile of LY2157299 included no medically significant cardiovascular toxicities as monitored by monthly serial plasma markers (troponin I, BNP) and echocardiography/Doppler imaging every 2 months. Contrary to the concern that blocking the TGFβ signaling pathway may potentially induce secondary malignancies (26), no signs of tumorigenesis were observed, including in patients who have been in treatment longer than 3 years. With the exception of diarrhea (grade 2) which presented in one patient during each of the 14-day on-treatment periods and two cases of grade 3/4 thrombocytopenia, no other events were judged to be possibly related to LY2157299 treatment. The causes for both cases of thrombocytopenia remain unclear, because LY2157299 has shown no direct bone marrow toxicity in animal studies or in human in vitro bone marrow assays. Both patients had previous bevacizumab treatment and had shown moderate platelet reduction during bevacizumab treatment. Perhaps in both patients the previous antibody treatment unmasked an underlying idiopathic thrombocytopenia (ITP), which is thought to be suppressed by regulatory T cells (27). As LY2157299 is designed to block TGFβ production including in regulatory T cells, its administration may have removed the regulatory T cell–mediated suppression of ITP. The concern that LY2157299 could have direct bone marrow toxicity was further weakened in part B, where the combination of LY2157299 and lomustine did not result in an increase of the known lomustine toxicity. Platelet recovery after lomustine administration was not diminished by giving LY2157299 during the recovery period (Supplementary Fig. S1A and S1B).
LY2157299 treatment showed single-agent activity in glioma patients who had progressed on previous treatments not only based on radiographic responses, but also by SD exceeding 5 cycles (SD ≥ 6 cycles, which is ~5 months). In part A, there was 1 patient and in part B there were 4 patients who had such a prolonged tumor growth control (Fig. 2C and D). The radiographic response often occurred after several cycles. Whether this pattern of response is similar to the one observed for ipilimumab in melanoma awaits further investigation (28). Similar to ipilimumab, LY2157299 may cause an activation of cytotoxic T cells by blocking the TGFβ signaling in T regulatory cells. Furthermore, the combination of LY2157299 and lomustine was justified because of its additive in vitro and in vivo effects (29). In two patients of part B, a tumor response was observed requiring a formal comparative phase II study to determine whether the combination is more active than the monotherapy. This phase II study recently completed enrollment and awaits study results (30).
The responses are also reminiscent of those reported for glioma patients treated with trabedersen, an antisense oligonucleotide targeting TGFβ2 (31). Similar to trabedersen, the responses appeared to be more common in patients with lower WHO grade glioma. Secondary or lower grade gliomas are commonly associated with IDH1 mutations (23), and IDH1 mutations have been recently associated with TGFβ signaling (32, 33). To further investigate this possibility, we assessed the IDH1/2 mutation in tumor tissue of the original diagnostic specimen. Given the limited amount of tissue available, it was not possible to assess the methylation status of the MGMT promotor status in the same patient group. Thus, it is not possible to determine whether MGMT promotor status also plays a role in determining patients with a possible response to LY2157299. Among the 5 patients with an IDH1/2–mutated tumor, 4 patients had either CR/PR or SD ≥ 6 cycles (4/5; 80%). Among the 11 patients with CR/PR/SD ≥ 6 cycles and compared with these 4 patients with IDH1/2 mutation (4/11; 36.3%), there were 7 other patients who did not have the IDH1/2 mutation (7/11; 63.6%). This observation suggests that the TGFβ signaling pathway may be enriched in the IDH1/2-mutated tumors, but also be present in other less molecularly characterized glioma, perhaps those with a mesenchymal phenotype (34). Therefore, future clinical studies with LY2157299 will likely benefit from a patient selection based on profiling tumors for a TGFβ-dependent signature.
In summary, LY2157299 has predictable pharmacokinetics and a favorable safety profile to continue its clinical investigation in phase II, and has shown clinical benefit at the recommended dose predicted by a preclinical PK/PD model.
Supplementary Material
Translational Relevance.
TGFβ signaling is a driver in tumorigenesis, but blocking this pathway with small-molecule inhibitors (SMI) is associated with severe cardiac toxicities in animals. By defining a therapeutic window for the SMI LY2157299, long-held reservations toward the clinical development of such SMIs have been addressed. Currently, LY2157299 is the only SMI in clinical development.
Acknowledgments
The authors thank patients for their willingness to participate in this study. The authors also thank all site staff at the four institutions and the trial personnel at Eli Lilly and Company, Quintiles, and ICON.
Grant Support
This study was supported by Eli Lilly and Company.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Prior presentation: The data from this study were presented in part at ASCO 2008, ASCO 2011, ASCO 2012, and ASCO 2013.
Disclosure of Potential Conflicts of Interest
N.S. Pillay is an employee of and has ownership interest (including patents) in Eli Lilly and Co. D. Desaiah and M.M. Lahn are employees of Eli Lilly and Co. L. Paz-Ares has speakers bureau honoraria from Eli Lilly and Co. No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions
Conception and design: J. Rodon, M.A. Carducci, I. Gueorguieva, A.L. Cleverly, N.S. Pillay, M.M. Lahn, J. Baselga
Development of methodology: J. Rodon, E. Calvo, J. Seoane, I. Gueorguieva, N.S. Pillay, J. Blakeley, M.M. Lahn, J. Baselga
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): J. Rodon, M.A. Carducci, J.M. Sepulveda-Sanchez, E. Calvo, J. Seoane, I. Braña, E. Sicart, N.S. Pillay, S.T. Estrem, L. Paz-Ares, M. Holdhoff, J. Blakeley, M.M. Lahn, J. Baselga
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): J. Rodon, M.A. Carducci, J.M. Sepulveda-Sanchez, J. Seoane, I. Gueorguieva, A.L. Cleverly, N.S. Pillay, D. Desaiah, S.T. Estrem, L. Paz-Ares, M. Holdhoff, M.M. Lahn, J. Baselga
Writing, review, and/or revision of the manuscript: J. Rodon, M.A. Carducci, J.M. Sepulveda-Sanchez, A. Azaro, E. Calvo, J. Seoane, I. Braña, E. Sicart, A.L. Cleverly, N.S. Pillay, D. Desaiah, S.T. Estrem, L. Paz-Ares, M. Holdhoff, J. Blakeley, M.M. Lahn, J. Baselga
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): N.S. Pillay, M.M. Lahn
Study supervision: J. Rodon, M.A. Carducci, E. Calvo, I. Gueorguieva, N.S. Pillay, L. Paz-Ares, M.M. Lahn
Other (study conduction): A. Azaro, M.M. Lahn
References
- 1.Roberts AB, Anzano MA, Lamb LC, Smith JM, Sporn MB. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981;78:5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massagué J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 3.Cheifetz S, Weatherbee JA, Tsang MLST, Anderson JK, Mole JE, Lucas R, et al. The transforming growth factor-B system, a complex pattern of cross-reactive ligands and receptors. Cell. 1987;48:409–415. doi: 10.1016/0092-8674(87)90192-9. [DOI] [PubMed] [Google Scholar]
- 4.Miyazono K, Ehata S, Koinuma D. Tumor-promoting functions of transforming growth factor-b in progression of cancer. Ups J Med Sci. 2012;117:143–152. doi: 10.3109/03009734.2011.638729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas DA, Massagué TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. J Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Bruna A, Greenwood W, LeQuesne J, Teschendorff A, Miranda-Saavedra D, Rueda OM, et al. TGFβ induces the formation of tumour-initiating cells in claudinlow breast cancer. Nat Commun. 2012;3:1055. doi: 10.1038/ncomms2039. [DOI] [PubMed] [Google Scholar]
- 7.Anido J, Sáez-Borderías A, Gonzàlez-Juncà A, Rodón L, Folch G, Carmona MA, et al. TGF-beta receptor Inhibitors target the CD44(high)/Id1(high) glioma-initiating cell population in human glioblastoma. Cancer Cell. 2010;18:655–668. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Viñals F, Pouysségur J. Transforming growth factor β1 (TGF-β1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF-α signaling. Mol Cell Biol. 2001;21:7218–7230. doi: 10.1128/MCB.21.21.7218-7230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawinkels LJ, Paauwe M, Verspaget HW, Wiercinska E, van der Zon JM, van der Ploeg K, et al. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene. 2014;33:97–107. doi: 10.1038/onc.2012.536. [DOI] [PubMed] [Google Scholar]
- 10.Calon A, Espinet E, Palomo-Ponce S, Tauriello DVF, Iglesias M, Cespedes MV, et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider T, Sailer M, Ansorge S, Firsching R, Reinhold D. Increased concentrations of transforming growth factor beta1 and beta2 in the plasma of patients with glioblastoma. J Neuro Oncol. 2006;79:61–65. doi: 10.1007/s11060-005-9116-7. [DOI] [PubMed] [Google Scholar]
- 12.Bruna A, Darken RS, Rojo F, Ocaña A, Peñuelas S, Arias A, et al. High TGF-beta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGFB gene. Cancer Cell. 2007;11:147–160. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Peñuelas S, Anido J, Prieto-Sánchez RM, Folch G, Barba I, Cuartas I, et al. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15:315–327. doi: 10.1016/j.ccr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Tritschler I, Gramatzki D, Capper D, Mittelbronn M, Meyermann R, Saharinen J, et al. Modulation of TGF-beta activity by latent TGF-beta-binding protein 1 in human malignant glioma cells. Int J Cancer. 2009;125:530–540. doi: 10.1002/ijc.24443. [DOI] [PubMed] [Google Scholar]
- 15.Fransvea E, Angelotti U, Antonaci S, Giannelli G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology. 2008;47:1557–1566. doi: 10.1002/hep.22201. [DOI] [PubMed] [Google Scholar]
- 16.Melisi D, Ishiyama S, Sclabas GM, Fleming JB, Xia Q, Tortora G, et al. LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther. 2008;7:829–840. doi: 10.1158/1535-7163.MCT-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou L, McMahon C, Bhagat T, Alencar C, Yu Y, Fazzari M, et al. Reduced SMAD7 leads to over activation of TGF-beta signaling in MDS that can be reversed by a specific inhibitor of TGF-beta receptor I kinase. Cancer Res. 2011;71:955–963. doi: 10.1158/0008-5472.CAN-10-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderton MJ, Mellor HR, Bell A, Sadler C, Pass M, Powell S, et al. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol Pathol. 2011;39:916–924. doi: 10.1177/0192623311416259. [DOI] [PubMed] [Google Scholar]
- 19.Stauber AJ, Credille KM, Truex LL, Ehlhardt WJ, Young JK. Nonclinical safety evaluation of a transforming growth factor β receptor I kinase inhibitor in Fischer 344 rats and beagle dogs. J Clin Toxicol. 2014;4:196. [Google Scholar]
- 20.Bueno L, de Alwis DP, Pitou C, Yingling J, Lahn M, Glatt S, et al. Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur J Cancer. 2008;44:142–150. doi: 10.1016/j.ejca.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Gueorguieva I, Cleverly AL, Stauber A, Pillay NS, Rodon JA, Miles CP, et al. Defining a therapeutic window for the novel TGF-β inhibitor LY2157299 monohydrate based on a pharmacokinetic/pharmacodynamics (PK/PD) model. Br J Clin Pharmacol. 2013;77:796–807. doi: 10.1111/bcp.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 23.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawyer JS, Beight DW, Britt KS, Anderson BD, Campbell RM, Goodson T, Jr, et al. Synthesis and activity of new aryl- and heteroaryl-substituted 5,6-dihydro-4H-pyrrolo[1,2-b]pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain. Bioorg Med Chem Lett. 2004;14:3581–3584. doi: 10.1016/j.bmcl.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 27.Bao W, Bussel JB, Heck S, He W, Karpoff M, Boulad N, et al. Improved regulatory T cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116:4639–4645. doi: 10.1182/blood-2010-04-281717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsons S, Sawyer S, Yan L, Foreman R, Weir S, Lahn M, et al. The combination of the small molecule TGFβR1 inhibitor, LY2157299 monohydrate, with CCNU substantially blocks SMAD phosphorylation and significantly suppresses human glioblastoma xenograft growth [abstract]. Proceedings of the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics; 2011 Nov 12–16; San Francisco, California. Abstract C201. [Google Scholar]
- 30.Carpentier A, Brandes B, Kesari S, Sepulveda J, Wheeler H, Chinot O, et al. Safety interim data from a 3-arm phase 2 study evaluating safety and pharmacokinetics of the oral transforming growth factor-beta (TGF-β) receptor I kinase inhibitor LY2157299 monohydrate in patients with glioblastoma at first progression. J Clin Oncol. 2013;31 (abstr 2061). [Google Scholar]
- 31.Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahapatra AK, Suri A, et al. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13:132–142. doi: 10.1093/neuonc/noq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki M, Knobbe CB, Munger JC, Lind EF, Brenner D, Brüstle A, et al. IDH1 (R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012;488:656–659. doi: 10.1038/nature11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grassian AR, Lin F, Barrett R, Liu Y, Jiang W, Korpal M, et al. Isocitrate dehydrogenase (IDH) mutations promote a reversible ZEB1/MicroRNA (miR)-200-dependent epithelial-mesenchymal transition (EMT) J Biol Chem. 2012;287:42180–42194. doi: 10.1074/jbc.M112.417832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.