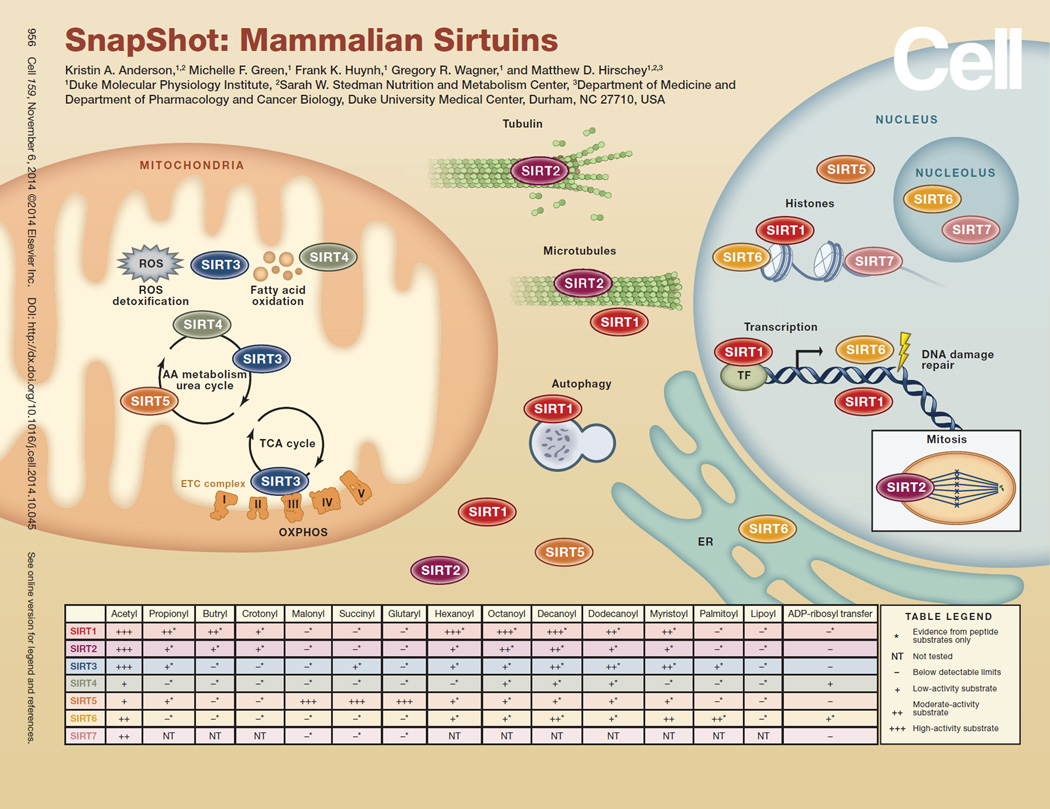
SIRT1
SIRT1, the most-studied sirtuin, is best known for mediating the lifespan extension effects of CR. Though this original claim has been refuted by some, SIRT1 consistently improves health during aging by protecting against or delaying the onset of metabolic disease, neurodegeneration, CVD, and some types of cancers (Finkel et al., 2009). SIRT1 deacetylates many different targets in the cytoplasm and nucleus, and several mechanisms have been repeatedly described to explain how SIRT1 carries out its protective roles (Cantó and Auwerx, 2012). SIRT1 reduces inflammation by reducing NF-κB activity; activates key transcription factors, such as FOXO; regulates lipid metabolism, via PGC1α; and acts as a tumor suppressor by preserving genomic integrity. Much evidence supports the idea that increased SIRT1 activity is protective against age-associated diseases, and current research is aimed at enhancing SIRT1 activity as a way to prevent or treat these diseases.
SIRT2
SIRT2 deacetylates a wide range of targets in the cytoplasm and nucleus, which allows it to influence diverse pathways such as cell-cycle regulation, microtubule dynamics, inflammation, and differentiation. SIRT2 is particularly abundant in the brain, where it influences the process of myelination. The physiological effects of SIRT2 are highly contextual. For example, loss of SIRT2 through small-molecule inhibition or genetic ablation is beneficial for treating a number of neurodegenerative diseases, but SIRT2 null mice also develop tumors of the mammary glands and liver.
SIRT3
SIRT3 is a deacetylase in mitochondria. Acetylation levels vary with nutritional status and regulate a majority of pathways in the mitochondria, especially related to energy metabolism. Phenotypes of mice lacking SIRT3 are consistent with the failure to maintain energy balance during stress. SIRT3 activation is generally thought to be beneficial, as loss of SIRT3 leads to cardiac dysfunction, hearing loss, metabolic syndrome, and cancer. Together, SIRT3 plays an important role in regulating energy production in the mitochondria.
SIRT4
SIRT4 has been shown to have ADP-ribosyltransferase as well as weak substrate-specific deacetylase activity in the mitochondria. Because these enzymatic activities are weak, some have suggested that the major enzymatic activity of SIRT4 still remains to be discovered. SIRT4 is protective against diet-induced obesity through its role in promoting fatty acid oxidation and inhibiting lipogenesis (Laurent et al., 2013). However, high-fat fed SIRT4 knockout mice still develop diet-induced glucose intolerance and insulin resistance (Laurent et al., 2013). Furthermore, a loss of SIRT4 leads to a shift in glutamine metabolism that favors tumor development (Zhu et al., 2014). Taken together, SIRT4 acts as a tumor suppressor by regulating lipid and glutamine metabolism in the mitochondria.
SIRT5
SIRT5 is a lysine desuccinylase, demalonylase, and deglutarylase primarily in the mitochondria that activates the urea cycle (Wagner and Hirschey, 2014). SIRT5 knockout mice develop hyperammonemia during fasting or when fed a high-protein diet but are otherwise phenotypically unremarkable (Nakagawa et al., 2009). Thus, SIRT5 plays an important role in regulating nitrogen balance and some aspects of mitochondrial metabolism. Hypersuccinylation of mitochondrial and nonmitochondrial proteins suggests that SIRT5 could play a role in regulating mitochondrial, cytosolic, and nuclear processes. Future efforts will be targeted at understanding the biological processes regulated by SIRT5.
SIRT6
SIRT6 has deacetylase activity against histone substrates and weak in vitro ADP-ribosyltransferase activity. SIRT6 can also remove long-chain acyl groups from peptides in vitro, which is more efficient than its deacetylase activity against peptides derived from H3K9 (Gertler and Cohen, 2013). The long-chain deacylase activity for SIRT6 modulates tumor necrosis factor α (TNFα) by controlling its secretion rate (Jiang et al., 2013). Overall, it appears that, through its effects on histone deacetylation, SIRT6 protects against aging and the diseases of aging. SIRT6 promotes genomic stability and helps to maintain telomere integrity. Remarkably, SIRT6 overexpression in male mice increased lifespan by ~15% (Gertler and Cohen, 2013). In addition, SIRT6 protects against several age-related diseases, including cancer and diabetes. Thus, SIRT6 plays an important role in maintaining both lifespan and healthspan.
SIRT7
SIRT7 is a deacetylase localized to the nucleus and nucleolus, where it positively regulates ribosomal DNA transcription and expression of RNA polymerase 1 as well as interacts with chromatin remodeling complexes to silence gene expression. Loss of SIRT7 also reduces the expression of nuclear-encoded mitochondrial genes (Ryu et al., 2014). These molecular signatures manifest as hypertrophic inflammatory cardiomyopathy, fatty liver disease, age-related hearing loss, and reduced mean and maximal lifespan in SIRT7-deficient mice, suggestive of multisystem mitochondrial dysfunction. SIRT7 knockdown in human cancer cells inhibits tumor growth in mouse xenograft models, suggesting that SIRT7 is also critical for maintaining oncogenic transformation (Barber et al., 2012).
Figure 1.
ACKNOWLEDGEMENTS
The authors contributed equally to this SnapShot and apologize to colleagues whose work was not cited due to space limitations or oversight. We would like to acknowledge funding support from the National Institutes of Health and the NIAAA grant R01AA022146, the National Institutes of Health and the NIA grant R01AG04535, and The Duke Pepper Older Americans Independence Center (OAIC) Program in Aging Research supported by the National Institute of Aging (P30AG028716-01). M.F.G. is supported by a postdoctoral fellowship on an NIH/NCI training grant to Duke University (CA059365-19); F.K.H. is supported by an American Diabetes Association/Canadian Diabetes Association Postdoctoral Fellowship (PF-3-13-4342-FH); G.R.W. is supported by an NIH/NIDDK training grant to Duke University Department of Endocrinology, Metabolism, and Nutrition (2T32-DK007012-36A1).
REFERENCES
- Barber MF, Michishita-Kioi E, Xi Y, Tasselli L, Kioi M, Moqtaderi Z, Tennen RI, Paredes S, Young NL, Chen K, et al. Nature. 2012;487:114–118. doi: 10.1038/nature11043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Auwerx J. Pharmacol. Rev. 2012;64:166–187. doi: 10.1124/pr.110.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Deng CX, Mostoslavsky R. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler AA, Cohen HY. Biogerontology. 2013;14:629–639. doi: 10.1007/s10522-013-9478-8. [DOI] [PubMed] [Google Scholar]
- Jiang H, Khan S, Wang Y, Charron G, He B, Sebastian C, Du J, Kim R, Ge E, Mostoslavsky R, et al. Nature. 2013;496:110–113. doi: 10.1038/nature12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G, German NJ, Saha AK, de Boer VC, Davies M, Koves TR, Dephoure N, Fischer F, Boanca G, Vaitheesvaran B, et al. Mol. Cell. 2013;50:686–698. doi: 10.1016/j.molcel.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Lomb DJ, Haigis MC, Guarente L. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu D, Jo YS, Lo Sasso G, Stein S, Zhang H, Perino A, Lee JU, Zeviani M, Romand R, Hottiger MO, et al. Cell Metab. 2014 doi: 10.1016/j.cmet.2014.08.001. Published online September 3, 2014. http://dx.doi.org/10.1016/j.cmet.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Wagner GR, Hirschey MD. Mol. Cell. 2014;54:5–16. doi: 10.1016/j.molcel.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Yan Y, Principe DR, Zou X, Vassilopoulos A, Gius D. Cancer Metab. 2014;2:15. doi: 10.1186/2049-3002-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]