Abstract
OBJECTIVE
To report race-based outcomes after radical prostatectomy (RP) in a cohort stratified by National Comprehensive Cancer Network (NCCN) risk category with updated follow-up.
MATERIALS AND METHODS
Studies describing racial disparities in outcomes after RP are conflicting. We studied 15,993 white and 1634 African American (AA) pretreatment-naïve men who underwent RP at our institution (1992–2013) with complete preoperative and pathologic data. Pathologic outcomes were compared between races using appropriate statistical tests; biochemical recurrence (BCR) for men with complete follow-up was compared using multivariate models that controlled separately for preoperative and postoperative covariates.
RESULTS
Very low- and low-risk AA men were more likely to have positive surgical margins (P <.01), adverse pathologic features (P <.01), and be upgraded at RP (P <.01). With a median follow-up of 4.0 years after RP, AA race was an independent predictor of BCR among NCCN low-risk (HR, 2.16; P <.001) and intermediate-risk (hazard ratio [HR], 1.34; P = .024) classes and pathologic Gleason score ≤6 (HR, 2.42; P <.001) and Gleason score 7 (HR, 1.71; P <.001). BCR-free survival for very low-risk AA men was similar to low-risk white men (P = .890); BCR-free survival for low-risk AA men was similar to intermediate-risk white men (P = .060).
CONCLUSION
When stratified by NCCN risk, AA men with very low-, low-, or intermediate-risk prostate cancer who undergo RP are more likely to have adverse pathologic findings and BCR compared with white men. AA men with “low risk” prostate cancer, especially those considering active surveillance, should be counseled that their recurrence risks can resemble those of whites in higher risk categories.
Racial differences in prostate cancer (PCa) incidence and mortality are well known. The incidence of PCa is about 60% higher in African American (AA) men compared with white men, and mortality rates for AA men are 2–3 times greater.1 However, there is controversy in the literature as to whether AA race is an independent predictor of adverse oncologic outcomes for patients with clinically localized PCa.2,3 A prior study at our institution analyzed outcomes in 326 AA men and 4962 white men who had been treated with radical prostatectomy (RP) between 1988 and 2004 and found that there was no association between AA race and adverse pathologic features or biochemical recurrence (BCR).4
However, there is growing evidence that AA men with PCa may harbor more aggressive tumors. Sanchez-Ortiz et al5 found that AA men with clinical stage T1c disease had higher postprostatectomy Gleason scores, greater cancer volume, and greater tumor volume per ng/mL of serum prostate-specific antigen (PSA) compared with matched white men. Synthesizing data from autopsy and surgical studies, Powell et al6 have suggested that PCa in AA men, compared with white men, grows and progresses more rapidly with 4-fold increased rates of subsequent metastatic disease.
The etiology of racial disparities in PCa incidence and outcomes is likely multifactorial, with increasing support for biologic differences as contributing factors.6,7 Given the conflicting conclusions of race as a predictor of oncologic outcomes, we sought to evaluate the role of race in pathologic and oncologic outcomes in a large cohort of AA and white men treated with RP from our institution in the PSA era and stratified by National Comprehensive Cancer Network (NCCN) risk category.
MATERIALS AND METHODS
This study was approved by the institutional review board. We analyzed the cohort of AA or white men who underwent RP at our institution from the beginning of the PSA era (1992) to August 2013 (n = 19,474). We chose to include the PSA era patients previously analyzed in our 2006 study4 to increase the power for our current analysis. Men were excluded from analysis if they had: previous prostate-directed pretreatments (n = 877: 799 white, 78 AA), unknown preoperative PSA values or incomplete pathologic staging (n = 548: 428 white, 120 AA), unknown biopsy Gleason score (n = 24: 20 white, 4 AA), or unknown clinical stage (n = 398: 282 white, 116 AA). The final study population included 17,627 men (15,993 white, 1634 AA) and was stratified by NCCN risk category.
A total of 50 different surgeons performed RP in this cohort from 1992 to August 2013. The 7 highest volume surgeons in this period performed 76.6% of the total RP cases and 71.7% of the total AA cases.
Biopsy and prostatectomy specimens were reviewed at Johns Hopkins by genitourinary pathologists as previously described.8 The following pathologic findings were evaluated: positive surgical margins (PSM), upgrading in the RP specimen, and Cancer of the Prostate Risk Assessment Postsurgical score (CAPRA-S). CAPRA-S is based on preoperative PSA level, pathologic Gleason score (GS), surgical margins, extracapsular extension, seminal vesicle invasion, and lymph node invasion. CAPRA-S scores ≥3 were used in this cohort to define adverse pathology. CAPRA-S scores ≥3 has been previously associated with a 27.2% or higher risk of 5-year progression or recurrence.9
Postoperative follow-up included serial symptom assessment and PSA measurements. Outcomes evaluated with follow-up were BCR, metastasis, and PCa-specific mortality (PCSM). BCR was defined as a single postoperative PSA level of ≥0.2 ng/ mL. Men with unknown follow-up information (n = 5321: 4768 white, 535 AA) were excluded from this subset analysis.
Preoperative characteristics were compared between races using the Wilcoxon-Mann-Whitney rank sum tests for non-normally distributed continuous variables and the chi-square tests for categorical variables. BCR-free survival (BFS), metastasis-free survival, and PCa-specific survival (time 0 was defined as the date of surgery) were compared between races using the Kaplan-Meier method and the log-rank statistic. Two multivariate Cox models to examine the association between race and follow-up outcomes were performed. The first model was adjusted for preoperative variables: PSA, body mass index (BMI ≥30 kg/m2), age, year of surgery, education and median income by zip code, biopsy GS (≥7), and clinical stage (≥T2). The second model was adjusted for postoperative variables: PSA, BMI (≥30 kg/m2), age, year of surgery, education and median income by zip code, PSM, pathologic GS (≥7), pathologic stage (>T2), and secondary radiation therapy. Education and income data were based on zip code tabulation areas accessible from the United States Census Bureau. Univariate and multivariate analyses were performed among the following subgroups: NCCN very low risk, low risk, intermediate risk, and high risk with the preoperative model and pathologic GS ≤6, 7, 8, and 9–10 with the postoperative model. All statistical analyses were performed using Stata 11.0 (StataCorp, College Station, TX). P values <.05 (2 tailed) were considered statistically significant.
RESULTS
Of the total study population of 17,627 men, AA men (n = 1634) presented at a younger age (median, 57 vs 59 years; P <.001), were more likely to be obese as defined by BMI ≥30 kg/m2 (40.3% vs 31.1%; P <.001), had higher preoperative PSA values (median, 5.8 vs 5.4 ng/mL; P <.001), and had higher preoperative PSA density (median, 0.14 vs 0.12 ng/mL/cc; P <.001) compared with white men (Table 1). AA men were more likely to have higher biopsy GS (P = .001) and higher NCCN risk disease (P <.001).
Table 1.
Baseline patient characteristics and cancer outcomes by race for Johns Hopkins radical prostatectomy series, 1992–2013
| Patient Characteristics | White | AA | P Value |
|---|---|---|---|
| N | 15,993 | 1634 | |
| Median age (IQR), y | 59.0 (54.0–63.0) | 57.0 (52.0–62.0) | <.001 |
| Median PSA level (IQR), ng/mL | 5.4 (4.1–7.6) | 5.8 (4.3–8.6) | <.001 |
| Median PSA density (IQR), ng/mL/cc | 0.12 (0.09–0.18) | 0.14 (0.09–0.22) | <.001 |
| BMI, kg/m2 | |||
| Median (IQR) | 26.6 (24.7–28.9) | 27.6 (25.8–30.2) | <.001 |
| ≥30, n (%) | 5004 (31.3) | 658 (40.3) | <.001 |
| Education level by zip code (IQR), % | |||
| Median high school graduation rate | 30.5 (23.2–39.0) | 31.6 (24.3–39.6) | .008 |
| Median college graduation rate | 24.4 (16.7–29.9) | 23.7 (15.1–29.2) | .002 |
| Median income by zip code(IQR), $ | 53,363 (41,549–70,974) | 52,224 (40,786–70,974) | .139 |
| Clinical stage, n (%) | <.001 | ||
| T1 | 11,325 (70.8) | 1289 (78.9) | |
| T2 | 4587 (28.7) | 343 (21.0) | |
| T3 | 81 (0.5) | 2 (0.1) | |
| Biopsy Gleason score, n (%) | <.001 | ||
| ≤6 | 11,484 (71.8) | 1034 (63.3) | |
| 7 | 3821 (23.9) | 504 (30.8) | |
| 8–10 | 686 (4.3) | 95 (5.8) | |
| NCCN risk category, n (%) | <.001 | ||
| Very low | 1562 (9.8) | 239 (14.6) | |
| Low | 7957 (49.8) | 616 (37.7) | |
| Intermediate | 5402 (33.8) | 629 (38.5) | |
| High | 1072 (6.7) | 150 (9.2) | |
| Pathologic stage, n (%) | <.001 | ||
| pT2N0 | 11,072 (69.2) | 1128 (69.0) | |
| pT3aN0 | 4066 (25.4) | 386 (23.6) | |
| pT3bN0 | 545 (3.4) | 88 (5.4) | |
| pN1 | 309 (1.9) | 32 (2.0) | |
| Pathologic Gleason score, n (%) | <.001 | ||
| ≤6 | 9414 (58.9) | 800 (49.0) | |
| 7 | 5610 (35.1) | 719 (44.0) | |
| 8–10 | 969 (6.1) | 115 (7.0) | |
| Positive margins, n (%) | |||
| All NCCN risk strata | 2086 (13.0) | 280 (17.1) | <.001 |
| Very low | 90 (5.8) | 25 (10.5) | .006 |
| Low | 839 (10.5) | 86 (14.0) | .008 |
| Intermediate | 872 (16.1) | 124 (19.7) | .022 |
| High | 285 (26.6) | 45 (30.0) | .378 |
| pT2N0 | 533 (4.8) | 77 (6.8) | .003 |
| ≥pT3aN0 | 1553 (31.6) | 203 (40.1) | <.001 |
| Upgraded, n (%) | |||
| All NCCN risk strata | 3511 (22.0) | 407 (24.9) | .006 |
| Very low | 241 (15.4) | 70 (29.3) | <.001 |
| Low | 1983 (24.9) | 190 (30.8) | .001 |
| Intermediate | 1025 (19.0) | 117 (18.6) | .821 |
| High | 262 (24.4) | 30 (20.0) | .232 |
| CAPRA-S score ≥3, n (%) | |||
| All NCCN risk strata | 4595 (28.7) | 599 (36.7) | <.001 |
| Very low | 104 (6.6) | 34 (14.2) | <.001 |
| Low | 1079 (13.6) | 121 (19.6) | <.001 |
| Intermediate | 2476 (45.8) | 315 (50.1) | .043 |
| High | 936 (87.3) | 129 (86.0) | .653 |
| CAPRA-S score ≥4, n (%) | |||
| All NCCN risk strata | 2917 (18.2) | 380 (23.3) | <.001 |
| Very low | 41 (2.6) | 10 (4.2) | .176 |
| Low | 520 (6.5) | 63 (10.2) | <.001 |
| Intermediate | 1597 (29.6) | 204 (32.4) | .137 |
| High | 759 (70.8) | 103 (68.7) | .591 |
| CAPRA-S score ≥5, n (%) | |||
| All NCCN risk strata | 1771 (11.1) | 245 (15.0) | <.001 |
| Very low | 10 (0.6) | 2 (0.8) | .728 |
| Low | 216 (2.7) | 25 (4.1) | .052 |
| Intermediate | 967 (17.9) | 132 (21.0) | .058 |
| High | 578 (53.9) | 86 (57.3) | .432 |
| Follow-up Outcomes | |||
| N | 11,207 | 1099 | |
| BCR, n (%) | |||
| All NCCN risk strata | 1463 (13.5) | 178 (16.7) | <.001* |
| Very low | 26 (2.6) | 7 (4.9) | .044* |
| Low | 424 (7.4) | 52 (12.3) | <.001* |
| Intermediate | 657 (19.7) | 76 (19.9) | .002* |
| High | 356 (47.9) | 42 (42.4) | .496* |
| Metastasis, n (%) | |||
| All NCCN risk strata | 375 (3.6) | 22 (2.2) | .525* |
| Very low | 4 (0.4) | 0 (0.0) | .572* |
| Low | 75 (1.4) | 4 (1.0) | .470* |
| Intermediate | 165 (5.2) | 7 (1.9) | .138* |
| High | 131 (18.5) | 11 (11.8) | .602* |
| PCSM, n (%) | |||
| All NCCN risk strata | 207 (1.9) | 12 (1.1) | .681* |
| Very low | 0 (0.0) | 0 (0.0) | — |
| Low | 42 (0.7) | 2 (0.5) | .769* |
| Intermediate | 88 (2.5) | 4 (1.0) | .626* |
| High | 77 (9.9) | 6 (6.0) | .803* |
| Follow-up (y after RP) for men without biochemical progression | |||
| Median, y | 4.0 | ||
| Mean, y | 5.8 | ||
| IQR, y | 2.0–9.0 | ||
| Range, y | 1.0–21.0 | ||
AA, African American; BCR, biochemical recurrence; BMI, body mass index; CAPRA-S, Cancer of the Prostate Risk Assessment Postsurgical score; IQR, interquartile range; NCCN, National Comprehensive Cancer Network; PCSM, prostate cancer—specific mortality; PSA, prostate-specific antigen; RP, radical prostatectomy;
Medians compared by the Wilcoxon-Mann-Whitney rank sum test; proportions compared by the chi-square test.
Bolded P values indicate statistical significance.
P values compare freedom from biochemical recurrence, metastasis, or prostate cancer—specific mortality between races and are derived from the log-rank test.
There was no difference in median income between the 2 races (P = .139); AA men had higher high school graduation rates (P = .008) and white men had higher college graduation rates (P = .002), but the magnitudes of these differences were quite small (Table 1).
Postoperatively, AA men were more likely to have higher pathologic GS (P <.001). On stratification by NCCN risk category, AA men were more likely to be upgraded at RP among patients with very low-risk PCa (29.3% vs 15.4%; P <.001) and low-risk PCa (30.8% vs 24.9%; P = .001). AA men in each NCCN risk group were more likely to have PSM than their white counterparts, with significant differences in the very low-risk (10.5% vs 5.8%; P = .006), low-risk (14.0% vs 10.5%; P = .008), and intermediate-risk (19.7% vs 16.1%; P = .022) groups. Additionally, AA men had higher rates of PSM in both organ-confined (6.8% vs 4.8%; P = .003) and extra-prostatic disease (40.1% vs 31.6%; P <.001). AA men in the very low-, low-, and intermediate-risk groups were also more likely to have higher pathologic CAPRA-S scores (Table 1).
We analyzed long-term oncologic outcomes for the subset of men with complete follow-up information (n = 12,306: 11,207 white, 1099 AA). Median follow-up time was 4.0 years after RP for men without biochemical progression. AA men had decreased 10-year BFS rates compared with white men overall (68.2% vs 81.4%; P <.001) and among the NCCN very low-risk (88.4% vs 95.3%; P = .044), low-risk (75.6% vs 89.4%; P <.001), and intermediate-risk (61.7% vs 71.9%; P =.002) groups (Tables 1, 2). Ten-year metastasis-free survival rates were 95.5% and 95.4% for AA and white men, respectively (P = .525); 10-year PCa-specific survival rates were 97.0% and 97.2%, respectively (P = .681).
Table 2.
Observed 5- and 10-year survival rates by race
| Oncologic Outcome | 5-Y Survival Rate
|
10-Y Survival Rate
|
||
|---|---|---|---|---|
| White | AA | White | AA | |
| Biochemical recurrence-free survival | ||||
| All NCCN risk strata | 0.869 (0.862–0.877) | 0.797 (0.760–0.828) | 0.814 (0.803–0.823) | 0.682 (0.626–0.732) |
| Very low | 0.973 (0.957–0.983) | 0.956 (0.890–0.983) | 0.953 (0.927–0.969) | 0.884 (0.726–0.953) |
| Low | 0.937 (0.929–0.944) | 0.863 (0.811–0.902) | 0.894 (0.883–0.905) | 0.756 (0.670–0.823) |
| Intermediate | 0.803 (0.786–0.819) | 0.728 (0.655–0.788) | 0.719 (0.697–0.740) | 0.617 (0.518–0.701) |
| High | 0.493 (0.450–0.535) | 0.468 (0.327–0.597) | 0.405 (0.357–0.452) | 0.267 (0.104–0.463) |
| Metastasis-free survival | ||||
| All NCCN risk strata | 0.979 (0.975–0.982) | 0.983 (0.968–0.991) | 0.954 (0.947–0.959) | 0.955 (0.917–0.976) |
| Very low | 0.998 (0.985–0.999) | — | 0.994 (0.976–0.999) | — |
| Low | 0.993 (0.990–0.995) | 0.991 (0.971–0.997) | 0.984 (0.979–0.988) | 0.974 (0.908–0.993) |
| Intermediate | 0.966 (0.957–0.973) | 0.981 (0.938–0.994) | 0.932 (0.918–0.944) | 0.970 (0.915–0.990) |
| High | 0.895 (0.864–0.919) | 0.920 (0.791–0.972) | 0.748 (0.694–0.793) | 0.727 (0.445–0.882) |
| Prostate cancer—specific survival | ||||
| All NCCN risk strata | 0.994 (0.992–0.996) | 0.995 (0.982–0.998) | 0.972 (0.967–0.977) | 0.970 (0.936–0.986) |
| Very low | — | — | — | — |
| Low | 0.998 (0.997–0.999) | 0.994 (0.956–0.999) | 0.990 (0.985–0.993) | 0.981 (0.920–0.996) |
| Intermediate | 0.992 (0.987–0.995) | 0.996 (0.973–0.999) | 0.963 (0.951–0.972) | 0.996 (0.973–0.999) |
| High | 0.966 (0.946–0.978) | 0.985 (0.899–0.998) | 0.851 (0.808–0.886) | 0.808 (0.575–0.921) |
Abbreviations as in Table 1.
Inadequate number of failure events to generate survival rates are indicated by “—.”
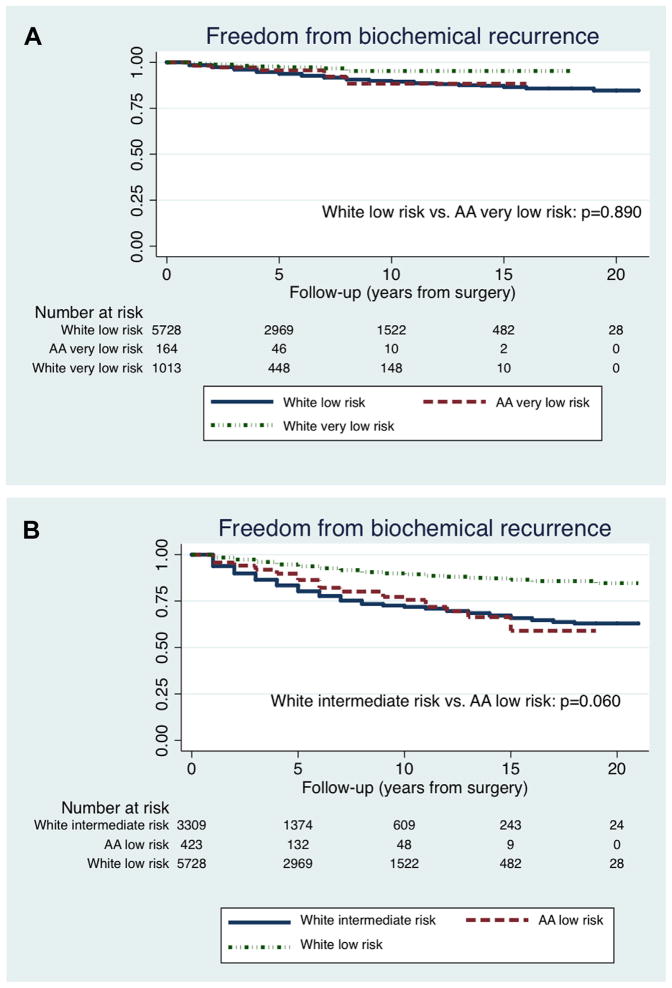
Because AA men had worse recurrence outcomes compared with white men of similar NCCN risk, we compared AA outcomes with whites in higher NCCN risk groups. There were no significant differences in BFS trends between very low-risk AA men and low-risk white men (P = .890) or between low-risk AA men and intermediate-risk white men (P = .060; Fig. 1).
Figure 1.
The Kaplan-Meier curves comparing biochemical recurrence-free survival trends between races in specified National Comprehensive Cancer Network risk categories. (A) White low-risk men vs African American very low-risk men (P = .890), (B) White intermediate-risk men vs African American low-risk men (P = .060). AA, African American. (Color version available online.)
In multivariate regression models adjusting for either preoperative or postoperative features and including socioeconomic covariates, AA race was an independent predictor of BCR in several risk strata (hazard ratios [HRs] and confidence intervals listed in Table 3). In the preoperative model, HR for BCR among AA men were 2.16 (P <.001) and 1.34 (P = .024) for NCCN low-risk and intermediate-risk disease, respectively. In the postoperative model, risk groups were stratified by pathologic GS and subdivided by pathologic stage. HRs for BCR among AA men with pathologic GS ≤6 were 2.42 (P <.001), 2.87 (P <.001) for pT2N0 disease, and 2.32 (P = .005) for pT3N0 disease. For AA men with pathologic GS 7, HR for BCR was 1.71 (P <.001), 1.43 (P =.164) for organ-confined disease, and 1.72 (P <.001) for non—organ-confined disease. AA race also had an HR of 4.06 (P = .012) for BCR among men with pathologic GS 8 and pT2N0 disease.
Table 3.
Multivariate models: hazard ratios of African American race associated with biochemical recurrence
| Biochemical Recurrence | HR | 95% CI | P Value |
|---|---|---|---|
| Preoperative models* | |||
| All patients | 1.62 | 1.37–1.93 | <.001 |
| NCCN very low risk | 2.01 | 0.75–5.36 | .162 |
| NCCN low risk | 2.16 | 1.53–3.04 | <.001 |
| NCCN intermediate risk | 1.34 | 1.04–1.72 | .024 |
| NCCN high risk | 1.21 | 0.86–1.71 | .277 |
| Postoperative models† | |||
| All patients | 1.69 | 1.41–2.03 | <.001 |
| Pathologic GS ≤6 | 2.42 | 1.62–3.61 | <.001 |
| pT2N0 | 2.87 | 1.63–5.03 | <.001 |
| pT3N0 | 2.32 | 1.29–4.16 | .005 |
| Pathologic GS 7 | 1.71 | 1.34–2.16 | <.001 |
| pT2N0 | 1.43 | 0.86–2.35 | .164 |
| pT3N0 | 1.72 | 1.28–2.31 | <.001 |
| Pathologic GS 8 | 1.61 | 0.94–2.74 | .082 |
| pT2N0 | 4.06 | 1.36–12.13 | .012 |
| pT3N0 | 1.54 | 0.78–3.06 | .216 |
| Pathologic GS 9–10 | 0.96 | 0.54–1.69 | .875 |
| pT2N0 | 1.81 | 0.23–14.31 | .572 |
| pT3N0 | 0.80 | 0.33–1.93 | .622 |
CI, confidence interval; GS, Gleason score; HR, hazard ratio; other abbreviation as in Table 1.
Bolded P values indicate statistical significance.
Preoperative model covariates: age, year of surgery, body mass index ≥30 kg/m2, prostate-specific antigen, biopsy Gleason score ≥7, clinical stage ≥T2, income, and education.
Postoperative model covariates: age, year of surgery, body mass index ≥30 kg/m2, prostate-specific antigen, pathologic GS ≥7, pathologic stage >T2, positive surgical margins, secondary radiation therapy, income, and education.
When surgeon experience was included as a covariate in each postoperative multivariate model (data not shown), the HR of AA race predicting BCR did not change appreciably and remained significant for pathologic GS ≤7.
AA race was not significantly associated with metastasis or PCSM in any risk group on multivariate analysis (data not shown), although follow-up was limited.
COMMENT
A previous retrospective analysis from our institution4 included, at that time, the largest cohort of AA patients to explore potential racial disparities and PCa outcomes. In that study, we analyzed outcomes of 326 AA and 4962 white men and found no association between AA race and pathologic or oncologic outcomes. Multiple prior studies, similarly, showed no race-based outcome disparities after RP.3,10,11 However, Freedland and Isaacs12 point out in a recent review that many studies are limited because of short follow-up, small sample sizes, and inclusion of a significant number of patients from the pre-PSA era.
Other studies, however, have demonstrated significant racial disparities in postprostatectomy outcomes.2,13 Those that stratified patients into pretreatment risk groups, too, are limited by the number of AA men and short follow-up.13 Our study adds to the current body of literature by including the largest sample size to date of AA patients (n = 1634) with updated and longer follow-up and identifies the racial disparities that exist among specific groups matched by preoperative or postoperative criteria. Compared with white men matched for preoperative risk characteristics, AA men with NCCN very low-, low-, and intermediate-risk PCa who undergo RP are more likely to have adverse oncologic outcomes, including GS upgrading, PSM, higher CAPRA-S scores, and BCR. Even when matched for pathologic grade, with adjustments for margin status, pathologic stage, and secondary radiation therapy, AA race was an independent predictor of BCR for men with pathologic GS ≤6 or GS 7. Given these findings, AA race appears to be an independent risk factor for BCR after RP, suggesting a hypothesis that AA men may have more aggressive tumor genetics contributing to elevated risks of recurrence.
Moreover, we showed that long-term freedom from BCR in AA men with very low-risk or low-risk disease is comparable with that of white men with low-risk or intermediate-risk disease, respectively. These findings have implications on the appropriate treatment options for AA men with “low risk” disease, especially if they are considering active surveillance. Although surveillance may be an option, these men should be counseled of their elevated oncologic risks, which can resemble those of whites in higher risk categories, and may benefit from more aggressive treatment plans than their conventional risk classification would suggest. Additionally, these data suggest that emerging molecular tests for PCa risk may need to be reassessed for AA men as most were developed with predominantly white cohorts.
The results of the present study are also in concordance with a recent study from our institution,14 which showed AA men with very low-risk PCa who were candidates for active surveillance but opted to undergo RP were more likely to have PSM, BCR, higher CAPRA-S scores, and >2-fold higher incidence of GS upgrading than white men. Also focusing on very low- or low-risk patients, Iremashvili et al15 (Miami), Abern et al16 (Duke), and our group17 have shown that among men on active surveillance in 3 separate cohorts, AA men are at higher risk of disease progression. Taken together with our present findings, these studies question the accuracy of current active surveillance entry criteria for AA men and underscore the need to develop race-specific risk stratifications.
On the other hand, a recent study by Jalloh et al18 did not find any racial differences in the rates of upgrading or upstaging in NCCN low-risk men. Although their analysis included patients from multiple institutions (University of California, San Francisco, and Cancer of the Prostate Strategic Urologic Research Endeavor databases), their cohort of low-risk AA men (n = 273, 6.5%) was small and lacked centralized pathology review. The authors did, however, find that low-risk AA men had significantly higher PSM rates compared with their white counterparts, which is consistent with results from our present analysis. These findings beckon the role of surgeon experience on margin status, particularly for the AA patients. The 7 busiest surgeons at our institution from 1992 to 2013 operated on 77% of the total RP cases and 72% of the AA cases during that time, suggesting that AA men were likely to have an experienced surgeon perform their operation. Although surgeon proficiency is a technical factor in margin status, other factors including obesity and variations in pelvic anatomy have been postulated to contribute to racial differences in margin status at RP.19,20 Increased rates of positive margins are important outcomes to consider in the AA patient as they may require the need for adjuvant radiation to reduce the risk of BCR and progression.21 It is known that PSM rates are associated with higher risks of BCR in AA men after RP.11 To account for the role of PSM on recurrence outcomes, margin status was included as a covariate in the postoperative multivariate model, and AA race remained an independent and strong risk factor for BCR among low-to-intermediate grade disease.
What are the underlying factors causing racial disparities in PCa outcomes? This question is controversial, and multiple explanations have been proposed. Studies show that social and economic factors can explain poorer outcomes for AA men22; yet, other studies (including our present report) demonstrate that even after adjustment for socioeconomic factors, long-term risks are still elevated for AA patients.23 For example, Chandrasekar et al24 recently analyzed >360,000 men with PCa in the California Cancer Registry and found that after adjustments for socioeconomic and insurance status, AA race also independently predicted PCSM (HR, 1.28; P <.0001) and all-cause mortality (HR, 1.18; P <.0001) in men with localized disease. These findings also support the hypothesis that race may play a biological role in long-term PCa disparities.
Examination of autopsy and prostatectomy specimens reveals that tumor biology in AA men is indeed more aggressive.5,6,25,26 AA men have larger tumor volumes than white men and are more likely to have multiple tumor nodules.5,25,26 Larger tumor volumes in AA men may be explained by a recent hypothesis by Powell et al6: the incidence of PCa starts at an early age in both AA and white men, but the tumor growth rates and transformation from latent to aggressive disease are more rapid in AA men. Although the hypothesis that biological factors underlie more aggressive PCa in AA men is a provocative one, exact genetic or molecular differences between races have yet to be proven and related factors important for diagnostic and therapeutic interventions remain unknown.
Our study is limited in its retrospective nature and reflects the experiences of a single tertiary center. A cohort from a largely referral-based center introduces the potential of selection bias and limits generalizability. Of note, the comparable socioeconomic status of both AA and white patients at our tertiary center may not reflect disparities seen at the community level. Additionally, our PCa database does not include information on other factors that could have impacted racial differences in outcomes, including intensity of screening before diagnosis or detailed information on previous biopsies. As such, the effect of these factors on our results is unknown. Our analysis did not find any significant racial differences in risks of metastasis or PCSM, although this lack of difference may likely be due to follow-up length. It is possible that longer follow-up may reveal further racial disparities in these important long-term outcomes. Additionally, AA men have higher rates of obesity, and obesity has been shown in multiple studies to be a significant predictor of PSA failure after treatment.27 Freedland et al27 found that race was no longer predictive of BCR when controlling for obesity. However, in the present study, AA men had higher risks of BCR even after adjusting for BMI. The main strength of this study is the cohort size, which permitted stratification of results by preoperative risk categories and postoperative tumor grade (as well as adjustment for significant covariates), allowing robust statistical analyses. Other study strengths attributable to the single-center study design included central pathology review of all biopsy and surgical specimens and consistency in providing surgeons throughout the study period.
CONCLUSION
In conclusion, this study shows that postprostatectomy outcomes vary significantly by race and supports a hypothesis that race-based differences may be related to underlying genetic differences in either cancer initiation, progression, or both. These racial disparities are most pronounced in NCCN very low-, low-, and intermediate-risk patients. If AA patients with very low-to-low-risk PCa are considering deferral of treatment, they should be counseled that they are at elevated risk for adverse pathologic outcomes and BCR and that their recurrence outcomes actually resemble those of white men in higher risk categories. Additionally, AA men presenting with intermediate-risk disease should be counseled that despite aggressive treatment, they too are at increased risk of adverse oncologic outcomes after prostatectomy. Race is an important factor in clinical decision-making, and these findings underscore the need to develop race-specific risk stratifications.
Footnotes
Financial Disclosure: Edward M. Schaeffer is funded by the Prostate Cancer Foundation and Howard Hughes Medical Institute. The remaining authors declare that they have no relevant financial interests.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: Apr, 2013. based on November 2012 SEER data submission, posted to the SEER website. [Google Scholar]
- 2.Moul JW, Douglas TH, McCarthy WF, McLeod DG. Black race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting. J Urol. 1996;155:1667–1673. [PubMed] [Google Scholar]
- 3.Freedland SJ, Jalkut M, Dorey F, et al. Race is not an independent predictor of biochemical recurrence after radical prostatectomy in an equal access medical center. Urology. 2000;56:87–91. doi: 10.1016/s0090-4295(00)00587-2. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen ME, Han M, Mangold L, et al. Black race does not independently predict adverse outcome following radical retropubic prostatectomy at a tertiary referral center. J Urol. 2006;176:515–519. doi: 10.1016/j.juro.2006.03.100. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Ortiz RF, Troncoso P, Babaian RJ, et al. African-American men with nonpalpable prostate cancer exhibit greater tumor volume than matched white men. Cancer. 2006;107:75–82. doi: 10.1002/cncr.21954. [DOI] [PubMed] [Google Scholar]
- 6.Powell IJ, Bock CH, Ruterbusch JJ, Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol. 2010;183:1792–1797. doi: 10.1016/j.juro.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chornokur G, Dalton K, Borysova ME, Kumar NB. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate. 2011;71:985–997. doi: 10.1002/pros.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein JI, Pizov G, Walsh PC. Correlation of pathologic findings with progression after radical retropubic prostatectomy. Cancer. 1993;71:3582–3593. doi: 10.1002/1097-0142(19930601)71:11<3582::aid-cncr2820711120>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Cooperberg MR, Hilton JF, Carrol PR. The CAPRA-S score: a straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer. 2011;117:5039–5046. doi: 10.1002/cncr.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moul JW, Wu H, Sun L, et al. Epidemiology of radical prostatectomy for localized prostate cancer in the era of prostate-specific antigen: an overview of the Department of Defense Center for Prostate Disease Research national database. Surgery. 2002;132:213–219. doi: 10.1067/msy.2002.125315. [DOI] [PubMed] [Google Scholar]
- 11.Iselin CE, Bo JW, Vollmer RT, et al. Surgical control of clinically localized prostate carcinoma is equivalent in African-American and white males. Cancer. 1998;83:2353–2360. doi: 10.1002/(sici)1097-0142(19981201)83:11<2353::aid-cncr15>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Freedland SJ, Isaacs WB. Explaining racial differences in prostate cancer in the United States: sociology or biology? Prostate. 2005;62:243–252. doi: 10.1002/pros.20052. [DOI] [PubMed] [Google Scholar]
- 13.Grossfeld GD, Latini DM, Downs T, et al. Is ethnicity an independent predictor of prostate cancer recurrence after radical prostatectomy? J Urol. 2002;168:2510–2515. doi: 10.1016/S0022-5347(05)64179-1. [DOI] [PubMed] [Google Scholar]
- 14.Sundi D, Ross AE, Humphreys EB, et al. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? J Clin Oncol. 2013;31:2991–2997. doi: 10.1200/JCO.2012.47.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iremashvili V, Soloway MS, Rosenberg DL, Manoharan M. Clinical and demographic characteristics associated with prostate cancer progression in patients on active surveillance. J Urol. 2012;187:1594–1600. doi: 10.1016/j.juro.2011.12.082. [DOI] [PubMed] [Google Scholar]
- 16.Abern MR, Bassett MR, Tsivian M, et al. Race is associated with discontinuation of active surveillance of low-risk prostate cancer: results from the Duke Prostate Center. Prostate Cancer Prostatic Dis. 2013;16:85–90. doi: 10.1038/pcan.2012.38. [DOI] [PubMed] [Google Scholar]
- 17.Sundi D, Faisal FA, Trock BJ, et al. Reclassification rates are higher among African American men that Caucasians on active surveillance [e-pub ahead of print] Urology. 2014 doi: 10.1016/j.urology.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jalloh M, Myers F, Cowan JE, et al. Racial variation in prostate cancer upgrading and upstaging among men with low-risk clinical characteristics. Eur Urol. 2014 Apr 5; doi: 10.1016/j.eururo.2014.03.026. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Jayachandran J, Banez LL, Aronson WJ, et al. Obesity as a predictor of adverse outcome across black and white race: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2009;115:5263–5271. doi: 10.1002/cncr.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Bodman C, Matikainen MP, Yunis LH, et al. Ethnic variation in pelvimetric measures and its impact on positive surgical margins at radical prostatectomy. Urology. 2010;76:1092–1096. doi: 10.1016/j.urology.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson IM, Valicenti R, Albertsen PC, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190:441–449. doi: 10.1016/j.juro.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz K, Powell IJ, Underwood W, et al. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology. 2009;74:1296–1302. doi: 10.1016/j.urology.2009.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans S, Metcalfe C, Ibrahim F, et al. Investigating Black-White differences in prostate cancer prognosis: a systematic review and meta-analysis. Int J Cancer. 2008;123:430–435. doi: 10.1002/ijc.23500. [DOI] [PubMed] [Google Scholar]
- 24.Chandrasekar T, Fish K, Evans C, et al. Disparities in survival for California men with prostate cancer are more associated with socioeconomics than either race or insurance status. J Urol. 2014;191:899. [Google Scholar]
- 25.Moul JW, Connelly RR, Mooneyhan RM, et al. Racial differences in tumor volume and prostate specific antigen among radical prostatectomy patients. J Urol. 1999;162:394–397. [PubMed] [Google Scholar]
- 26.Sundi D, Kryvenko ON, Carter HB, et al. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in black American men. J Urol. 2014;191:60–67. doi: 10.1016/j.juro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]