Abstract
Purpose
To assess the possible radiosensitizing capabilities of two different poly(ADP-ribose) polymerase (PARP) inhibitors in combination with external beam and 131I-tositumomab in a non-Hodgkin’s lymphoma cell line.
Methods and materials
Epstein–Barr virus-infected human Raji lymphoma cells with lentivirally transfected green fluorescent protein and luciferase in log-phase growth were incubated with various doses of AZD-2281 and ABT-888 24 h before external beam radiation exposure. A 500nmol/l concentration of AZD-2281 and ABT-888 was used to assess the growth curve of Raji lymphoma cells over 5 days. The number of double-stranded breaks was visually assessed using a H2AX antibody and confocal microscopy. Intracellular PARP activity was measured 2 h after incubation with AZD-2281 (500nmol/l) and ABT-888 using a colorimetric PARP assay kit. The radiosensitizing effect of AZD-2281 (500 nmol/l) with various doses of 131I-tositumomab was assessed after 24 h.
Results
A volume of 500 nmol/l of AZD-2281 and 500 nmol/l of ABT-888, in combination with 0, 4, 8, and 12 Gy external beam radiation, showed a 5.2, 7.1, 10.1, and 33.1% radiosensitization. A measure of 500 nmol/l AZD-2281 and ABT-888 significantly reduced the percentage of viable cells on days 3–5 compared with controls. The maximal relative reduction in viable cells was 78.5%, and this occurred with AZD-2281 (500 nmol/l) on day 5. AZD-2281 revealed a higher number of double-stranded breaks with confocal microscopy than did ABT-888. Two hours after incubation of Raji cells with 500 nmol/l of AZD-2281 or ABT-888, the colorimetric PARP activity assay showed a reduction of 30.36% with ABT-888 and of 47.8% with AZD-2281. Combining AZD-2281 (500 nmol/l) with 0, 5 μCi (0.185MBq), 10 μCi (0.37MBq) and 20 μCi (0.74MBq) 131I-tositumomab revealed a significant reduction in cell viability after 24 h with 5 μCi (0.185MBq) (P< 0.01) and 10 μCi (0.37MBq) (P < 0.01) radiation dose.
Conclusion
PARP inhibitors AZD-2281 and ABT-888 are highly radiosensitizing agents when used before external beam radiation and 131I-tositumomab.
Keywords: ABT-888, AZD-2281, lymphoma, poly(ADP-ribose) polymerase inhibitor, radioimmunotherapy
Introduction
All cells have overlapping mechanisms to protect DNA from damage. It is well recognized that resistance to chemotherapy is linked to enhanced DNA repair [1]. In recent years, DNA repair has become an increasingly important topic in cancer therapy. Poly(ADP-ribose) polymerase-1 (PARP-1) has emerged as a promising and novel target for cancer therapy. PARPs comprise a family of enzymes that catalyze the polymerization of poly(ADP-ribose) chains on target proteins and modify their activity [2]. PARP-1 is an enzyme that plays an important role in the recognition and repair of single-stranded DNA breaks through the base excision repair pathway [3]. A recent clinical phase I trial of PARP-1 showed substantial antitumor activity in patients with breast cancer who had BRCA1 or BRCA2 mutations [4].
Although ionizing radiation is a cornerstone in cancer therapy, external beam radiation is limited by its local activity and side effects due to high radiation burden. Radioimmunotherapy is a relatively new and highly effective treatment for lymphoma. Proof of concept of iodine-131 bound to anti-CD20 antibodies was published in the early 1990s in patients with relapsed CD20- positive follicular lymphoma [5]. Several studies have now reported the benefit of 90Y-ibritumomab tiuxetan in relapsed/refractory CD20-positive follicular lymphomas [6–8].
A recent large prospective phase III trial investigated 90Y-ibritumomab tiuxetan in the setting of consolidation of first-line therapy and showed high efficacy with no unexpected toxicities [9]. However, only a fraction of the infused radioactive antibodies reached the tumor site, and further strategies are needed to elicit an effective tumor response. Radiosensitizers, in combination with external beam radiation, are used in a variety of cancers [10].
PARP inhibitors show antitumor activity through inhibition of the repair of single-stranded DNA breaks through the base excision repair pathway. Ionizing radiation is effective because of the induction of double-stranded breaks in cancer cells [11]. In addition to their clearly defined role in the repair of single-stranded breaks, PARP inhibitors have an equivalent role in the repair of double-stranded breaks [12]. The combination of downregulation of DNA repair by PARP inhibitors and induction of DNA damage by means of ionizing radiation is an intriguing concept.
We assessed the possible radiosensitizing capabilities of two different PARP inhibitors in combination with various doses of external beam radiation and 131I-tositumomab. A possible concept to further increase the effectiveness of ionizing radiation, which should lead to enhanced antitumor efficacy, is presented in this manuscript.
Methods
Cell lines and cell culture
We used Epstein–Barr virus-infected Raji lymphocyte tumor cells transfected through a lentivirus vector with green fluorescent protein and luciferase for all in-vitro experiments. The cell lines were established by Dr Zhaohui Ye in collaboration with the laboratory of Dr Linzhao Cheng at The Johns Hopkins University [13]. Cells in the exponential phase of growth were incubated in 10 ml of CellGro RPMI medium (Mediatech, Manassas, Virginia, USA) containing 10% fetal bovine serum (Hyclone, Fisher Scientific, Leicestershire, UK) and 1% penicillin–streptomycin (Invitrogen Corp., Carlsbad, California, USA) and maintained at 37°C with 5% CO2 (in a Forma Series II Water-Jacketed Incubator; Thermo Fisher Scientific Inc., Leicestershire, UK).
Cell viability assay
Luciferin (MIP, Molecular Imaging Products, Bend, Oregon, USA) was added to a known number of Epstein–Barr virus-infected Raji lymphocyte tumor cells. Tumor cells were initially counted with a BrightLine hemocytometer (Reichert, Depew, New York, USA). The known amount of tumor cells was incubated for 2 min in the dark before 30 s of luminescence counting (Monolight 3010 luminometer; Pharmingen/Becton–Dickinson, Franklin Lakes, New Jersy, USA). All steps were followed according to the manufacturer’s instructions. Standard curves were established to ensure a linear correlation between manually counted cell numbers and measured photon luminescence. Verification of the standard curves revealed a linear correlation between 3 × 102 and 3 × 107 tumor cells.
Chemicals
AZD-2281 (AstraZeneca Pharmaceuticals LP, Wilmington, Delaware, USA) and ABT-888 (Abbott, Illinois, USA) are highly potent and selective PARP inhibitors with a potency of approximately 5 nmol/l in vitro [14,15]. Both chemicals were purchased from Selleck Chemicals LLC (Houston, Texas, USA). All experiments were conducted with freshly diluted ABT-888 (fresh PBS) and AZD-2281 [dimethyl sulfoxide (DMSO)] and appropriate controls (PBS and DMSO). Dilution of DMSO as carrier for AZD- 2281 in cell culture was less than 1%.
Radiation
Transfected Raji lymphoma cells were exposed to various amounts of external source cesium-based radiation (none, 4, 8, or 12 Gy) on a Gammacell 3000 Elon apparatus (MDS-Nordion, Ottawa, Ontario, Canada). Ionizing radiation was delivered at a rate of 5 Gy/min. Control cells that were not irradiated were removed from the incubator and transported to the radiation site to expose them to the same environmental conditions as the irradiated cells.
Laser confocal microscopy and fluorescent H2AX antibody
Double-stranded breaks were visually assessed using laser confocal microscopy and an Alexa Fluor 647 anti-H2A.X-phosphorylated (Ser139) antibody (Biolegend, San Diego, California, USA). Briefly, H2AX is a member of the H2 histone family and is phosphorylated on serine 139 after double-stranded DNA breaks. Phosphorylated H2AX promotes DNA repair and maintains genomic stability. The anti-H2A.X-phosphorylated (Ser139) antibody reacts with phosphorylated human H2AX. Laser confocal microscopy was performed in the Johns Hopkins Microscope Facility under the guidance of the in-house staff of the microscope facility.
All experiments were conducted on a Zeiss LSM510- Meta single-point, laser scanning confocal microscope using multimode and a 1024 × 1024 matrix. One million Epstein–Barr virus-infected Raji lymphocyte tumor cells were inoculated with 500nmol/l of ABT-888 or AZD-2281 2 h before 0, 4, 8, and 12Gy external beam radiation. Immediately after external beam radiation, cell samples were fixed and further permeabilized (FIX & PERM; Trevigen, Gaithersburg, Maryland, USA) according to the manufacturers’ manual. After permeabilization, the Alexa Fluor 647 anti-H2A.X-phosphorylated (Ser139) antibody was used to stain for double-stranded breaks. Image data were analyzed using ImageJ software (Version 1.42q; National Institutes of Health, USA). The procedure used is described by Zhongli et al. [16] using the analyze particles function. Thresholds were chosen equally for all data sets.
Cell viability after various doses of ABT-888 and AZD-2281 and external beam radiation
A total of 1 000 000 cells were incubated with various amounts of ABT-888 and AZD-2281 (control, 100, 250, 500, 750, and 1000nmol/l) and with various doses of external beam radiation (0, 4, 8, and 12 Gy). Cell viability was measured after 24 h.
Cell growth after 500 nmol/l of ABT-888 and AZD-2281 in combination with external beam radiation
A total of 100 000 cells in the exponential phase of growth were incubated in 10 ml of CellGro RPMI medium (Mediatech) containing 10% fetal bovine serum (Hyclone) and 1% penicillin–streptomycin (Invitrogen Corp.) and maintained at 37°C with 5% CO2 (in a Forma Series II Water-Jacketed Incubator; Thermo Fisher Scientific Inc.). The cells were incubated with 500nmol/l of ABT-888 or AZD-2281 2 h before external beam radiation. External beam radiation consisted of a radiation dose of 0, 4, 8, or 12Gy. Cell number was assessed daily with the described cell viability assay at 24 h intervals. All experiments were conducted in six-well plates in triplicate.
Colorimetric poly(ADP-ribose) polymerase activity assay
A 96-well PARP Assay Kit (Trevigen) was used to measure the incorporation of biotinylated poly(ADP-ribose) onto histone proteins in a 96-well strip-well format. Reagent preparation, the processing of cells and data interpretation were performed according to the manufacturer’s instructions. A standard curve using the manufacturer’s controls revealed a correlation coefficient of R=0.966. Activity was measured in Epstein–Barr virus-infected Raji lymphocyte tumor cells inoculated with 500nmol/l of ABT-888 or AZD-2281 for 2 h, as were the corresponding controls (24 h of PBS and 1% DMSO). All experiments were conducted in six-well plates in triplicate.
Radioimmunotherapy with 131I-tositumomab (Bexxar) combined with AZD-2281
A total of 100 000 Epstein–Barr virus-infected Raji lymphocyte tumor cells were inoculated with AZD-2281 (500 nmol/l) or with the 1% DMSO as control. Two hours later the cells were challenged with 0, 5 μCi (0.185 MBq), 10 μCi (0.37 MBq), or 20 μCi (0.74 MBq) of 131I-tositumomab for 2 h. All cells were washed three times and resuspended in six-well plates. Cell kill was assessed after 24 h. Experiments were conducted in triplicate with adequate shielding between the dosing groups.
Results
Cell growth after 500 nmol/l of ABT-888 and AZD-2281 in combination with external beam radiation
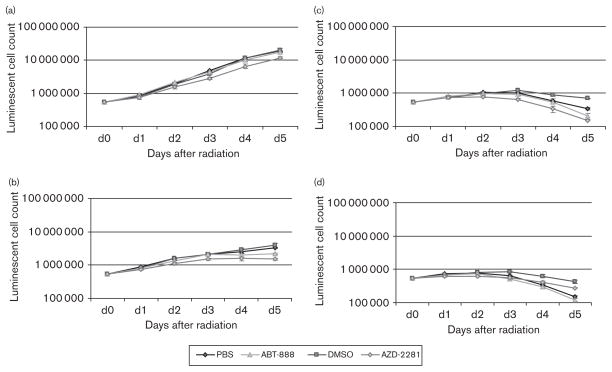
A volume of 500 nmol/l ABT-888 and AZD-2281 showed a moderate intrinsic effect on cell proliferation on days 3–5 (Fig. 1a). ABT-888 and AZD-2281 showed a significant (P<0.05) reduction in lymphoma cell growth on days 2–5 (Fig. 1b–d). The maximum reduction in cell proliferation was measured after 8 Gy of external beam radiation. ABT-888 produced a reduction in cell proliferation of 38.5%, and AZD-2281 produced a reduction in cell growth of 78.5% compared with controls (P<0.01), on day 5. DSMO (1%) had a strong and significant radioprotective effect compared with PBS at 4, 8, and 12 grays, which increased over the incubation time and with increasing radiation dose (P<0.05). The maximal radioprotection of 65.0% was measured after 5 days in the 12Gy group.
Fig. 1.
Logarithmic growth curves of human Burkitt lymphoma cells over 5 days with 500 nmol/l of ABT-888 and AZD-2281 in combination with 0Gy (a), 4Gy (b), 8Gy (c), and 12 Gy (d) of external beam radiation. The maximal relative reduction was 65.5% of viable cells and occurred with AZD-2281 (500 nmol/l) on day 5. DMSO, dimethyl sulfoxide.
Laser confocal microscopy and fluorescent H2AX antibody
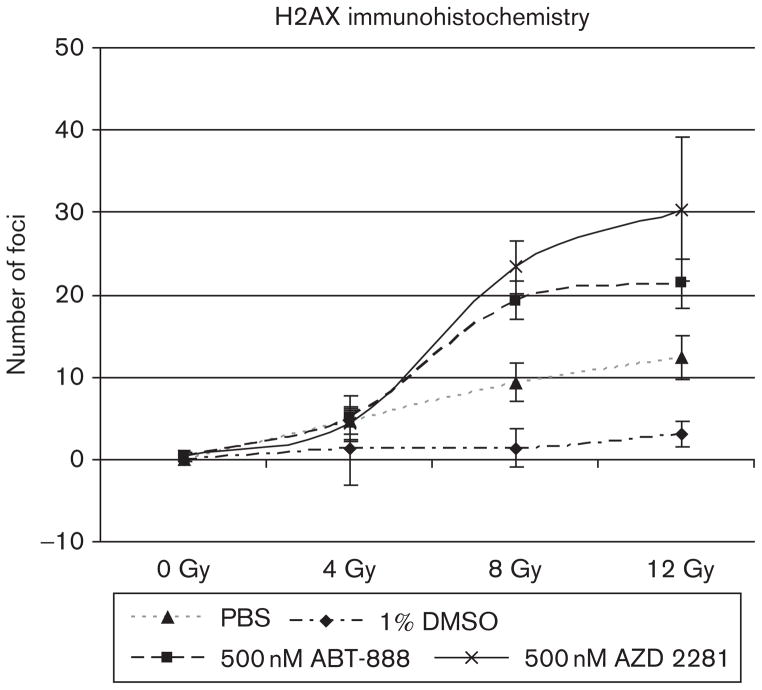
The Fluor-647 anti-H2A.X-phosphorylated (Ser139) antibody stained significantly more double-stranded breaks in cells treated with ABT-888 and AZD-2281 compared with controls at 8 and 12 Gy (P<0.05). There was no difference in the 0 and 4Gy groups in treated and untreated cells. Cells in 1% DMSO had significantly fewer H2AX double-stranded breaks than did cells in PBS (P<0.05) (Fig. 2).
Fig. 2.
Number of foci detected using laser confocal microscopy and fluorescent Fluor 647 anti-H2A.X-phosphorylated (Ser139) antibody. Double-stranded breaks (red) are clearly augmented in cells incubated with 500 nmol/l of ABT-888 and 500 nmol/l of AZD-2281 compared with PBS and 1% dimethyl sulfoxide controls. Image analysis was performed using ImageJ and the ‘analyze particle’ function.
Colorimetric PARP activity assay in Epstein–Barr virus-infected Raji lymphocyte tumor cells after dosing with 500 nmol/l of ABT-888 or AZD-2281
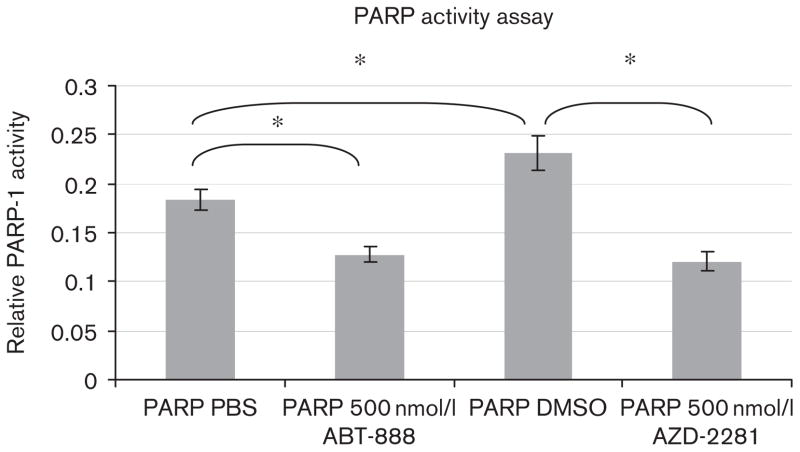
PARP activity significantly decreased after 2 h of incubation with ABT-888 or AZD-2281. In contrast, measured PARP activity was increased after 2 h of incubation with 1% DMSO in comparison with PBS (Fig. 3). There was a 30.4% reduction in PARP-1 activity after 2 h of incubation with 500 nmol/l of ABT-888 compared with PBS (P<0.01) and a 47.8% reduction in PARP-1 activity after 2 h of incubation with 500nmol/l of AZD-2281 compared with 1% DMSO (P<0.01). We measured an augmented PARP-1 activity of 26% in cells incubated for 2 h in 1% DMSO compared with PBS. All results were acquired in triplicate and were significant (P<0.01).
Fig. 3.
Colorimetric poly(ADP-ribose) polymerase (PARP) activity assay showing the relative activity of the PARP-1 enzyme in Raji lymphocyte tumor cells. Results show a highly significant difference in PARP activity in the controls [PBS and dimethyl sulfoxide (DMSO)] compared with 24 h incubation with 500 nmol/l of ABT-888 and 500 nmol/l of AZD-2281. A significant increase in PARP enzyme activity is shown in DMSO-incubated cells compared with PBS control. *P<0.05.
Radioimmunotherapy with 131I-tositumomab (Bexxar) combined with ABT-888 and AZD-2281
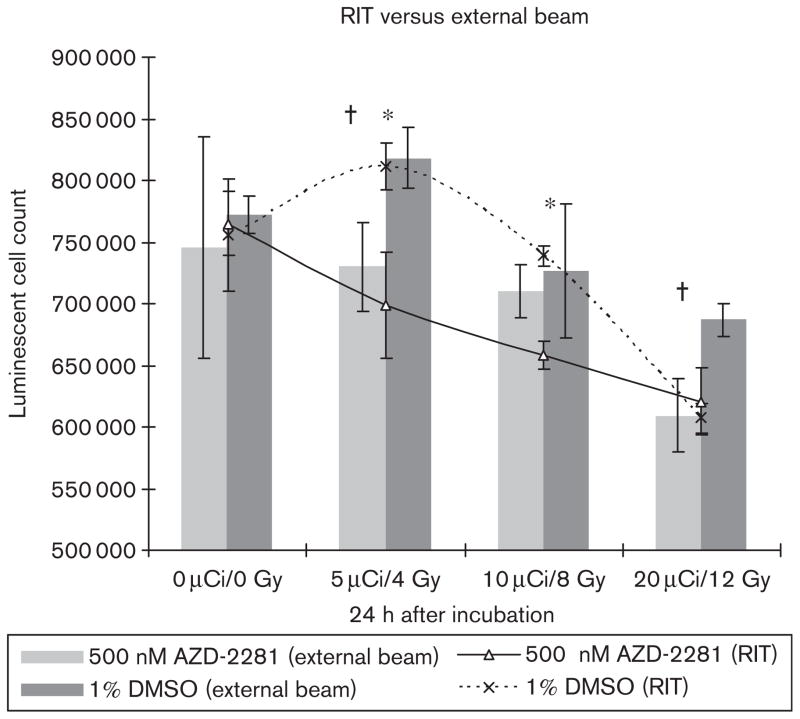
A total of 100 000 Epstein–Barr virus-infected Raji lymphocyte tumor cells were incubated with 500nmol/l of AZD-2281 2 h before radioimmunotherapy with 131I-tositumomab. Cell viability after 24 h was significantly reduced in the 5 μCi (0.185 MBq) and 10 μCi (0.37 MBq) groups (P<0.05) compared with DMSO control [ – 18.2% in 5 μCi (0.185 MBq), – 13.9% in 10 μCi (0.37 MBq), and – 1.1% in 20 μCi (0.74 MBq)] (Fig. 4).
Fig. 4.
The combination of AZD-2281 (500 nmol/l) with various doses of 131I-tositumomab shows a significant reduction (P<0.05) in cell growth after 24 h with 5 μCi (500 nmol/l) and 10 μCi (0.37MBq) of 131I-tositumomab (line). The combination of 500 nmol/l AZD-2281 with various doses of external beam shows a significant reduction in cell viability for 4 and 12 Gy but not for 8 Gy (bars). *P<0.05 significant difference in RIT with Bexxar, †P<0.05 significant difference in external beam.
Discussion
We examined the PARP inhibitors ABT-888 and AZD- 2281 in combination with various doses of external beam radiation and 131I-tositumomab. We found a strong additive effect of both when ABT-888 and AZD-2281 were combined with radiation. However, AZD-2281 in combination with radiation in lymphoma seems to have a more potent effect. We also found a clear increase in double-stranded breaks in cells treated with ABT-888 and AZD-2281 in combination with 8 and 12 Gy of external beam radiation. Our results were verified with a colorimetric PARP enzyme assay, which showed a decrease in PARP-1 for ABT-888 and AZD-2281 and an increase in PARP activity in 1% DSMO-treated cells. When we combined PARP inhibitors with 131I-tositumomab we saw a synergistic effect of PARP inhibition and radioimmunotherapy.
PARP-1 is a 113 kDa nuclear enzyme encoded by the ADPRT-1 gene, located at the q41-q42 position on human chromosome 1. PARP-1 activation facilitates DNA repair by the base-excision repair pathway. The role of PARP-1 in the cellular reaction to genotoxic stress led to the development of inhibitors for use in combination with DNA-damaging therapies. Initial data describing ABT- 888 were recently published in a phase 0 trial [17]. AZD-2281 was investigated in female patients with breast cancer who had a BRCA-1 or BRCA-2 mutation [18]. Objective tumor responses were seen in patients with mutated BRCAs who had ovarian, breast, and prostate cancer, and they had only a few side effects.
Recurrence-free and overall survival was increased in tumor-bearing mice with BRCA-1-deficient breast cancer treated with AZD-2281 in combination with platinum drugs, suggesting that AZD-2281 potentiates the effect of these DNA-damaging agents [19]. ABT-888 showed pronounced activity in combination with temozolamide in mismatch repair-deficient leukemia cells [20]. Recent reports describe a synergistic effect of PARP-1 inhibition and external beam radiation in a model of head and neck cancer [21]. We used 500 nmol/l of ABT-888 and 500 nmol/l of AZD-2281. This concentration is the dose that is reached in the blood levels of patients in the phase 0 trial reported by Kummar et al. [17].
Molecular radiotherapy such as radioimmunotherapy is a promising method of cancer treatment [22]. However, because of the nature of diverse carrier systems, only a fraction of the systemic radiation reaches the tumor cells. Tumor response can be improved in three ways: first, increasing the radiation dose to the tumor; second, improving the carrier systems; and third, selectively radiosensitizing the target cancer cells.
Augmented radiation doses can be reached by means of an appropriate choice of radioisotope. Clearly, the efficiency of conventional, β-emitting radionuclides to kill isolated cells is limited by the deposition of most of the β particle energy far from the targeted cell. Although the use of α emitters that deliver large amounts of energy (several MeV) in an area of less than 100 μm has been proposed [23], the limited availability of radionuclides with appropriate physical properties limits widespread use of this promising concept.
Optimized carrier systems for radioimmunotherapy are under investigation. The most advanced concept, the pretargeting technique, is currently being assessed in clinical trials [24,25]. This innovative technology decouples the phase of tumor targeting with an antitumor antibody and the phase of the delivery of the radionuclide [26]. The separation into several steps allows the unlabeled antibody to bind to tumor targets over a few days and allows the small molecule of the radiolabeled effector to reach the prelocalized antibody within a few hours.
An improved tumor effect can also be reached by a sensitization of tumor cells. Gemcitabene, taxotere, methotrexate and cetuximab, among others, have been reported to enhance the efficacy of radioimmunotherapy [27–29]. To our knowledge, our current report is the first to investigate the combination of radioimmunotherapy with PARP inhibitors. Our data suggest that a combination of radioimmunotherapy with PARP inhibitors might be a promising concept to enhance the activity of radioimmunotherapy. This would be particularly true if there were selective deficiencies in PARP activity in tumor cells so that the effect is more specific for tumor cells than for normal cells. However, the toxicity of PARP inhibitors in combination with radiation in normal cells has to be explored.
Our data also demonstrate a radioprotective effect of 1% DMSO. This finding is confirmed by significantly enhanced PARP-1 enzyme activity in Raji Burkitt lymphoma cells after 24 h of incubation with 1% DMSO in comparison with PBS. This observation was previously made by Howell et al. [30], who described the radioprotective effect of 5% DMSO against unbound 32P. To the best of our knowledge, this has not been described in relation to radioimmunotherapy.
Laser confocal microscopy revealed significantly more double-stranded breaks after 8 and 12 Gy of radiation in combination with either AZD-2281 or ABT-888. PARP inhibitors have been implicated in the repair of single-stranded breaks. Repair of radiation-induced DNA double-stranded breaks involves the DNA-dependent protein kinase and PARP-1 [31]. Measurement of doublestranded breaks is therefore a reasonable method to assess the efficacy of PARP inhibitors in combination with ionizing radiation. The higher frequency of double-stranded breaks suggests a firm biological rationale for the radiation enhancement effects we have described.
Conclusion
The two PARP inhibitors AZD-2281 and ABT-888 are highly radiosensitizing agents in combination with external beam and 131I-tositumomab. These data suggest that PARP inhibition may have potential for combination with external beam radiation or radioimmunotherapy and may offer the potential for selective enhancement of therapeutic efficacy.
Footnotes
Conflicts of interest
Dr Richard L. Wahl is one of the inventors of anti CD-20 radioimmunotherapy and receives payments from the University of Michigan as part of a patent licensing agreement related to royalties from the US sales of the radioimmunotherapy agents Bexxar and Zevalin. Other coinvestigators on this manuscript have no financial conflict of interest related to the study.
References
- 1.Peltomaki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol. 2003;21:1174–1179. doi: 10.1200/JCO.2003.04.060. [DOI] [PubMed] [Google Scholar]
- 2.Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD + into a nuclear signal. Genes Dev. 2005;19:1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber V, Dantzer F, Ame J-C, De Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 4.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 5.Kaminski MS, Zasadny KR, Francis IR, Milik AW, Ross CW, Moon SD, et al. Radioimmunotherapy of B-cell lymphoma with [131I]anti-B1 (anti-CD20) antibody. N Engl J Med. 1993;329:459–465. doi: 10.1056/NEJM199308123290703. [DOI] [PubMed] [Google Scholar]
- 6.Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 7.Wiseman GA, Gordon LI, Multani PS, Witzig TE, Spies S, Bartlett NL, et al. Ibritumomab tiuxetan radioimmunotherapy for patients with relapsed or refractory non-Hodgkin lymphoma and mild thrombocytopenia: a phase II multicenter trial. Blood. 2002;99:4336–4342. doi: 10.1182/blood.v99.12.4336. [DOI] [PubMed] [Google Scholar]
- 8.Witzig TE, Flinn IW, Gordon LI, Emmanouilides C, Czuczman MS, Saleh MN, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J Clin Oncol. 2002;22:3262–3269. doi: 10.1200/JCO.2002.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Morschhauser F, Radford J, Van Hoof A, Vitolo U, Soubeyran P, Tilly H, et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol. 2008;26:5156–5164. doi: 10.1200/JCO.2008.17.2015. [DOI] [PubMed] [Google Scholar]
- 10.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 11.Menegakis A, Yaromina A, Eicheler W, Dörfler A, Beuthien-Baumann B, Thames HD, et al. Prediction of clonogenic cell survival curves based on the number of residual DNA double strand breaks measured by gammaH2AX staining. Int J Radiat Biol. 2009;85:1032–1041. doi: 10.3109/09553000903242149. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell J, Smith GC, Curtin NJ. Poly(ADP-Ribose) polymerase-1 and DNA-dependent protein kinase have equivalent roles in double strand break repair following ionizing radiation. Int J Radiat Oncol Biol Phys. 2009;75:1520–1527. doi: 10.1016/j.ijrobp.2009.07.1722. [DOI] [PubMed] [Google Scholar]
- 13.Baba S, Cho SY, Ye Z, Cheng L, Engles JM, Wahl RL. How reproducible is bioluminescent imaging of tumor cell growth? Single time point versus dynamic measurement approach. Mol Imaging. 2007;6:315–322. [PubMed] [Google Scholar]
- 14.Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 15.Menear KA, Adcock C, Boulter R, Cockcroft XL, Copsey L, Cranston A, et al. 4-[3-(4-cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one: a novel bioavailable inhibitor of poly(ADP-ribose) polymerase-1. J Med Chem. 2008;51:6581–6591. doi: 10.1021/jm8001263. [DOI] [PubMed] [Google Scholar]
- 16.Zhongli C, Vallis KA, Reilly RM. Computational analysis of the number, area and density of c-H2AX foci in breast cancer cells exposed to 111In-DTPA-hEGF or c-rays using Image-J software. Int J Radiat Biol. 2009;85(3):262–271. doi: 10.1080/09553000902748757. [DOI] [PubMed] [Google Scholar]
- 17.Kummar S, Kinders R, Gutierrez ME, Rubinstein L, Parchment RE, Phillips LR, et al. Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. J Clin Oncol. 2009;27:2705–2711. doi: 10.1200/JCO.2008.19.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 19.Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A. 2008;105:17079–17084. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horton TM, Jenkins G, Pati D, Zhang L, Dolan ME, Ribes-Zamora A, et al. Poly(ADP-ribose) polymerase inhibitor ABT-888 potentiates the cytotoxic activity of temozolomide in leukemia cells: influence of mismatch repair status and O6-methylguanine-DNA methyltransferase activity. Mol Cancer Ther. 2009;8:2232–2242. doi: 10.1158/1535-7163.MCT-09-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan K, Araki K, Wang D, Li G, Li X, Zhang J, et al. Head and neck cancer radiosensitization by the novel poly(ADP-ribose) polymerase inhibitor GPI-15427. Head Neck. 2010;32:381–391. doi: 10.1002/hed.21195. [DOI] [PubMed] [Google Scholar]
- 22.Sharkey RM, Karacay H, Goldenberg DM. Improving the treatment of non-Hodgkin lymphoma with antibody-targeted radionuclides. Cancer. 2010;116 (S4):1134–1145. doi: 10.1002/cncr.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jurcic JG, Larson SM, Sgouros G, McDevitt MR, Finn RD, Divgi CR, et al. Targeted alpha particle immunotherapy for myeloid leukemia. Blood. 2002;100:1233–1239. [PubMed] [Google Scholar]
- 24.Forero A, Weiden PL, Vose JM, Knox SJ, LoBuglio AF, Hankins J, et al. Phase 1 trial of a novel anti-CD20 fusion protein in pretargeted radioimmunotherapy for B-cell non-Hodgkin lymphoma. Blood. 2004;104:227–236. doi: 10.1182/blood-2003-09-3284. [DOI] [PubMed] [Google Scholar]
- 25.Grana C, Chinol M, Robertson C, Mazzetta C, Bartolomei M, De Cicco C, et al. Pretargeted adjuvant radioimmunotherapy with yttrium-90-biotin in malignant glioma patients: a pilot study. Br J Cancer. 2002;86:207–212. doi: 10.1038/sj.bjc.6600047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karacay H, Sharkey RM, Gold DV, Ragland DR, McBride WJ, Rossi EA, et al. Pretargeted radioimmunotherapy of pancreatic cancer xenografts: TF10-90Y-IMP-288 alone and combined with gemcitabine. J Nucl Med. 2009;50:2008–2016. doi: 10.2967/jnumed.109.067686. [DOI] [PubMed] [Google Scholar]
- 27.Karacay H, Sharkey RM, Gold DV, Ragland DR, McBride WJ, Rossi EA, et al. Pretargeted radioimmunotherapy of pancreatic cancer xenografts: TF10-90Y-IMP-288 alone and combined with gemcitabine. J Nucl Med. 2009;50:2008–2016. doi: 10.2967/jnumed.109.067686. [DOI] [PubMed] [Google Scholar]
- 28.Kelly MP, Lee ST, Lee FT, Smyth FE, Davis ID, Brechbiel MW, Scott AM. Therapeutic efficacy of 177Lu-CHX-A″-DTPA-hu3S193 radioimmunotherapy in prostate cancer is enhanced by EGFR inhibition or docetaxel chemotherapy. Prostate. 2009;69:92–104. doi: 10.1002/pros.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costantini DL, Bateman K, McLarty K, Vallis KA, Reilly RM. Trastuzumab-resistant breast cancer cells remain sensitive to the auger electron-emitting radiotherapeutic agent 111In-NLS-trastuzumab and are radiosensitized by methotrexate. J Nucl Med. 2008;49:1498–1505. doi: 10.2967/jnumed.108.051771. [DOI] [PubMed] [Google Scholar]
- 30.Howell RW, Goddu SM, Bishayee A, Rao DV. Radioprotection against lethal damage caused by chronic irradiation with radionuclides in vitro. Radiat Res. 1998;150:391–399. [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell J, Smith GC, Curtin NJ. Poly(ADP-Ribose) polymerase-1 and DNA-dependent protein kinase have equivalent roles in double strand break repair following ionizing radiation. Int J Radiat Oncol Biol Phys. 2009;75:1520–1527. doi: 10.1016/j.ijrobp.2009.07.1722. [DOI] [PubMed] [Google Scholar]