Abstract
Background
Diffusion-weighted magnetic resonance (MR) imaging (DWI) has been suggested for staging liver fibrosis. The aim of this study was to evaluate the diagnostic accuracy of DWI for the noninvasive assessment of hepatic fibrosis.
Methods
We retrospectively compared DWI from clinically acquired MR scans with histologic methods. Liver biopsy specimens were staged F0–F4 in accordance with the METAVIR score. Hepatic steatosis was classified on a 5-point scale. Hepatic iron was graded on a 3-point scale. Liver inflammation was scored according to the modified hepatic activity index. Nonparametric methods, linear regression models, and receiver operating characteristic analyses were used to determine diagnostic accuracy and apparent diffusion coefficient (ADC) cutoff values.
Results
Liver ADC values were inversely correlated with fibrosis stage: P = −0.54 (P < 0.0001). Although there was substantial overlap in the ADC distributions, the differences in ADC values by METAVIR stages F0 versus (vs.) F1–4, F0–1 versus F > 1, F0–2 versus F3–4 and F0–3 versus F4 were all significant. For prediction of fibrosis stage 1, stage 2, stage 3, and stage 4 area under the receiver operating characteristic curve of 0.79, 0.77, 0.77, and 0.79 were obtained, respectively. Inflammation also correlated significantly with ADC values (P = −0.23, P = 0.03), but iron content (P = 0.17) or steatosis (P = 0.63) did not correlate with ADC measurements.
Conclusions
Liver ADC can be used to predict liver fibrosis with acceptable diagnostic accuracy. DWI should be included in further prospective studies to validate a comprehensive MR imaging protocol for the noninvasive assessment of hepatic fibrosis.
Keywords: fibrosis, magnetic resonance imaging, diffusion-weighted imaging, noninvasive staging
HEPATIC FIBROSIS
The incidence of chronic liver disease is projected to increase, mostly secondary to nonalcoholic fatty liver disease and hepatitis C. Hepatic fibrosis is an integral part in the progression of chronic liver disease, and may ultimately lead to cirrhosis and hepatocellular carcinoma.1 Early detection of liver disease and prevention of disease progression could reduce healthcare costs and overall disease burden.2 However, many patients with liver disease remain asymptomatic until decompensation occurs.3 A number of serum markers, an inexpensive and simple way to diagnose liver disease, have shown high sensitivity for the detection of fibrosis but limited specificity.4 To date, histology remains the gold standard for staging liver disease. On the other hand, liver biopsy is an invasive procedure, with several other known drawbacks (costly, sampling and observation errors) and the use of liver biopsy to diagnose and stage liver diseases affecting large segments of the population is impractical.5–7
MAGNETIC RESONANCE IMAGING
Using magnetic resonance imaging (MRI)-based methods for the diagnosis of liver fibrosis has advantages with respect to its potential broad application in heterogeneous populations. These include: (1) integration into a comprehensive hepatic MRI examination, including morphologic and perfusion imaging for the detection of hepatocellular carcinomas and the assessment of liver structure and function, (2) operator independence, (3) reliability regardless of body habitus, and (4) potential to assess the entire liver with an appropriate rapid multislice method. Thus, MRI provides an overall estimate of hepatic disease rather than the relatively small samples obtained with ultrasound-based elastography (transient elastography) or liver biopsy.
DIFFUSION-WEIGHTED MRI
Diffusion-weighted MRI (DWI) depends on the microscopic mobility of water protons. This mobility, classically called Brownian motion, is due to thermal agitation and is highly influenced by the cellular environment of water. Findings on DWI could be an early harbinger of biologic abnormality. The most established clinical indication for DWI is the assessment of cerebral ischemia, where DWI findings precede abnormalities in all other MR techniques.8 Indeed, DWI provides important information about the movement and functional environment of water in tissue and reflects cellular status of normal and pathological tissue. Further, DWI is sensitive to changes in the micro diffusion of water within the intracellular and extracellular spaces. Apparent diffusion coefficient (ADC) is an indicator of the movement of water within the tissue. It provides an average value of the flow and distance a water molecule has moved. In hepatic fibrosis, it is thought that collagen is limited in free water and that the complex assembly of collagen fibers, glycosaminoglycans, and proteoglycans that constitute liver fibrosis may restrict the molecular diffusion measured by DWI.9 Therefore, the accumulation of fibrosis should cause a reduction in the amount of water diffusion in affected liver tissue. DWI has been successfully applied to differentiate cirrhotic from healthy tissue.10,11 Attempts to differentiate between earlier stages of fibrosis in humans have resulted in contradictory results.9,12–14 However, these studies used small numbers of patients and various hardware and sequencing profiles, rendering comparisons difficult. Clearly more research is needed to determine whether or not DWI can be a useful tool for detection and staging of hepatic fibrosis.
The purpose of this study was to retrospectively evaluate DWI data acquired in patients with suspected or known liver disease to determine if DWI parameters correlate with the degree of fibrosis by liver biopsy.
PATIENTS AND METHODS
Patients
The institutional review board approved this retrospective assessment of imaging and clinical data, along with a waiver of informed consent. In all, 4036 consecutive patients underwent an elective liver biopsy for various clinical indications between 2005 and 2008. Total 198 of these 4036 patients also had clinically indicated MRI of the abdomen within 12 months of liver biopsy. Of these 198 patients, 110 had to be excluded from this study owing to the following reasons: in 72 cases, MRI did not include DWI, in 14 cases the DWI sequence could not be analyzed owing to artifacts (respiratory motion), and in 2 cases the biopsy sample was less than 10 mm long. After these exclusions, 88 individuals remained. Demographics and clinical data of these individuals are shown in Table 1.
TABLE 1.
Demographic and Clinical Characteristics of the Study Population (n = 88)
| Characteristic | Value (%) |
|---|---|
| Median age (y) | 50.6 (range, 44.2–55.2) |
| Male | 53 (60.2) |
| White | 52 (59.1) |
| African American | 34 (38.6) |
| Asian | 2 (2.3) |
| Liver disease | |
| Healthy living donor | 7 (7.9) |
| Hepatitis C | 45 (51.1) |
| Hepatitis B | 2 (2.3) |
| Alcoholic liver disease | 5 (5.7) |
| Nonalcoholic fatty liver disease | 3 (3.4) |
| Drug induced hepatitis | 1 (1.1) |
| Cryptogenic hepatitis | 2 (2.3) |
| Ischemic hepatitis | 3 (3.4) |
| Cytomegalovirus | 2 (2.3) |
| Cholestatic liver disease/biliary obstructions | 9 (10.2) |
| Primary sclerosing cholangitis | 8 (9.1) |
| Budd-Chiari syndrome | 1 (1.1) |
| Tissue origin | |
| Needle biopsy | 64 (72.7) |
| Specimen sample length in mm | 22 (range 15–29) |
| Wedge biopsy | 7 (7.9) |
| Resection | 16 (18.2) |
| Explant | 1 (1.1) |
Data are number (%) of participants for categorical variables or median and interquartile range (IQR) for continuous variables.
Liver Histology
Batched slides were read by an experienced hepatopathologist who was blinded to the clinical data. The adequacy of the final tissue sample was judged by the pathologist. The hepatopathologist graded the degree of inflammation with use of the Ishak scoring system15 and staged fibrosis according to the METAVIR scoring system.16 Hepatic steatosis was classified on a 5-point scale.17 Hepatic iron was graded as none, mild, and moderate to severe. The necroinflammatory activity was assessed according to the modified histologic activity index (MHAI) of Ishak.15 The presence and absence of necrosis in the tissue sample was also noted and the presence of cholestasis was ranked on a 4-point scale (none, mild, moderate, marked).
MRI
All MRI was performed on 1 of 2 clinical 1.5-T systems. Thirty-six patients (40.9%) were imaged using a GE Signa (GE Medical System, Waukesha, WI), and 52 patients (59.1%) were imaged using a Siemens Magnetom Avanto (Siemens Healthcare, Erlangen, Germany). Axial single-shot breath-hold, gradient echo, echo-planar DWI covering the whole liver was acquired during a 25-second breath hold using the following parameters: repetition time 5000 to 6500 ms, echo time 110 ms, 128 × 128 matrix, 8-mm slice thickness, 2-mm interslice gap, 0 and 750 s/mm2 b value, 64-kHz receiver bandwidth. All image analysis was done by a reader (S.B.) who was blinded to clinical or histopathological information on the participants.
Measurement of the ADC
The DWI sequences produced a set of images corresponding to the 2 applied b-values and an ADC map was automatically calculated by a commercially available software package (Advantage Workstation, release 4.1; GE Medical Systems, Waukesha, WI, or Leonardo Syngo; Siemens, Erlangen, Germany). The ADC was calculated with a linear regression analysis of the function S= S0 · exp(−b × ADC), where b is the diffusion factor, S is the signal intensity after application of the diffusion gradient, and S0 is the signal intensity at b = 0 mm2/s. Quantitative analysis of the ADCs of liver parenchyma was performed by positioning 5 separate circular regions of interest (ROIs) of a 1-cm diameter minimum (left lobe lateral segment, left lobe medial segment, right lobe anterior segment, and 2 ROIs in the right lobe posterior segment) on the ADC maps of 3 axial slices. The ROIs were between 1.5 and 5 cm2 (mean 2.1 cm2) in size. Care was taken to avoid artifacts from the abdominal wall and vascular motion. The ROIs were positioned far from visible vascular and biliary structures and at least 1 cm apart from the glissonian capsule. Because low signal to noise ratio (SNR) can artificially decrease ADCs,18 an estimated liver SNR was calculated on images obtained with a b value of 750 s/mm2. All patients included in this study had an SNR > 2. The spleen was selected to serve as a reference within each patient. Two ROIs of the spleen were obtained in each subject. For ADC measurements of the spleen, the greatest diameter was chosen to place the ROI. Spleen capsule and vessels were excluded as far as possible. Normalized liver ADC was calculated as the ratio of liver ADC to spleen ADC.
Statistical Analysis
Nonparametric methods [Spearman rank correlation (Ñ), Mann-Whitney-Wilcoxon test (MWT)], and linear regression models were used. Stochastic independency was assumed for each observation. Hence advanced models for irregular repeated measures were not deployed. Unless otherwise specified, variables are shown in the format of mean values ± standard deviations (SD). Multiple logistic regressions were done to assess the influence of inflammation, steatosis, and iron content on ADC values. The diagnostic value of DWI relative to histological fibrosis staging (METAVIR score, F0–F4) was determined on the basis of assessment of sensitivity, specificity, and predictive values and was determined by varying the threshold values and constructing receiver operating characteristic (ROC) curves. The primary comparisons were made to distinguish significant fibrosis (METAVIR score, F2, F3, or F4) from no or mild fibrosis (METAVIR score, F0 or F1) and to identify cirrhosis (METAVIR score, F4). Area under the ROC curve analysis was employed as a global measure of the diagnostic accuracy of DWI for the noninvasive assessment of fibrosis. Optimal ADC cutoff values for classification of the dichotomous histological outcomes were determined by maximizing the combination of sensitivity and specificity and the proportion of samples that were correctly classified. Similar diagnostic accuracy resulted from the use of Ishak fibrosis scoring (data not shown). A P value less than 0.05 was considered statistically significant. Statistical Software was STATA version 10.1 (StataCorp LP, College Station, TX).
RESULTS
Histopathological Findings
The majority of participants [53 (60.2%)] had no or minimal (F < 2) fibrosis (Table 2). Of 35 (39.8%) participants with significant fibrosis (METAVIR score, F2, F3, or F4), 27 (30.7% of all patients) had cirrhosis (METAVIR score, F4). The prevalence of significant hepatic inflammation, as determined by a total modified histologic activity index > 5, was 32.0%. Of 88 participants who underwent steatosis grading, 64.0% had no detectable fatty change; significant steatosis, defined as a fatty change of > 30%, was seen in 19% (in 17 of 88 participants).The mean interval between MRI and histologic analysis was 73 days (range, 0–322 d).
TABLE 2.
Tissue Histology Results
| Ishak fibrosis score | |
| 0 | 33 (37.5%) |
| 1 | 12 (13.6%) |
| 2 | 8 (9.1%) |
| 3 | 2 (2.3) |
| 4 | 1 (1.1) |
| 5 | 6 (6.8) |
| 6 | 26 (29.5) |
| METAVIR fibrosis score | |
| F0 | 33 (37.5) |
| F1 | 20 (22.7) |
| F2 | 2 (2.3) |
| F3 | 6 (6.8) |
| F4 | 27 (30.7) |
| Overall MHAI score | |
| None (MHAI 0) | 8 (9.1) |
| Minimal (MHAI 1–4) | 52 (59.1) |
| Mild (MHAI 5-9) | 15 (28.4) |
| Moderate (MHAI 9–12) | 3 (3.4) |
| Marked (MHAI 13–18) | 0 |
| Necrosis present | |
| Yes | 70 (79.5) |
| No | 18 (20.4) |
| Macrovesicular fatty change* | |
| None | 56 (63.6%) |
| Mild, < 5% | 15 (17.1%) |
| Mild to Moderate, 5–30% | 10 (11.4%) |
| Moderate, 30–60% | 3 (3.4%) |
| Severe, > 60% | 4 (4.5%) |
| Hepatocellular iron | |
| None | 52 (59.1) |
| Mild | 12 (13.6) |
| Moderate | 5 (5.7) |
| Severe | 1 (1.1) |
| N/A (iron stain not available) | 18 (20.4) |
| Cholestasis | |
| None | 72 (81.8) |
| Mild | 3 (3.4) |
| Moderate | 3 (3.4) |
| Marked | 10 (11.4) |
Data are number (%) of participants for categorical variables or median and interquartile range (IQR) for continuous variables.
Fatty changes were mostly azonal [29 of 31 (93.5%)]
Distribution of ADC Values Within the Liver and Spleen
There were minimal variations in ADC values within the liver and spleen (Table 3 and Fig. 1) but differences among ADC values of the liver segments (P ≥ 0.44) or between the 2 spleen ROIs (P = 0.41) were not statistically significant. However, spleen measurements values revealed significantly lower ADC values compared with all liver measurements (P < 0.0001). The average SD between the ROIs placed within the liver was small (0.1 × 10−3 mm2/s with a maximum of 0.34 × 10−3 mm2/s, median 0.093−3 mm2/s, IQR 0.055 to 0.129 × 10−3 mm2/s).
TABLE 3.
Distribution of ADC Values (× 10−3 mm2/s) Within the Liver and Spleen
| Location | Mean ADC Value in × 10−3 mm2/s of All 88 Patients |
|---|---|
| Liver averaged | 1.45 (0.25) |
| Left lobe lateral segment | 1.43 (0.27) |
| Left lobe medial segment | 1.46 (0.28) |
| Right lobe anterior segment | 1.47 (0.28) |
| Right lobe posterior segment 1 | 1.43 (0.24) |
| Right lobe posterior segment 2 | 1.46 (0.22) |
| Spleen averaged | 0.97 (0.18) |
| Spleen 1 | 0.98 (0.20) |
| Spleen 2 | 0.97 (0.13) |
Data are mean values (and standard deviation).
ADC indicates apparent diffusion coefficient.
FIGURE 1.
A, ADC map with little variation. B, ADC map with large variations. ADC indicates apparent diffusion coefficient.
Diagnostic Accuracy of Liver ADC Values
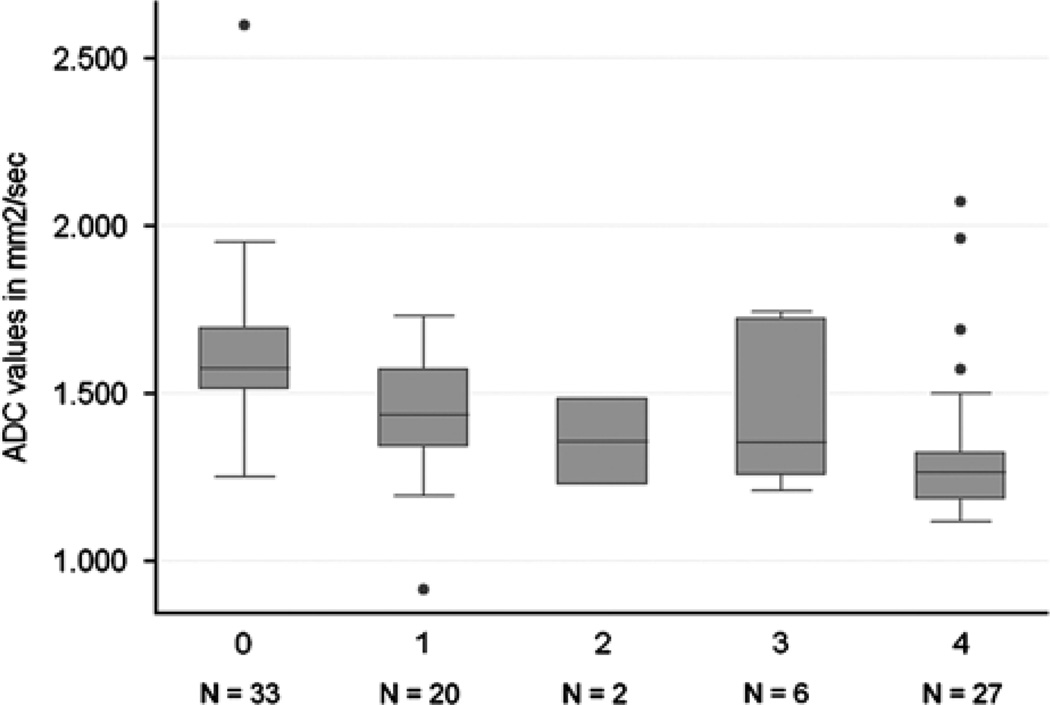
Distribution of hepatic ADC values in patients stratified by METAVIR fibrosis stage is shown in Table 4 and Figures 2 and 3. Using analysis of covariance, there were significant differences between all grades of fibrosis (P = 0.003). The Spearman rank correlation test showed an inverse correlation between fibrosis stage and ADC measurements: P = −0.54 (P < 0.0001). Although there was overlap in the ADC distributions (Fig. 2), the differences in ADC values by METAVIR stages F0 versus (vs.) F1–4 (t < 0.0001, MWT P < 0.0001), F0–1 versus F > 1 (t = 0.0003, MWT P < 0.0001), F0–2 versus F3–4 (t = 0.0006, MWT P < 0.0001) and F0–3 versus F4 (t = 0.006, MWT P < 0.0001) were all significant. The diagnostic accuracy of ADC for the assessment of hepatic fibrosis compared with liver biopsy is shown in Table 5.
TABLE 4.
Distribution of Hepatic Apparent Diffusion Coefficient Values (× 10−3 mm2/s) in 88 Patients Stratified by METAVIR Fibrosis Stage
| METAVIR | Mean Liver ADC ± SD |
Mean Spleen ADC ± SD |
Ratio of Liver ADC to Spleen ADC ± SD |
|---|---|---|---|
| 0 | 1.61 ± 0.24 | 0.97 ± 0.19 | 1.77 ± 0.93 |
| 1 | 1.43 ± 0.19 | 0.94 ± 0.21 | 1.62 ± 0.65 |
| 2 | 1.36 ± 0.18 | 1.08 | 1.13 |
| 3 | 1.44 ± 0.24 | 1.04 ± 0.21 | 1.39 ± 0.15 |
| 4 | 1.33 ± 0.24 | 0.98 ± 0.13 | 1.38 ± 0.30 |
Data are mean values. ADC indicates apparent diffusion coefficient; SD, Standard deviation.
FIGURE 2.
Box plot of liver ADC values for each fibrosis stage. The top of the bottom of the boxes are the first and third quartiles, respectively. The length of the box thus represents the interquartile range within which 50% of the values were located. The line through the middle of each box represents the median. The error bars show the minimum and maximum adjacent values and the dots mark outside values. ADC indicates apparent diffusion coefficient.
FIGURE 3.
Graph shows results of receiver operator characteristic (ROC) analysis using apparent diffusion coefficients (ADCs) for differentiation of METAVIR groups (A) F0 versus F1–2–3–4, (B) F0–1 versus F2–3–4, (C) F0–1–2 versus F3–4; and (D) F0–1–2–3 versus F4.
TABLE 5.
Diagnostic Accuracy of Apparent Diffusion Coefficient Values in 88 Subjects Across METAVIR Stages
| METAVIR | AUROC | ADC* cutoff | Correctly Classified | Sensitivity | Specificity | LR+ | LR− |
|---|---|---|---|---|---|---|---|
| F0 vs. F > 0 | 0.79 | > = 1.51 | 77.3% | 75.8% | 78.2% | 3.5 | 0.3 |
| F ≤ 1 vs. F > 1 | 0.77 | > = 1.33 | 79.6% | 84.9% | 71.4% | 3.0 | 0.2 |
| F ≤ 2 vs. F > 2 | 0.77 | > = 1.31 | 79.6% | 87.3% | 63.6% | 2.6 | 0.2 |
| F ≤ 3 vs. F4 | 0.78 | > = 1.30 | 79.6% | 86.9% | 63.0% | 2.4 | 0.2 |
ADC values are given in × 10−3 mm2/second.
ADC indicates apparent diffusion coefficient; LR+, positive likelihood ratio; LR−, negative likelihood ratio.
No significant differences were observed in the ADC values of the spleen between different METAVIR stages and no correlation between splenic ADC values and METAVIR fibrosis stage was found (P = −0.02, P = 0.88; Table 4). The ADC values of the spleen did not correlate with MR hardware, patient age, sex, race, hepatic iron content, steatosis, or inflammation either. Fibrosis stage was inversely correlated with liver ADC normalized by splenic ADC measurements: P = −0.38 (P < 0.003).
Linear regression analysis showed that hepatic ADC values were a good predictor of fibrosis stage (R2 = 0.19, P < 0.0001). There was no significant correlation between ADC values and hepatic iron content (P = 0.17), steatosis (P = 0.63), necrosis (P = 0.28), cholestasis (P = 0.54), age (P = 0.71), race (P = 0.05), or sex (P = 0.48) of the patient. However, overall MHAI score (P = −0.23, P = 0.03), more specifically MHAI1 (interface hepatitis) (P = −0.31, P = 0.0026) did correlate significantly with ADC values. The influence of inflammation on ADC values was also nonsignificant in a multiple regression model, which included overall MHAI score and MHAI1 score. Furthermore, there was a significant correlation between MR hardware (P = 0.39, P = 0.002) and ADC values, however, multiple regression found that the impact of the MR hardware on relationship between METAVIR fibrosis staging and ADC values was borderline nonsignificant (P = 0.05) as the correlation between MR hardware and ADC values was based on an uneven distribution of fibrosis grades (Table 6). A comparison of mean ADC values by METAVIR stage and MR scanner is shown in Table 6. ADC values from the GE Signa scanner compared with the Siemens Avanto were significantly larger in cases with METAVIR F = 1. The ADC values of the 2 scanners were not significantly different within each METAVIR category for F = 0, F = 2, F = 3, and F = 4.
TABLE 6.
Distribution of Hepatic Apparent Diffusion Coefficient Values (× 10−3 mm2/s) in 88 Patients Stratified by MR Hardware and METAVIR Fibrosis Stage
| Siemens Avanto (n=52) | GE Signa (N=36) | ||||
|---|---|---|---|---|---|
| METAVIR | No. Subjects (%) | Mean Liver ADC ± SD | No. Subjects (%) | Mean Liver ADC ± SD | P* |
| 0 | 18 (34.6%) | 1.57 ± 0.31 | 15 (41.7%) | 1.65 ± 0.10 | 0.32 |
| 1 | 9 (17.3%) | 1.31 ± 0.19 | 11 (30.6%) | 1.53 ± 0.12 | 0.006 |
| 2 | 1 (1.9%) | 1.23 | 1 (2.8%) | 1.48 | n/a |
| 3 | 3 (5.8%) | 1.39 ± 0.28 | 3 (8.3%) | 1.48 ± 0.23 | 0.70 |
| 4 | 21 (40.4%) | 1.32 ± 2.60 | 6 (16.7%) | 1.35 ± 0.16 | 0.77 |
P for Student T test comparing mean ADC of the 2 MR scanners within each METAVIR stage.
SD indicates standard deviation.
DISCUSSION
DWI provides noninvasive quantification of water diffusion and can be used for in vivo quantification of the combined effects of capillary perfusion and diffusion. There are limited data on correlation between ADC and degree of fibrosis and other histopathological findings (inflammation, steatosis, iron content, necrosis, cholestasis) in the liver. Patient populations (hepatitis C, alcoholic liver disease), grouping of the study population (significant fibrosis versus nonsignificant fibrosis, cirrhosis versus healthy volunteers) and technical parameters (manufacturer, field strength, repetition time, echo time, b values) differ between studies, immensely hindering comparison of these studies.9,10,12–14,19,20 Our aim was to determine the diagnostic performance of liver ADC measured with conventional DWI for the diagnosis of liver fibrosis.
Our findings, in common with most of those studies, showed lower liver ADC in patients with fibrosis owing to chronic liver disease when compared with normal controls. Fibrosis stage was inversely correlated with ADC measurements: P = −0.54 (P < 0.0001). This correlation is similar to those previously reported between ADC values and fibrosis stage, which ranged from P = −0.36 (significance not reported) to r = −0.448 to r = −0.654 (P = 0.0003–0.01680).17,20 Several recent studies have investigated the diagnostic value of ADC for the detection and staging of fibrosis. Do et al21 found significant differences in liver ADC between control livers and intermediate stages of fibrosis (stages 2 to 3) and cirrhosis (stage 4) and between stages 1 and 4 using ADC cutoff values of 1.68 × 10−3 mm2/s, 1.53 × 10−3 mm2/s, and 1.68 × 10−3 mm2/s for the detection of METAVIR stages ≥ 2, ≥ 3, and 4, respectively. For the same METAVIR stages our study obtained cutoff values of 1.33 × 10−3 mm2/s, 1.31 × 10−3 mm2/s, and 1.30 × 10−3 mm2/s (for the detection of METAVIR stages ≥ 2, ≥ 3, and 4, respectively). Differences in b values (0, 50, and 500 s/mm2 used by Do et al vs. 0, and 750 s/mm2 in our study) could explain these differences. Fujimoto et al22 determined an excellent diagnostic accuracy (AUC of 0.842 to 0.926) using b values of 0 and 1000 s/mm2 and ADC cutoff values of 1.35 × 10−3 mm2/s (METAVIR ≥ F1), 1.32 × 10−3 mm2/s (METAVIR ≥ F2), 1.27 × 10−3 mm2/s (METAVIR ≥ F3), and 1.23 × 10−3 mm2/s (METAVIR ≥ F4). These cutoff values are closer to the results we obtained. However, Fujimoto et al22 observed a greater specificity (AUC of 0.94 for METAVIR ≥ F1) with entropy ADC, a measure of the variation in a volume histogram of ADC. Using b values of 50, 500, and 1000 s/mm2, Wang et al23 found lower diagnostic accuracy values for ADC (AUC of 0.86) compared with magnetic resonance elastography (AUC of 0.98) and their cutoff values were again different from others: 0.91 × 10−3 mm2/s (for all groups of METAVIR). All these studies underline the importance of obtaining specific cutoff values for the imaging parameters used.
Do et al21 reported that normalizing liver ADC with spleen ADC improved diagnostic accuracy for detection of liver fibrosis and cirrhosis. Contrary to these findings, normalization of the hepatic ADC did decrease the correlation between DWI and fibrosis score by biopsy in our study. The spleen was an obvious choice for normalization of liver ADC values as it is positioned within the field of view obtained for a liver examination. Previous studies found that the spleen is a reliable internal standard.21,24,25 In the study performed by Kim et al,26 ADCs of the spleen in patients with chronic liver disease did not differ from those of volunteers or patients without liver dysfunction, which suggested that splenomegaly caused by portal hypertension did not affect ADC of the spleen and that ADC value of spleen remains unaffected by liver pathology. In this study, the distribution of splenic ADC values appeared to be normally distributed and the ADC of the spleen did not differ between the METAVIR stages of fibrosis supporting the assumption that the diffusion of the spleen may be unaffected. However, using splenic ADC values did not improve the correlation or diagnostic accuracy of hepatic ADC for the assessment of hepatic fibrosis. Thus, the utility of normalization is of questionable value for hepatic ADC measurements.
Our findings extend those from published previously as we attempted to distinguish different stages of hepatic fibrosis and had histopathological confirmation of “normal” subjects with no fibrosis on biopsy using biopsy data from healthy liver donors. Most studies assumed that the hepatic parenchyma of “healthy controls” without any symptoms of liver disease would be nonfibrotic and free of iron, steatosis, and inflammation. However, as most patients with acute liver disease and even chronic liver disease with cirrhosis remain asymptomatic until decompensation occurs,3 this may be an incorrect assumption.
Furthermore, we investigated the influence of hepatic inflammation, iron content, necrosis, cholestasis, and steatosis on ADC values and fibrosis stage. The influence of inflammation on ADC values has been described in the literature and we confirmed that inflammation correlated with decrease in ADC vales. However, most of this decrease was due to an increase in fibrosis. In a multiple regression model, inflammation did not significantly contribute to the ADC values. Fujimoto et al22 found that mean ADC significantly differed between inflammatory activity grades, however, their analysis was focused on the diagnostic value of ADC for the staging of fibrosis or inflammation and did not investigate the influence of inflammation on fibrosis or vice versa.
We did not find a significant correlation between hepatic steatosis or iron content and ADC values, but cholestasis and steatosis were slightly more prevalent in subjects who had a higher degree of fibrosis on METAVIR or Ishak scoring, indicating that hepatic steatosis as well as the underlying cause of liver disease may have to be taken into account when using diffusion as a surrogate marker of fibrosis. However, hepatic steatosis itself should not influence ADC values as a water selective spatial-spectral excitation pulse is used in the DWI sequence. Similarly, iron should not influence the actual ADC value as the T2-weighting between images at different b values is identical.
We also found that MR hardware (P = 0.39, P = 0.002) did correlate significantly with hepatic ADC values. However, the ADC values of the spleen did not correlate with MR hardware and a closer look at the distribution of fibrosis stage between the 2 machines showed that the prevalence of cirrhosis (METAVIR F = 4) was higher on the Siemens Avanto (21 of 52 cases, 40%) compared with the GE Signa (6 of 36 cases, 17%), explaining some of the discrepancy (Table 6). However, after stratifying by METAVIR stage, the number of subjects was too small to reliably assess for differences. We suggest that future studies should employ a standardized phantom to ensure that ADC values can be compared across vendors and perhaps even between different imaging parameters (b values). Nevertheless, care should be taken to minimize hardware and imaging parameter differences between studies to assure the comparability of ADC values.
On the other hand, interpretation of the ADC is complicated by perfusion effects and some authors have suggested that reduced ADC values in advanced liver disease may be due to perfusion changes and not owing to diffusion changes.27,28 The same may be true for diffusion measurements of the spleen. An animal study in 25 rats showed that decreased ADC correlated with increased liver fibrosis in living rats, but this correlation disappeared after death, suggesting that perfusion and not diffusion was a major factor.19 Other technical issues include sensitivity to susceptibility and motion-related artifacts, and difficulty in obtaining images with sufficient quality for reliable quantitative analysis on a consistent basis. More important, the ADC value depends on imaging parameters.29 Field strength, repetition time, echo time, and b values all affect the ADC. The manner in which a particular b value is achieved is also relevant. The b value is determined by the gradient strength, gradient duration, and gradient separation. Different combinations of gradient strength, gradient duration, and gradient separation may achieve the same b value but yield dissimilar ADC measurements: in general, for a fixed b value, increasing the gradient separation reduces the ADC.11,30,31 Because technical factors lead to differences in estimated ADC, reported ADCs are variable, with considerable overlap between normal and abnormal ranges and there is still limited data on correlation between ADC and degree of fibrosis and inflammation in the liver and studies have reported inconsistent results for staging liver fibrosis with DWI.9,13,19,31,32
Our study has several limitations. First, the results are limited by the sample size, especially a small number of patients with fibrosis stage F = 2 (2 patients) and F = 3 (6 patients). A large scale study with an equal number of patients in each fibrosis stage needs to be performed to validate a statistically significant correlation between ADC and increasing degrees of fibrosis and inflammation, as well to determine its role in clinical practice. Second, we performed a retrospective study of DWI of the liver performed within a clinical MRI scan. The scans were done on 2 different MR scanners, performed by different technologists and used only 2 b values. Third, there are some known problems with liver biopsy and METAVIR scoring in particular as a gold standard for the investigation of surrogate markers. Aside from sampling errors and interobserver variability, METAVIR is not a continuous scale and the increase of fibrous tissue accumulation from one stage to another is not linear.7 In addition, a recent publication also addressed the limitations of using biopsy as a gold standard for the evaluation of surrogate markers of liver disease. Even in the “best” scenario with high accuracy of liver biopsy accuracy (sensitivity and specificity of biopsy are 90%) and the prevalence of significant disease 40%, the calculated area under the ROC curve would be 0.90 for a perfect marker. Biopsy error caused the true validity of surrogate tests to be underestimated by an amount that would make a clinician falsely misperceive the test as inaccurate.33
In conclusion, the results of our study show the potential usefulness of liver ADC as surrogate marker of liver fibrosis as well as its limitations. To make DWI a useful clinical tool, further studies are needed to optimize and standardize liver DWI. After those studies, a multi-parametric MRI protocol of the liver, including DWI, chemical shift-based fat-water separation, dynamic contrast-enhanced MRI, and MR elastography should be established to provide a comprehensive imaging examination including screening for Hepatocellular carcinoma or other malignancies and evaluation of progression or treatment response in patients with diffuse liver disease.
Comments
Background
Hepatic fibrosis is part of the progression of chronic liver disease and can lead to end-stage liver disease, portal hypertension, and hepatocellular carcinoma. Liver biopsy is the gold standard for liver biopsy performed to assess degree of fibrosis, but it is invasive and is prone to interobserver variability and sampling error. Early non-invasive detection and staging of fibrosis is essential for treatment decisions, but no technique has been fully established yet.
Research Frontiers
Several recent studies have focused on the evaluation of noninvasive methods for the assessment of liver fibrosis. DWI has been shown to correlate with hepatic cirrhosis and fibrosis. There are limited data, however, on the influence of hepatic inflammation, necrosis, iron content, and steatosis on DWI measures in patients with chronic liver disease.
Related Publications
To provide the sources or hyperlinks of any published articles related to the article so that readers may obtain a broad and extensive knowledge so as to better understand the article.
Innovations and Breakthroughs
This is the first study to report the influence of hepatic inflammation, iron content, necrosis, cholestasis, and steatosis on the relationship between ADC values and fibrosis stage. The influence of inflammation on decreased ADC values could be confirmed but most of this decrease was due to a parallel increase in fibrosis. In a multiple regression model, inflammation did not significantly contribute to the ADC values. We did not find a significant correlation between hepatic steatosis or iron content and ADC values, but cholestasis and steatosis were slightly more prevalent in subjects who had a higher degree of fibrosis on METAVIR or Ishak scoring, indicating that hepatic steatosis as well as the underlying cause of liver disease may have to be taken into account when using diffusion as a surrogate marker of fibrosis.
Applications
The results of this study indicated that DWI is a useful clinical tool for the staging of fibrosis. However, DWI is not accurate enough by itself as changes in ADC could be attributed to several mechanisms. DWI should be used in conjunction with other MR-based technique, that is MR elastography, and hepatocyte-specific enhancement, to provide a comprehensive assessment of liver disease status.
Terminology
DWI can quantify water diffusion by calculation of the ADC. ADC can provide quantitative measure of the combined effects of capillary perfusion and diffusion. A decrease of ADC values in hepatic fibrosis is thought to be due to the increase in fibrous tissue and restriction of water diffusion.
Peer Review
Footnotes
Financial disclosures: N/A.
Conflict of Interest: Dr Bonekamp is receiving salary support from Bracco Diagnostics, Bayer Healthcare, and Siemens Medical. Dr Kamel is receiving grant support from Bracco Diagnostics, Bayer Healthcare, and Siemens Medical.
REFERENCES
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim YS, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. 2008;12:733–746. vii. doi: 10.1016/j.cld.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Angulo P. GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;25:883–889. doi: 10.1111/j.1365-2036.2007.03246.x. [DOI] [PubMed] [Google Scholar]
- 4.Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160–1174. doi: 10.1111/j.1572-0241.2004.30110.x. [DOI] [PubMed] [Google Scholar]
- 5.Piccinino F, Sagnelli E, Pasquale G, et al. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165–173. doi: 10.1016/s0168-8278(86)80075-7. [DOI] [PubMed] [Google Scholar]
- 6.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 7.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Johnston KC, Wagner DP, Wang XQ, et al. Validation of an acute ischemic stroke model: does diffusion-weighted imaging lesion volume offer a clinically significant improvement in prediction of outcome? Stroke. 2007;38:1820–1825. doi: 10.1161/STROKEAHA.106.479154. [DOI] [PubMed] [Google Scholar]
- 9.Aube C, Racineux PX, Lebigot J, et al. Diagnosis and quantification of hepatic fibrosis with diffusion weighted MR imaging: preliminary results. J Radiol. 2004;85:301–306. doi: 10.1016/s0221-0363(04)97582-8. [DOI] [PubMed] [Google Scholar]
- 10.Girometti R, Furlan A, Bazzocchi M, et al. Diffusion-weighted MRI in evaluating liver fibrosis: a feasibility study in cirrhotic patients. Radiol Med (Torino) 2007;112:394–408. doi: 10.1007/s11547-007-0149-1. [DOI] [PubMed] [Google Scholar]
- 11.Girometti R, Furlan A, Esposito G, et al. Relevance of b-values in evaluating liver fibrosis: a study in healthy and cirrhotic subjects using two single-shot spin-echo echo-planar diffusion-weighted sequences. J Magn Reson Imaging. 2008;28:411–419. doi: 10.1002/jmri.21461. [DOI] [PubMed] [Google Scholar]
- 12.Lewin M, Poujol-Robert A, Boelle PY, et al. Diffusion-weighted magnetic resonance imaging for the assessment of fibrosis in chronic hepatitis C. Hepatology. 2007;46:658–665. doi: 10.1002/hep.21747. [DOI] [PubMed] [Google Scholar]
- 13.Koinuma M, Ohashi I, Hanafusa K, et al. Apparent diffusion coefficient measurements with diffusion-weighted magnetic resonance imaging for evaluation of hepatic fibrosis. J Magn Reson Imaging. 2005;22:80–85. doi: 10.1002/jmri.20344. [DOI] [PubMed] [Google Scholar]
- 14.Boulanger Y, Amara M, Lepanto L, et al. Diffusion-weighted MR imaging of the liver of hepatitis C patients. NMR Biomed. 2003;16:132–136. doi: 10.1002/nbm.818. [DOI] [PubMed] [Google Scholar]
- 15.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 16.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 17.Sandrasegaran K, Akisik FM, Lin C, et al. Value of diffusion-weighted mri for assessing liver fibrosis and cirrhosis. Am J Roentgenol. 2009;193:1556–1560. doi: 10.2214/AJR.09.2436. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich O, Heiland S, Sartor K. Noise correction for the exact determination of apparent diffusion coefficients at low SNR. Magn Reson Med. 2001;45:448–453. doi: 10.1002/1522-2594(200103)45:3<448::aid-mrm1059>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Annet L, Peeters F, Abarca-Quinones J, et al. Assessment of diffusion-weighted MR imaging in liver fibrosis. J Magn Reson Imaging. 2007;25:122–128. doi: 10.1002/jmri.20771. [DOI] [PubMed] [Google Scholar]
- 20.Taouli B, Tolia AJ, Losada M, et al. Diffusion-weighted MRI for quantification of liver fibrosis: preliminary experience. AJR Am J Roentgenol. 2007;189:799–806. doi: 10.2214/AJR.07.2086. [DOI] [PubMed] [Google Scholar]
- 21.Do RK, Chandanara H, Felker E, et al. Diagnosis of liver fibrosis and cirrhosis with diffusion-weighted imaging: value of normalized apparent diffusion coefficient using the spleen as reference organ. AJR Am J Roentgenol. 2010;195:671–676. doi: 10.2214/AJR.09.3448. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto K, Tonan T, Azuma S, et al. Evaluation of the mean and entropy of apparent diffusion coefficient values in chronic hepatitis C: correlation with pathologic fibrosis stage and inflammatory activity grade. Radiology. 2011;258:739–748. doi: 10.1148/radiol.10100853. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Ganger DR, Levitsky J, et al. Assessment of Chronic Hepatitis and Fibrosis: Comparison of MR Elastography and Diffusion-Weighted Imaging. AJR Am J Roentgenol. 2011;196:553–561. doi: 10.2214/AJR.10.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshikawa T, Kawamitsu H, Mitchell DG, et al. ADC measurement of abdominal organs and lesions using parallel imaging technique. AJR Am J Roentgenol. 2006;187:1521–1530. doi: 10.2214/AJR.05.0778. [DOI] [PubMed] [Google Scholar]
- 25.Kandpal H, Sharma R, Madhusudhan KS, et al. Respiratory-triggered versus breath-hold diffusion-weighted MRI of liver lesions: comparison of image quality and apparent diffusion coefficient values. AJR Am J Roentgenol. 2009;192:915–922. doi: 10.2214/AJR.08.1260. [DOI] [PubMed] [Google Scholar]
- 26.Kim T, Murakami T, Takahashi S, et al. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999;173:393–398. doi: 10.2214/ajr.173.2.10430143. [DOI] [PubMed] [Google Scholar]
- 27.Annet L, Materne R, Danse E, et al. Hepatic flow parameters measured with MR imaging and Doppler US: correlations with degree of cirrhosis and portal hypertension. Radiology. 2003;229:409–414. doi: 10.1148/radiol.2292021128. [DOI] [PubMed] [Google Scholar]
- 28.Luciani A, Vignaud A, Cavet M, et al. Liver cirrhosis: intravoxel incoherent motion MR imaging–pilot study. Radiology. 2008;249:891–899. doi: 10.1148/radiol.2493080080. [DOI] [PubMed] [Google Scholar]
- 29.Kwee TC, Takahara T, Koh DM, et al. Comparison and reproducibility of ADC measurements in breathhold, respiratory triggered, and free-breathing diffusion-weighted MR imaging of the liver. J Magn Reson Imaging. 2008;28:1141–1148. doi: 10.1002/jmri.21569. [DOI] [PubMed] [Google Scholar]
- 30.Moteki T, Horikoshi H. Evaluation of hepatic lesions and hepatic parenchyma using diffusion-weighted echo-planar MR with three values of gradient b-factor. J Magn Reson Imaging. 2006;24:637–645. doi: 10.1002/jmri.20682. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Song B, Wu B, et al. Assessment of disease activity and liver fibrosis in chronic viral hepatitis by magnetic resonance diffusion-weighted imaging. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2009;31:155–159. [PubMed] [Google Scholar]
- 32.Taouli B, Chouli M, Martin AJ, et al. Chronic hepatitis: role of diffusion-weighted imaging and diffusion tensor imaging for the diagnosis of liver fibrosis and inflammation. J Magn Reson Imaging. 2008;28:89–95. doi: 10.1002/jmri.21227. [DOI] [PubMed] [Google Scholar]
- 33.Mehta SH, Lau B, Afdhal NH, et al. Exceeding the limits of liver histology markers. J Hepatol. 2009;50:36–41. doi: 10.1016/j.jhep.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]