Prostate cancer is the most commonly diagnosed cancer after skin cancer and the second leading cause of cancer death for American men, behind only lung cancer.1 The American cancer society estimates that there will be about 238,590 new cases of prostate cancer, and 29,720 men will die from the disease in 2013.1
Prostate cancer mortality rate is declining in developed countries, however it is not clear whether this is due to the increasing use of screening procedures based on prostate-specific antigen (PSA) blood test, improved treatment,2 or combination of these and/or other factors.3 In spite of declining prostate cancer mortality, striking racial disparities in prostate cancer outcomes exist in the U.S. Compared with Caucasian men, African American are more likely to be diagnosed at advanced stage disease and die from prostate cancer in the U.S.1,4
Studies have shown differences in prostate cancer treatment among patients with various races/ethnicities or socioeconomic backgrounds.5–8 Existing literature suggests that African American men have not been receiving optimal treatment for prostate cancer and have been experiencing delays in treatment.9–11 Such differences in treatment must be understood better and explained as they might be one cause of the racial disparities in prostate cancer mortality observed in the U.S. This information is critical for the development of appropriate policy and intervention strategies to eliminate long-term racial/ethnic disparities.
Several factors have been suggested to influence time-to-treatment in cancer patients, including socioeconomic status and other demographic characteristics.12–15 In breast cancer, for instance, studies have shown that time-to-treatment may be linked to socioeconomic status as well as race. The same studies suggested that delays of greater than three months after an initial diagnosis may decrease breast cancer survival by 12%.13–16
Some studies found that men with prostate cancer experience longer wait time for diagnosis and treatment than those observed for other cancers.17–19 Studies in both the urologic and medical literature have paid increasing attention to the question of overdiagnosis in prostate cancer. As evidence, a study reported overdiagnosis rates of 15% among White men and 37% among Black men.20 The association between wait time and prognosis of prostate cancer is inconclusive.21–26 Even though we recognize the current literature’s debate on whether or not prostate cancer has been over-treated, the intent of this project was not to make a clinical judgment about this matter. This study intended to investigate factors contributing to time-to-treatment and examine whether there was a difference in wait time or treatment rate between African American and Caucasian men in Florida.
Methods
Population studied
Caucasian and African American men 40 years of age or older diagnosed with prostate cancer between Oct. 2001 and Dec. 2007 in Florida. Other races were excluded due to small numbers in the dataset.
Data sources
The study used data from three sources. First, prostate cancer incidence data between Oct. 2001–Dec. 2007 were obtained from the Florida Cancer Data System (FCDS) that is managed by State of Florida Department of Health and housed at the University of Miami through a contract. The FCDS is the largest population-based, cancer incidence registry in the nation.27 It contains information on patient demographic characteristics, residence, prostate tumor characteristics, and other information such as tobacco use and primary payer of health insurance.
Second, comorbidity data were obtained from the Florida Agency for Health Care and Administration (AHCA). The AHCA maintains two databases (Hospital Patient Discharge Data and Ambulatory Outpatient Data) on all patient encounters within hospitals and freestanding ambulatory surgical and radiation therapy centers in Florida. Comorbidity was computed following the Elixhauser Index method28 based on diagnoses information from AHCA. The study used a total of 45 conditions, including 29 from the Elixhauser Index plus 16 new additional conditions based on clinical characteristics of the study population.
Third, data on demographic and area-level socioeconomic characteristics were extracted at the census tract level from the U.S. Census Bureau public use files (Census 2000, Summary File-3) for the State of Florida. Data obtained from the three sources were merged into a single dataset for analyses.
Statistical analysis
Time from diagnosis to initial treatment of prostate cancer was calculated. Patients who did not receive any treatment by last recorded follow up, including watchful waiting cases, were censored at time of last follow-up. Descriptive statistics were used to summarize sample characteristics, t-tests and chi-square tests were used for bivariate racial comparisons in Table 1; Kaplan-Meier estimator was applied to generate estimated survival probability curves in figures (probability waiting time is beyond a given time in our case). Wei, Lin and Weissfeld (WLW) survival model29 was utilized to examine effects of exploratory variables on time-to-treatment while accounting for possible correlation of patients from same census tract. This model choice was supported by the ratio of the robust standard error estimate relative to the model-based estimate that ranged from 0.6 to 1.5, where departure from one indicates improvement while accounting for correlation. Hazard ratios and p-values were calculated. The event of interest in this study was receiving initial treatment instead of death as frequently seen in publications involving time-to-event analysis. Therefore “hazard” in this study is interpreted as treatment rate instead of death rate, and a higher treatment rate corresponds to a shorter waiting time before treatment. Survival probability at any time t in figures is interpreted as the chance that waiting time before initial treatment is longer than time t i.e. chance that patient is still waiting at time t. Statistical analyses were carried out using SAS/STAT® software, Version 9.3 of the SAS System for Windows.
Table 1.
CHARACTERISTICS OF THE STUDY POPULATION (N =11,284)
| Variable | n | Mean(SD) or %a | Caucasiana,c | African Americana,c | P-Value | |
|---|---|---|---|---|---|---|
| Age | 11284 | 66.36 (8.98) | 66.84 (8.90) | 62.97 (8.84) | <.0001 | |
| Treatment | Active surveillance | 883 | 7.84 | 7.70 | 8.77 | .0003 |
| Surgery + radiation | 224 | 1.99 | 2.00 | 1.93 | ||
| Surgery + hormone | 351 | 3.11 | 2.94 | 4.35 | ||
| Radiation only | 2617 | 23.22 | 23.73 | 19.69 | ||
| Hormone only | 493 | 4.37 | 4.19 | 5.71 | ||
| Radiation + hormone | 1512 | 13.42 | 13.34 | 13.98 | ||
| Surgery only | 5189 | 46.05 | 46.11 | 45.58 | ||
| Year of dx | 2001b | 579 | 5.13 | 5.13 | 5.13 | .1664 |
| 2002 | 2213 | 19.61 | 19.91 | 17.53 | ||
| 2003 | 1538 | 13.63 | 13.54 | 14.26 | ||
| 2004 | 1605 | 14.22 | 14.20 | 14.40 | ||
| 2005 | 1616 | 14.32 | 14.16 | 15.47 | ||
| 2006 | 1804 | 15.99 | 16.17 | 14.68 | ||
| 2007 | 1929 | 17.10 | 16.89 | 18.53 | ||
| Race | Caucasian | 9881 | 87.57 | |||
| African American | 1403 | 12.43 | ||||
| Marital status | Married | 8923 | 79.08 | 80.74 | 67.36 | <.0001 |
| Unmarried | 2361 | 20.92 | 19.26 | 32.6 | ||
| Insurance | Publicly insured | 6395 | 56.67 | 58.47 | 46.68 | <.0001 |
| Privately insured | 4684 | 41.51 | 40.01 | 49.28 | ||
| Uninsured | 205 | 1.82 | 1.52 | 4.05 | ||
| Tumor grade | Well-moderately diff | 7078 | 62.73 | 63.24 | 59.09 | .0015 |
| Poorly differentiated | 3508 | 31.09 | 30.82 | 33.00 | ||
| Undiff or unknown | 698 | 6.19 | 5.94 | 7.91 | ||
| Stage | Early (localized) | 9861 | 87.39 | 87.94 | 83.54 | <.0001 |
| Late (regional & distant) | 1423 | 12.61 | 12.06 | 16.46 |
May not sum to 100% due to rounding
The study used partial data for the year 2001 (October 1, 2001–December 31, 2001)
Mean (SD) or Column percentages
Results
Population characteristics
Characteristics of the study population are summarized in Table 1. A total of 11,284 men diagnosed with prostate cancer in Florida during Oct. 2001–Dec. 2007 were included in the study, among whom 12.61% were diagnosed at late-stage. Patient age in the study ranged from 40 to 99 at diagnosis, with a median diagnosis age of 66 years. The average age at diagnosis was 66.36 years. About 87.57% of the study population was Caucasian and 12.43% was African American. Most patients were married (79.08%). The majority of the sample had public health insurance (56.67%). Public health insurance includes Medicare, Medicaid, Department of Defense (Tricare), military personnel (military), veteran affairs, or Indian/Public Health Service. Since the study only included three months of 2001 data, proportion of 2001 cases was lower than those of later years. Compared with Caucasian men, African American men were diagnosed at a younger age on average, had a lower percentage of patients treated with radiation only, a lower proportion of married patients, a higher uninsured rate, a lower share of patients with well-moderately differentiated tumor, and a larger fraction of late stage diagnosis.
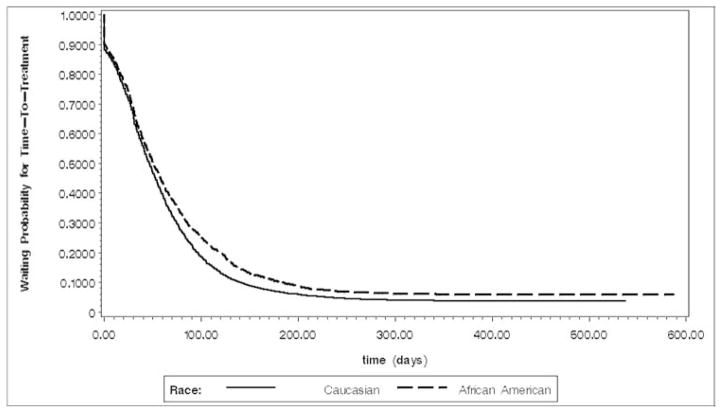
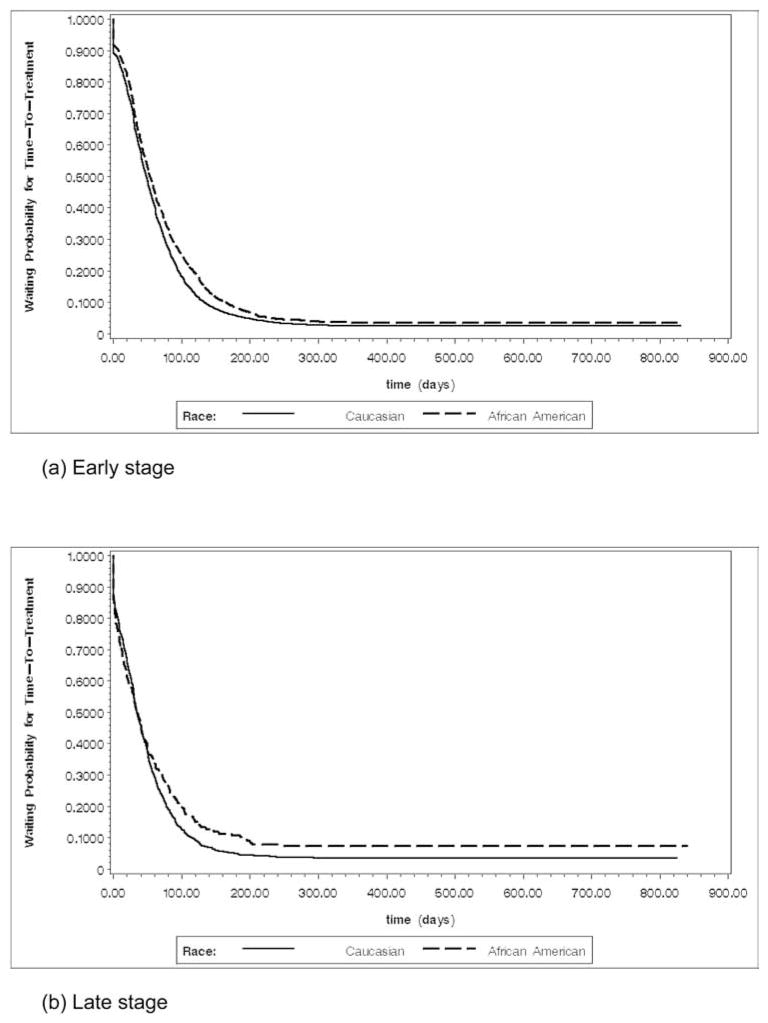
The observation period ranged from 0 days to 2,408 days (approximately six and a half years). The median time-to-treatment was 47 days, indicating 50% of patients received initial treatment within 47 days after diagnosis. Curves in Figures 1 and 2 dramatically drop down to approximately 90% at around diagnosis time, indicating approximately 10% of patients sought initial treatment shortly after diagnosis. Overall racial comparison of waiting probability for time-to-treatment in Figure 1 and racial comparisons by stage in Figure 2 both indicate that in general African American men had a higher chance to wait a longer time before receiving initial treatment.
Figure 1.
Waiting probability estimates by race (early follow-up period)
Figure 2.
Waiting probability estimates for early and late stage by race (early follow-up period)
Multilevel analysis
The analysis was performed for early-stage and late-stage patients separately. The results are shown in Tables 2 and 3. The comorbidity conditions that were not significant in the analysis were listed separately in Appendix 1 and 2. Among patients diagnosed with early prostate cancer, higher rate of treatment was associated with being diagnosed at for-profit facilities and/or hospitals. Wait time increased for African American patients and those who lived in areas with higher percentages of African American population. These effects did not change over time. For patients diagnosed with early-stage prostate cancer, presence of diabetes without chronic complications was associated with shorter wait time. Two comorbidity conditions were associated with longer waiting time: psychoses, and benign neoplasm and in-situ cancer.
Table 2.
MULTIVARIATE ANALYSIS FOR EARLY-STAGE CASES (N = 9,861)
| Variables | Hazard Ratio | 95% CI | P-Value | |
|---|---|---|---|---|
| African American vs. Caucasian | 0.921 | 0.857 | 0.990 | .0260* |
| Age | 1.003 | 1.000 | 1.007 | .0565 |
| Married vs. unmarried | 1.013 | 0.959 | 1.071 | .6396 |
| Uninsured vs. private insurance | 0.862 | 0.724 | 1.027 | .0964 |
| Public insurance vs. private insurance | 1.008 | 0.958 | 1.062 | .7472 |
| Current smoker vs. non-current smoker | 0.992 | 0.936 | 1.052 | .7905 |
| Ambulatory vs. hospital | 0.441 | 0.369 | 0.527 | <.0001* |
| For-profit vs. not-for-profit | 1.199 | 1.137 | 1.264 | <.0001* |
| Percentage of African American | 0.998 | 0.997 | 0.999 | .0045* |
| Comorbidity | ||||
| Diabetes w/o chronic complications | 1.092 | 1.017 | 1.171 | .0146* |
| Psychoses | 0.617 | 0.406 | 0.939 | .0242* |
| Benign neoplasm and in-situ cancer | 0.859 | 0.750 | 0.983 | .0272* |
| Year of Diagnosis | ||||
| 2007 vs. 2001 | 0.815 | 0.730 | 0.910 | .0003* |
| 2006 vs. 2001 | 0.903 | 0.808 | 1.009 | .0724 |
| 2005 vs. 2001 | 0.876 | 0.783 | 0.980 | .0207* |
| 2004 vs. 2001 | 0.993 | 0.887 | 1.113 | .9081 |
| 2003 vs. 2001 | 0.996 | 0.889 | 1.117 | .9469 |
| 2002 vs. 2001 | 0.964 | 0.864 | 1.077 | .5197 |
significant at 5% level
Table 3.
MULTIVARIATE ANALYSIS FOR LATE-STAGE CASES (N = 1,423)
| Variables | Hazard Ratio | 95% CI | P-Value | |
|---|---|---|---|---|
| African American vs. Caucasian | 0.995 | 0.817 | 1.211 | .9589 |
| Age | 1.004 | 0.995 | 1.013 | .3882 |
| Married vs. unmarried | 0.999 | 0.873 | 1.142 | .9838 |
| Uninsured vs. private insurance | 0.953 | 0.726 | 1.252 | .7315 |
| Public insurance vs. private insurance | 1.002 | 0.879 | 1.142 | .9749 |
| Current smoker vs. non-current smoker | 0.918 | 0.799 | 1.054 | .2244 |
| Ambulatory vs. hospital | 0.640 | 0.203 | 2.021 | .4467 |
| For-profit vs. not-for-profit | 1.032 | 0.889 | 1.198 | .6815 |
| Percentage of African American | 0.996 | 0.993 | 1.000 | .0285* |
| Comorbidity | ||||
| Liver disease | 2.096 | 1.350 | 3.255 | .0010* |
| Solid tumor w/out metastasis | 1.619 | 1.133 | 2.315 | .0082* |
| Rheumatoid arthritis/collagen vascular | 1.851 | 1.005 | 3.409 | .0482* |
| Deficiency Anemias | 1.243 | 1.003 | 1.541 | .0465* |
| Drug abuse | 2.369 | 1.231 | 4.557 | .0098* |
| Genitourinary system disease | 1.272 | 1.099 | 1.472 | .0013* |
| Year of Diagnosis | ||||
| 2007 vs. 2001 | 0.710 | 0.549 | 0.917 | .0087* |
| 2006 vs. 2001 | 0.752 | 0.580 | 0.975 | .0313* |
| 2005 vs. 2001 | 0.637 | 0.489 | 0.831 | .0009* |
| 2004 vs. 2001 | 0.702 | 0.537 | 0.919 | .0100* |
| 2003 vs. 2001 | 0.961 | 0.728 | 1.269 | .7815 |
| 2002 vs. 2001 | 0.800 | 0.617 | 1.037 | .0923 |
significant at 5% level
Similar to the association observed among early-stage cases, late-stage patients living in area with higher percentage of African American population had longer waiting time and this effect did not change over time. More comorbidity conditions became significant factors of shorter waiting time for late-stage patients. Specifically, treatment rate was higher among men with liver disease, solid tumor without metastasis, rheumatoid arthritis/collagen vascular disease, deficiency anemias, drug abuse, and genitourinary system disease.
Patients diagnosed in more recent years had lower treatment rate than those diagnosed in early years. This pattern was observed for both early and late stage.
Discussion
Our study differs from other studies investigating time-to-treatment among cancer patients in two major ways: 1) it utilized both the state cancer registry data and patient’s diagnosis of other conditions to present a much more complete sickness profile of men with prostate cancer, and 2) early and late-stage prostate cancer cases were analyzed separately.
Prostate cancer typically tends to grow slowly, which can explain why men diagnosed with late-stage prostate cancer wait less to receive treatment than those diagnosed at early-stage. Significantly shorter wait times were also observed for older patients, which is consistent with a study by Johnston and colleagues30 and may be partly due to easier access to health care. Indeed, in the U.S. the elderly are more likely to hold health insurance and, thus interact with health care provider more frequently than younger men. On the other hand, a population-based study in Canada with universal health coverage reported no difference in treatment wait time.31 Other studies have also suggested that due to rising prevalence of coexisting diseases with age and certain physiologic changes in the elderly, which may reduce their capacity to tolerate therapeutic complications, elderly patients were less likely to undergo treatments for prostate cancer.32,33
Another finding of our study is that characteristics of facility where prostate cancer was diagnosed have some impact on time-to-treatment. Men who were diagnosed in hospital settings with early-stage prostate cancer had a shorter wait time compared with those in ambulatory settings. For late-stage patients, treatment rates were comparable among diagnosis facilities. For early-stage disease, men who were diagnosed at for-profit facilities were more likely to wait longer. However, the reasons for this difference are not well understood as we have no data on treatment decision making. It is unclear whether longer time-to-treatment is related to treatment capacity issues, or provider and patient decisions or preferences.
The presence of some comorbidity reduced wait time to prostate cancer treatment. Men with other solid tumors without metastasis received treatment sooner regardless of prostate cancer stage. For men diagnosed with early-stage prostate cancer, having diabetes without chronic complications or other metastatic cancers, was associated with shorter wait time. Since these men are already in the health care system being treated for these co-existing conditions, they should be more likely to be treated early for prostate cancer. It is hard to explain why time-to-treatment of early-stage prostate cancer was lengthened when men had diabetes with other chronic complications and/ or other circulatory diseases. Among men with late-stage prostate cancer, wait time was shorter for those with conditions including but not limited to liver disease, lymphoma, rheumatoid arthritis, deficiency anemia, and drug abuse. This study revealed that AIDS as comorbidity was a deterrent in seeking early treatment for late-stage prostate cancer. Treatments for late-stage prostate cancer tend to be more aggressive, and could adversely affect patients with AIDS due to their weakened immune system. Treatment of both AIDS and cancer can be complex,34,35 so it is may be necessary for these treatments to be coordinated.
Although our study did not reveal racial differences in time-to-treatment at the individual level, a contextual factor was found to affect treatment rate significantly: the rate was lower in areas with high percentages of African American for analyses of both early-stage and late-stage patients, independent of age at diagnosis, health insurance, and the other factors. In other words, men who resided in predominantly African neighborhoods waited longer to have their prostate cancer treated than did those who lived in less African American-concentrated areas. Such a disparity may be explained in that predominantly African American neighborhoods have poorer health care facilities with less technology and fewer medical specialists, than do Caucasian neighborhoods.36,37
African American men have been found to be less likely than Caucasians to receive definitive treatment after being diagnosed with prostate cancer.38 Since the value of treating prostate cancer has been questioned, and concrete evidence of benefit from definitive treatment is lacking, it is not clear whether the disproportionate receipt of definitive treatment we observed for African Americans represents inappropriate care. Certain studies have contended that the preferences of African American men may differ from Caucasians or that African American men may weigh the risks of definitive treatment differently.38 Another view has been expressed that African American men are offered optimal treatment less frequently than their Caucasian counterparts.8 These are certainly issues worthy of investigation in future studies.
The current study has a number of limitations. First, only cases diagnosed from 2001 through 2007 were analyzed. As a result, the observations made in this analysis may not necessarily reflect the most current trends. Second, census-tract socioeconomic data was used due to lack of individual-level information on socio-economic status which would have provided more accurate information for the analyses. Third, we have no data on treatment decision making to determine whether the wait time is related to physician/patient decision making, or patient preferences. Fourth, cancer registry data are subject to some limitations. Registry data lack information about events leading up to screening. Follow-up information are often limited to vital status, and there are no detailed information on side effects of treatment or treatment compliance. They offer very little information about recurrence of disease.
Despite these limitations, the study was able to maximize the utility of currently available information by linking three data sources and presented a comprehensive picture of patient outcomes. Specifically, patient comorbidity was taken into consideration, which is highly relevant to examining treatment rate among men diagnosed with prostate cancer.
Individual-level factors, such as comorbid conditions and sociodemographic characteristics should be considered in prostate cancer treatment. The findings of this study have implications for clinical practice in prostate cancer. They show a difference in the time-to-treatment and also highlight the need for further study to understand treatment decision making for this disease. Further research is needed, especially in the midst of the ongoing debate about possible overdiagnosis and overtreatment of prostate cancer, to investigate the association between time-to-treatment and prostate cancer-specific patient outcomes such as survival and quality of life.
Acknowledgments
This study was jointly funded by American Cancer Society Scholar Grant #RSGT-10-082-01-CPHPS, the National Institute of Minority Health and Health Disparities of the National Institutes of Health through RCMI Grant Number 2 G12 RR003020 and 8 G12 MD007582-2. The authors thank the Florida Department of Health and Florida Cancer Data System for providing the prostate cancer incidence data.
Appendix 1. NONSIGNIFICANT COMORBIDITIES INCLUDED IN THE MULTIVARIATE ANALYSIS FOR EARLY-STAGE CASES
| Variables | Hazard Ratio | 95% CI | P-Value | |
|---|---|---|---|---|
| Congestive heart failure | 0.922 | 0.742 | 1.146 | .4649 |
| Valvular disease | 0.992 | 0.852 | 1.155 | .9203 |
| Pulmonary circulation disease | 0.718 | 0.411 | 1.255 | .2452 |
| Peripheral vascular disease | 0.837 | 0.697 | 1.005 | .0567 |
| Paralysis | 1.178 | 0.694 | 2.001 | .5440 |
| Other neurological disorders | 0.911 | 0.739 | 1.123 | .3830 |
| Chronic pulmonary disease | 1.019 | 0.942 | 1.103 | .6399 |
| Diabetes w/ chronic complications | 0.729 | 0.515 | 1.032 | .0746 |
| Hypothyroidism | 1.040 | 0.905 | 1.194 | .5802 |
| Renal failure | 0.893 | 0.719 | 1.109 | .3063 |
| Liver disease | 0.734 | 0.527 | 1.022 | .0669 |
| Peptic ulcer Disease excluding bleeding | 1.163 | 0.817 | 1.657 | .4017 |
| Acquired immune deficiency syndrome (AIDS) | 1.151 | 0.568 | 2.331 | .6971 |
| Lymphoma | 0.877 | 0.625 | 1.231 | .4484 |
| Metastatic cancer | 1.130 | 0.952 | 1.342 | .1622 |
| Solid tumor w/out metastasis | 1.079 | 0.957 | 1.216 | .2125 |
| Rheumatoid arthritis/collagen vascular | 1.170 | 0.854 | 1.604 | .3284 |
| Coagulopathy | 0.924 | 0.699 | 1.221 | .5785 |
| Obesity | 1.080 | 0.949 | 1.230 | .2437 |
| Weight loss | 0.661 | 0.407 | 1.074 | .0945 |
| Fluid and electrolyte disorders | 1.059 | 0.926 | 1.211 | .4037 |
| Chronic blood loss anemia | 1.032 | 0.764 | 1.394 | .8376 |
| Deficiency Anemias | 1.029 | 0.896 | 1.181 | .6879 |
| Alcohol abuse | 0.877 | 0.662 | 1.162 | .3595 |
| Drug abuse | 1.142 | 0.533 | 2.447 | .7329 |
| Depression | 1.063 | 0.918 | 1.231 | .4154 |
| Endocrine disorders, nutritional and metabolic, immunity | 1.046 | 0.990 | 1.105 | .1111 |
| Ischemic heart disease | 1.015 | 0.945 | 1.090 | .6862 |
| Digestive system disease | 1.037 | 0.975 | 1.102 | .2461 |
| Genitourinary system disease | 1.034 | 0.977 | 1.095 | .2491 |
| Injury and poisoning | 1.052 | 0.963 | 1.149 | .2651 |
| Respiratory disorders | 0.932 | 0.823 | 1.055 | .2625 |
| Infection | 0.962 | 0.808 | 1.146 | .6658 |
| Other circulatory disease | 0.891 | 0.773 | 1.026 | .1099 |
| Other nervous system and sense organs disorders | 0.992 | 0.870 | 1.132 | .9052 |
| Skin and subcutaneous tissue disease | 1.118 | 0.907 | 1.378 | .2971 |
| Muscularskeletal and connective tissue disease | 0.972 | 0.906 | 1.043 | .4266 |
| Other mental disorders | 0.894 | 0.696 | 1.147 | .3784 |
| Other anemias | 1.052 | 0.820 | 1.348 | .6916 |
| Congenital anomalies | 1.117 | 0.823 | 1.517 | .4781 |
| Brain and other Neurological disorders | 0.973 | 0.779 | 1.215 | .8095 |
| Hypertension | 0.965 | 0.923 | 1.009 | .1166 |
Appendix 2. NONSIGNIFICANT COMORBIDITIES INCLUDED IN THE MULTIVARIATE ANALYSIS FOR LATE-STAGE CASES
| Variables | Hazard Ratio | 95% CI | P-Value | |
|---|---|---|---|---|
| Congestive heart failure | 1.111 | 0.685 | 1.803 | .6700 |
| Valvular disease | 0.740 | 0.482 | 1.137 | .1693 |
| Pulmonary circulation disease | 1.130 | 0.438 | 2.912 | .8005 |
| Peripheral vascular disease | 0.774 | 0.484 | 1.237 | .2837 |
| Paralysis | 1.775 | 0.919 | 3.428 | .0876 |
| Other neurological disorders | 0.763 | 0.395 | 1.472 | .4195 |
| Chronic pulmonary disease | 0.924 | 0.744 | 1.146 | .4706 |
| Diabetes w/o chronic complications | 1.083 | 0.901 | 1.302 | .3935 |
| Diabetes w/ chronic complications | 0.618 | 0.284 | 1.347 | .2264 |
| Hypothyroidism | 1.084 | 0.820 | 1.433 | .5711 |
| Renal failure | 0.683 | 0.445 | 1.046 | .0797 |
| Peptic ulcer Disease excluding bleeding | 0.560 | 0.222 | 1.410 | .2182 |
| Acquired immune deficiency syndrome (AIDS) | 0.227 | 0.050 | 1.033 | .0551 |
| Lymphoma | 1.683 | 0.748 | 3.785 | .2081 |
| Metastatic cancer | 1.144 | 0.968 | 1.352 | .1147 |
| Coagulopathy | 1.184 | 0.799 | 1.754 | .3997 |
| Obesity | 1.075 | 0.822 | 1.408 | .5968 |
| Weight loss | 0.967 | 0.580 | 1.612 | .8968 |
| Fluid and electrolyte disorders | 1.123 | 0.882 | 1.430 | .3472 |
| Chronic blood loss anemia | 1.330 | 0.867 | 2.039 | .1915 |
| Alcohol abuse | 1.044 | 0.694 | 1.570 | .8366 |
| Psychoses | 0.465 | 0.213 | 1.016 | .0548 |
| Depression | 0.817 | 0.534 | 1.248 | .3495 |
| Endocrine disorders, nutritional and metabolic, immunity | 1.016 | 0.894 | 1.156 | .8037 |
| Ischemic heart disease | 0.943 | 0.779 | 1.143 | .5497 |
| Digestive system disease | 1.095 | 0.946 | 1.268 | .2252 |
| Injury and poisoning | 1.186 | 0.968 | 1.454 | .1002 |
| Respiratory disorders | 1.040 | 0.837 | 1.292 | .7260 |
| Infection | 1.074 | 0.794 | 1.454 | .6422 |
| Other circulatory disease | 0.843 | 0.639 | 1.112 | .2259 |
| Benign neoplasm and in-situ cancer | 0.922 | 0.619 | 1.374 | .6903 |
| Other nervous system and sense organs disorders | 1.157 | 0.873 | 1.533 | .3093 |
| Skin and subcutaneous tissue disease | 0.928 | 0.629 | 1.368 | .7053 |
| Muscularskeletal and connective tissue disease | 1.070 | 0.925 | 1.239 | .3622 |
| Other mental disorders | 1.049 | 0.610 | 1.803 | .8633 |
| Other anemias | 0.925 | 0.570 | 1.503 | .7543 |
| Congenital anomalies | 1.112 | 0.698 | 1.772 | .6542 |
| Brain and other Neurological disorders | 0.976 | 0.687 | 1.387 | .8916 |
| Hypertension | 0.983 | 0.876 | 1.104 | .7775 |
Contributor Information
Hong Xiao, Professor at Florida A&M University (FAMU), College of Pharmacy and Pharmaceutical Sciences, and can be reached at (850) 599-3375; 1520 Martin Luther King, Jr. Boulevard, 200 Dyson Pharmacy Building, Tallahassee, Florida 32312.
Fei Tan, Assistant Professor of Mathematical Sciences at Indiana University-Purdue Indianapolis in Indianapolis, Indiana.
Pierre Goovaerts, Chief Scientist at BioMedware Inc. in Ann Arbor, Michigan and Courtesy Associate Professor at the University of Florida.
Georges Adunlin, Graduate Research Assistant at Florida A&M University, College of Pharmacy and Pharmaceutical Sciences.
Askal Ayalew Ali, Graduate Research Assistant at Florida A&M University, College of Pharmacy and Pharmaceutical Sciences.
Youjie Huang, Email: hong.xiao@famu.edu, Epidemiologist at the Bureau of Epidemiology, Florida Department of Health, Tallahassee.
Clement K. Gwede, Associate Member at H. Lee Moffitt Cancer Center & Research Institute, and Associate Professor of Oncologic Sciences at University of South Florida, College of Medicine in Tampa.
Notes
- 1.American Cancer Society. Cancer Facts & Figures 2013. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- 2.Cipolle RJ, Strand LM, Morley PC. Pharmaceutical care practice: the clinician’s guide. 2. New York, NY: McGraw-Hill Companies, Inc; 2004. [Google Scholar]
- 3.Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: Geographical distribution and secular trends. Mol Nutr Food Res. 2009 Feb;53(2):171–84. doi: 10.1002/mnfr.200700511. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman RM, Gilliland FD, Eley JW, et al. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2001 Mar 7;93(5):388–95. doi: 10.1093/jnci/93.5.388. [DOI] [PubMed] [Google Scholar]
- 5.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002 Mar 6;94(5):334–57. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman RM, Harlan LC, Klabunde CN, et al. Racial differences in initial treatment for clinically localized prostate cancer. J Gen Intern Med. 2003 Oct;18(10):845–53. doi: 10.1046/j.1525-1497.2003.21105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall SE, Holman CDAJ, Wisniewski ZS, et al. Prostate cancer: socio-economic, geographical and private-health insurance effects on care and survival. BJU Int. 2005 Jan;95(1):51–8. doi: 10.1111/j.1464-410X.2005.05248.x. [DOI] [PubMed] [Google Scholar]
- 8.Underwood W, Demonner S, Ubel P, et al. Racial/ethnic disparities in the treatment of localized/regional prostate cancer. J Urol. 2004 Apr;171(4):1504–7. doi: 10.1097/01.ju.0000118907.64125.e0. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz K, Powell IJ, Underwood W, et al. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology. 2009 Dec;74(6):1296–302. doi: 10.1016/j.urology.2009.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shavers VL, Brown M, Klabunde CN, et al. Race/ethnicity and the intensity of medical monitoring under ‘watchful waiting’ for prostate cancer. Med Care. 2004 Mar;42(3):239–50. doi: 10.1097/01.mlr.0000117361.61444.71. [DOI] [PubMed] [Google Scholar]
- 11.Spencer BA, Fung CH, Wang M, et al. Geographic variation across veterans affairs medical centers in the treatment of early stage prostate cancer. J Urol. 2004 Dec;172(6 Pt 1):2362–5. doi: 10.1097/01.ju.0000144064.54670.7b. [DOI] [PubMed] [Google Scholar]
- 12.Meyer BJF, Talbot AP, Ranalli C. Why older adults make more immediate treatment decisions about cancer than younger adults. Psychol Aging. 2007 Sep;22(3):505–24. doi: 10.1037/0882-7974.22.3.505. [DOI] [PubMed] [Google Scholar]
- 13.Gorin SS, Heck JE, Cheng B, et al. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006 Nov 13;166(20):2244–52. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- 14.Smith ER, Adams SA, Das IP, et al. Breast cancer survival among economically disadvantaged women: The influences of delayed diagnosis and treatment on mortality. Cancer Epidemiology Biomarkers & Prevention. 2008 Oct;17(10):2882–90. doi: 10.1158/1055-9965.EPI-08-0221. Epub 2008 Oct 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pocock B, Nash S, Klein L, et al. Disparities in time to definitive surgical treatment between black and white women diagnosed with ductal carcinoma in situ. Am J Surg. 2007 Oct;194(4):521–3. doi: 10.1016/j.amjsurg.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Neal R, Allgar V. Sociodemographic factors and delays in the diagnosis of six cancers: Analysis of data from the “National survey of NHS patients: Cancer”. Br J Cancer. 2005 Jun 6;92(11):1971–5. doi: 10.1038/sj.bjc.6602623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simunovic M, Gagliardi A, McCready D, et al. A snapshot of waiting times for cancer surgery provided by surgeons affiliated with regional cancer centres in Ontario. CMAJ. 2001 Aug 21;165(4):421–5. [PMC free article] [PubMed] [Google Scholar]
- 18.Subramonian K, Puranik S, Mufti G. How will the two-weeks-wait rule affect delays in management of urological cancers? J R Soc Med. 2003 Aug;96(8):398–9. doi: 10.1258/jrsm.96.8.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: Lessons from US prostate cancer incidence trends. J Natl Cancer Inst. 2002 Jul 3;94(13):981–90. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 20.Nam R, Jewett M, Krahn M, et al. Delay in surgical therapy for clinically localized prostate cancer and biochemical recurrence after radical prostatectomy. Can J Urol. 2003 Jun 10;10(3):1891–8. [PubMed] [Google Scholar]
- 21.Andrews SF, Horwitz EM, Feigenberg SJ, et al. Does a delay in external beam radiation therapy after tissue diagnosis affect outcome for men with prostate carcinoma? Cancer. 2005 Jul 15;104(2):299–304. doi: 10.1002/cncr.21184. [DOI] [PubMed] [Google Scholar]
- 22.Khan MA, Mangold LA, Epstein JI, et al. Impact of surgical delay on long-term cancer control for clinically localized prostate cancer. J Urol. 2004 Nov;172(5 Pt 1):1835–9. doi: 10.1097/01.ju.0000140277.08623.13. [DOI] [PubMed] [Google Scholar]
- 23.Freedland SJ, Kane CJ, Amling CL, et al. Delay of radical prostatectomy and risk of biochemical progression in men with low risk prostate cancer. J Urol. 2006 Apr;175(4):1298–302. doi: 10.1016/S0022-5347(05)00646-4. discussion 1302–3. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen PL, Whittington R, Koo S, et al. The impact of a delay in initiating radiation therapy on prostate-specific antigen outcome for patients with clinically localized prostate carcinoma. Cancer. 2005 May 15;103(10):2053–9. doi: 10.1002/cncr.21050. [DOI] [PubMed] [Google Scholar]
- 25.Saad F, Finelli A, Dranitsaris G, et al. Does prolonging the time to prostate cancer surgery impact long-term cancer control: A systematic review of the literature. Can J Urol. 2006 Jun;13( Suppl 3):16–24. [PubMed] [Google Scholar]
- 26.Florida Cancer Data System (FCDS) FCDS. Miami, FL: 2013. [Google Scholar]
- 27.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. Journal of the American Statistical Association. 1989 Dec;84(408):1065–73. [Google Scholar]
- 29.Johnston GM, MacGarvie VL, Elliott D, et al. Radiotherapy wait times for patients with a diagnosis of invasive cancer, 1992–2000. Clin Invest Med. 2004 Jun;27(3):142–56. [PubMed] [Google Scholar]
- 30.Siemens DR, Schulze KM, Mackillop WJ, et al. A population-based study of the waiting times for prostatectomy in Ontario. Can J Urol. 2005 Apr;12(2):2568–74. [PubMed] [Google Scholar]
- 31.Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011 Jan;29(2):235–41. doi: 10.1200/JCO.2010.30.2075. Epub 2010 Dec 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Post P, Hansen B, Kil P, et al. The independent prognostic value of comorbidity among men aged< 75 years with localized prostate cancer: a population-based study. BJU Int. 2001 Jun;87(9):821–6. doi: 10.1046/j.1464-410x.2001.02189.x. [DOI] [PubMed] [Google Scholar]
- 33.Rudek MA, Flexner C, Ambinder RF. Use of antineoplastic agents in patients with cancer who have HIV/AIDS. Lancet Oncol. 2011 Sep;12(9):905–12. doi: 10.1016/S1470-2045(11)70056-0. Epub 2011 May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Achenbach CJ, Cole SR, Kitahata MM, et al. Mortality after cancer diagnosis in HIV-infected individuals treated with antiretroviral therapy. AIDS. 2011 Mar 13;25(5):691–700. doi: 10.1097/QAD.0b013e3283437f77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landrine H, Corral I. Separate and unequal: Residential segregation and black health disparities. Ethn Dis. 2009 Spring;19(2):179–84. [PubMed] [Google Scholar]
- 36.Hayanga AJ, Kaiser HE, Sinha R, et al. Residential segregation and access to surgical care by minority populations in US counties. J Am Coll Surg. 2009 Jun;208(6):1017–22. doi: 10.1016/j.jamcollsurg.2009.01.047. Epub 2009 Apr 17. [DOI] [PubMed] [Google Scholar]
- 37.Robbins AS, Whittemore AS, Thom DH. Differences in socioeconomic status and survival among white and black men with prostate cancer. Am J Epidemiol. 2000 Feb 15;151(4):409–16. doi: 10.1093/oxfordjournals.aje.a010221. [DOI] [PubMed] [Google Scholar]
- 38.Knight SK, Siston AK, Chmiel JS, et al. Ethnic variation in localized prostate cancer: A pilot study of preferences, optimism, and quality of life among black and white veterans. Clin Prostate Cancer. 2004 Jun;3(1):31–7. doi: 10.3816/cgc.2004.n.010. [DOI] [PubMed] [Google Scholar]