Abstract
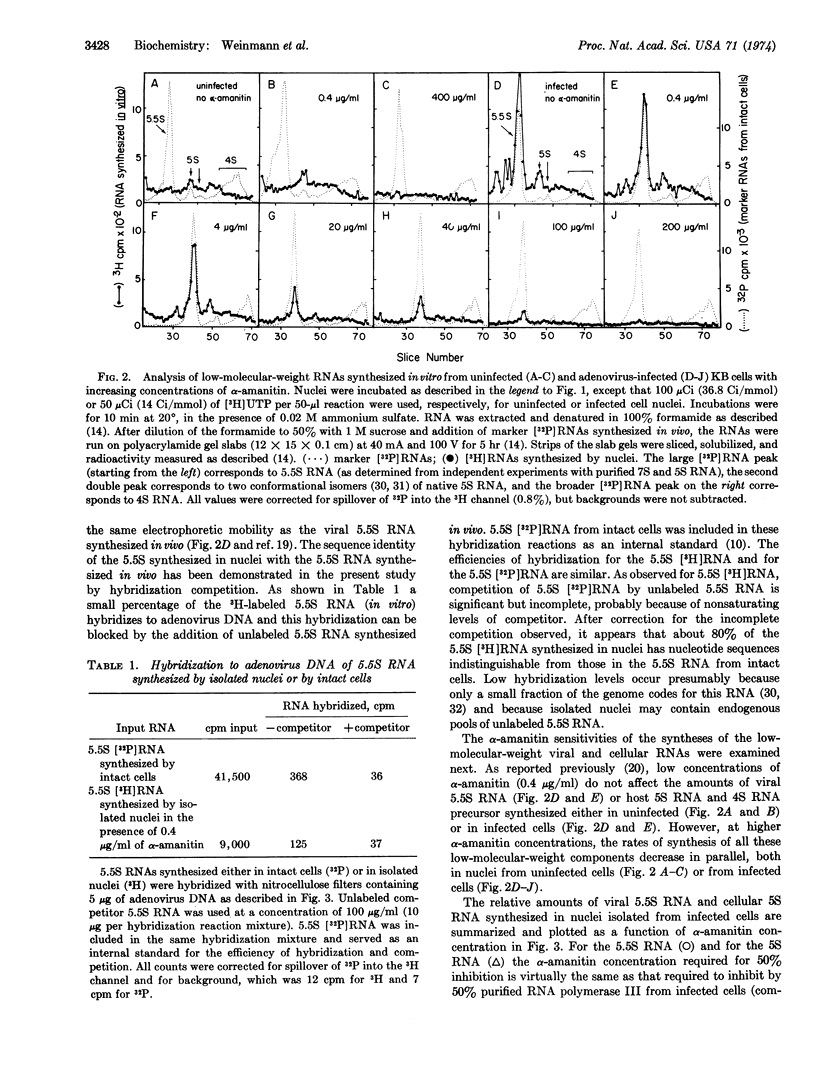
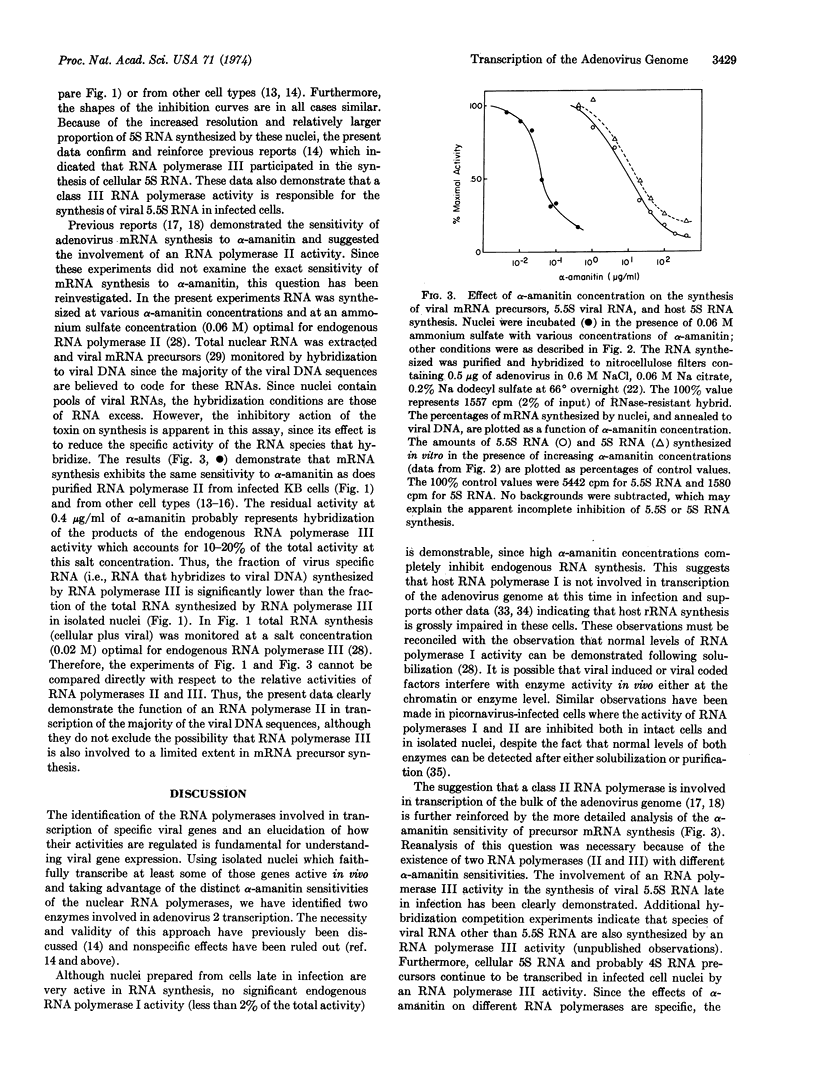
DNA-dependent RNA polymerases I, II, and III were isolated and partially purified from KB (human) cells 18 hr after infection with adenovirus 2. As reported previously for the enzymes from other animal cells, RNA polymerase II was completely sensitive to low concentrations of α-amanitin (50% inhibition at 0.02 μg/ml), RNA polymerase III was completely sensitive to high concentrations of α-amanitin (50% inhibition at 20 μg/ml) and RNA polymerase I was totally resistant to concentrations of α-amanitin less than or equal to 200 μg/ml. RNA synthesis by the endogenous RNA polymerase activities in nuclei isolated from infected cells was completely sensitive to α-amanitin, thus suggesting that RNA polymerase I is not involved in viral DNA transcription even though it is present in these cells. The α-amanitin inhibition curve was biphasic and showed inflection points at about 0.02 and 20 μg/ml, suggesting the participation of both RNA polymerases II and III in the synthesis of RNA in these nuclei. Furthermore, at least a large fraction of the synthesis of the nuclear precursors to viral mRNA, monitored by hybridization to viral DNA, showed the same sensitivity to α-amanitin as did RNA polymerase II; and the synthesis of both viral 5.5S RNA and (presumably cellular) 5S RNA in the isolated nuclei exhibited the same sensitivity to α-amanitin as did purified RNA polymerase III. Thus, these data provide strong supporting evidence for previous studies which suggested the involvement of an RNA polymerase II in transcription of the adenovirus genome and demonstrate the role of an RNA polymerase III activity in the synthesis of viral 5.5S RNA and cellular 5S RNA.
Keywords: RNA polymerase, α-amanitin, 5.5S viral RNA, 5S host RNA
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin G. E., Bello L. J., Furth J. J. DNA dependent RNA polymerase of KB cells. I. Isolation of the enzymes and transcription of viral DNA, mammalian DNA and chromatin. Biochim Biophys Acta. 1973 Nov 14;324(4):488–500. doi: 10.1016/0005-2787(73)90208-6. [DOI] [PubMed] [Google Scholar]
- Bhaduri S., Raskas H. J., Green M. Procedure for the preparation of milligram quantities of adenovirus messenger ribonucleic acid. J Virol. 1972 Dec;10(6):1126–1129. doi: 10.1128/jvi.10.6.1126-1129.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B. G., Weissman S. M. Nucleotide sequence of KB cell 5S RNA. Science. 1967 Dec 29;158(3809):1695–1699. doi: 10.1126/science.158.3809.1695. [DOI] [PubMed] [Google Scholar]
- Fujinaga K., Green M. Mechanism of viral carcinogenesis by DNA mammalian viruses. VII. Viral genes transcribed in adenovirus type 2 infected and transformed cells. Proc Natl Acad Sci U S A. 1970 Feb;65(2):375–382. doi: 10.1073/pnas.65.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedinger C., Gniazdowski M., Mandel J. L., Jr, Gissinger F., Chambon P. Alpha-amanitin: a specific inhibitor of one of two DNA-pendent RNA polymerase activities from calf thymus. Biochem Biophys Res Commun. 1970 Jan 6;38(1):165–171. doi: 10.1016/0006-291x(70)91099-5. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T., Philipson L. Isolation and characterization of adenovirus messenger ribonucleic acid in productive infection. J Virol. 1972 Nov;10(5):909–919. doi: 10.1128/jvi.10.5.909-919.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell T. J., Weinberg F., Morris P. W., Roeder R. G., Rutter W. J. Specific inhibition of nuclear RNA polymerase II by alpha-amanitin. Science. 1970 Oct 23;170(3956):447–449. doi: 10.1126/science.170.3956.447. [DOI] [PubMed] [Google Scholar]
- Ohe K. Virus-coded origin of a low molecular weight RNA from KB cells infected with adenovirus 2. Virology. 1972 Mar;47(3):726–733. doi: 10.1016/0042-6822(72)90562-4. [DOI] [PubMed] [Google Scholar]
- Ohe K., Weissman S. M., Cooke N. R. Studies on the origin of a low molecular weight ribonucleic acid from human cells infected with adenoviruses. J Biol Chem. 1969 Oct 10;244(19):5320–5332. [PubMed] [Google Scholar]
- Ohe K., Weissman S. M. The nucleotide sequence of a low molecular weight ribonucleic acid from cells infected with adenovirus 2. J Biol Chem. 1971 Nov 25;246(22):6991–7009. [PubMed] [Google Scholar]
- Price R., Penman S. A distinct RNA polymerase activity, synthesizing 5-5 s, 5 s and 4 s RNA in nuclei from adenovirus 2-infected HeLa cells. J Mol Biol. 1972 Oct 14;70(3):435–450. doi: 10.1016/0022-2836(72)90551-7. [DOI] [PubMed] [Google Scholar]
- Price R., Penman S. Transcription of the adenovirus genome by an -amanitine-sensitive ribonucleic acid polymerase in HeLa cells. J Virol. 1972 Apr;9(4):621–626. doi: 10.1128/jvi.9.4.621-626.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskas H. J., Thomas D. C., Green M. Biochemical studies on adenovirus multiplication. XVII. Ribosome synthesis in uninfected and infected KB cells. Virology. 1970 Apr;40(4):893–902. doi: 10.1016/0042-6822(70)90135-2. [DOI] [PubMed] [Google Scholar]
- Reeder R. H., Roeder R. G. Ribosomal RNA synthesis in isolated nuclei. J Mol Biol. 1972 Jun 28;67(3):433–441. doi: 10.1016/0022-2836(72)90461-5. [DOI] [PubMed] [Google Scholar]
- Roeder R. G. Multiple forms of deoxyribonucleic acid-dependent ribonucleic acid polymerase in Xenopus laevis. Isolation and partial characterization. J Biol Chem. 1974 Jan 10;249(1):241–248. [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969 Oct 18;224(5216):234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Specific nucleolar and nucleoplasmic RNA polymerases. Proc Natl Acad Sci U S A. 1970 Mar;65(3):675–682. doi: 10.1073/pnas.65.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Dahlberg J. E. Small RNAs of Rous sarcoma virus: characterization by two-dimensional polyacrylamide gel electrophoresis and fingerprint analysis. J Virol. 1973 Dec;12(6):1226–1237. doi: 10.1128/jvi.12.6.1226-1237.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts C., Pettersson U., Johansson K., Philpson L. Relationship of mRNA from productively infected cells to the complementary strands of adenovirus type 2 DNA. J Virol. 1974 Feb;13(2):370–377. doi: 10.1128/jvi.13.2.370-377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Philipson L., Darnell J. E. Processing of adenovirus specific nuclear RNA during virus replication. Virology. 1972 Oct;50(1):27–34. doi: 10.1016/0042-6822(72)90342-x. [DOI] [PubMed] [Google Scholar]
- Wallace R. D., Kates J. State of adenovirus 2 deoxyribonucleic acid in the nucleus and its mode of transcription: studies with isolated viral deoxyribonucleic acid-protein complexes and isolated nuclei. J Virol. 1972 Apr;9(4):627–635. doi: 10.1128/jvi.9.4.627-635.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann R., Roeder R. G. Role of DNA-dependent RNA polymerase 3 in the transcription of the tRNA and 5S RNA genes. Proc Natl Acad Sci U S A. 1974 May;71(5):1790–1794. doi: 10.1073/pnas.71.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylber E. A., Penman S. Products of RNA polymerases in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2861–2865. doi: 10.1073/pnas.68.11.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]



