Abstract
The NCCN Guidelines for Central Nervous System Cancers provide multidisciplinary recommendations for the clinical management of patients with cancers of the central nervous system. These NCCN Guidelines Insights highlight recent updates regarding the management of metastatic brain tumors using radiation therapy. Use of stereotactic radiosurgery (SRS) is no longer limited to patients with 3 or fewer lesions, because data suggest that total disease burden, rather than number of lesions, is predictive of survival benefits associated with the technique. SRS is increasingly becoming an integral part of management of patients with controlled, low-volume brain metastases.
NCCN: Continuing Education
Accreditation Statement
This activity is designated to meet the educational needs of physicians, nurses, and pharmacists involved in the management of patients with cancer. There is no fee for this article. The National Comprehensive Cancer Network (NCCN) is accredited by the ACCME to provide continuing medical education for physicians. NCCN designates this journal-based CE activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
NCCN is accredited as a provider of continuing nursing education by the American Nurses Credentialing Center’s Commission on Accreditation.
NCCN designates this educational activity for a maximum of 1.0 contact hour. Accreditation as a provider refers to recognition of educational activities only; accredited status does not imply endorsement by NCCN or ANCC of any commercial products discussed/displayed in conjunction with the educational activity. Kristina M. Gregory, RN, MSN, OCN, is our nurse planner for this educational activity.
National Comprehensive Cancer Network is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. NCCN designates this continuing education activity for 1.0 contact hour(s) (0.1 CEUs) of continuing education credit in states that recognize ACPE accredited providers. This is a knowledge-based activity. UAN: 0836-0000-14-011-H01-P.
All clinicians completing this activity will be issued a certificate of participation. To participate in this journal CE activity: 1) review the learning objectives and author disclosures; 2) study the education content; 3) take the posttest with a 66% minimum passing score and complete the evaluation at http://education.nccn.org/node/56627; and 4) view/print certificate.
Release date: November 4, 2014; Expiration date: November 4, 2015.
Learning Objectives
Upon completion of this activity, participants will be able to:
Integrate into professional practice the updates to NCCN Guidelines for Central Nervous System Cancers
Describe the rationale behind the decision-making process for developing the NCCN Guidelines for Central Nervous System Cancers
Overview
Metastases to the brain are the most common intracranial tumors in adults and may occur up to 10 times more frequently than primary brain tumors. Population-based data reported that approximately 8% to 10% of patients with cancer are affected by symptomatic metastatic tumors in the brain.1,2 As a result of advances in diagnosis and treatment, many patients improve with proper management and do not die from progression of these metastatic lesions. Primary lung cancers are the most common source, accounting for half of intracranial metastases, although melanoma has been documented to have the highest predilection to spread to the brain. Diagnosis of central nervous system (CNS) involvement is becoming more common in patients with breast cancer as therapy for metastatic disease is improving.3
Nearly 80% of brain metastases occur in the cerebral hemispheres, an additional 15% occur in the cerebellum, and 5% occur in the brainstem.4 These lesions typically follow a pattern of hematogenous spread to the gray–white junction, where the relatively narrow blood vessels tend to trap tumor emboli. Most cases have multiple brain metastases evident on MRI scans. The presenting signs and symptoms of metastatic brain lesions are similar to those of other mass lesions in the brain, such as headache, seizures, and neurologic impairment.
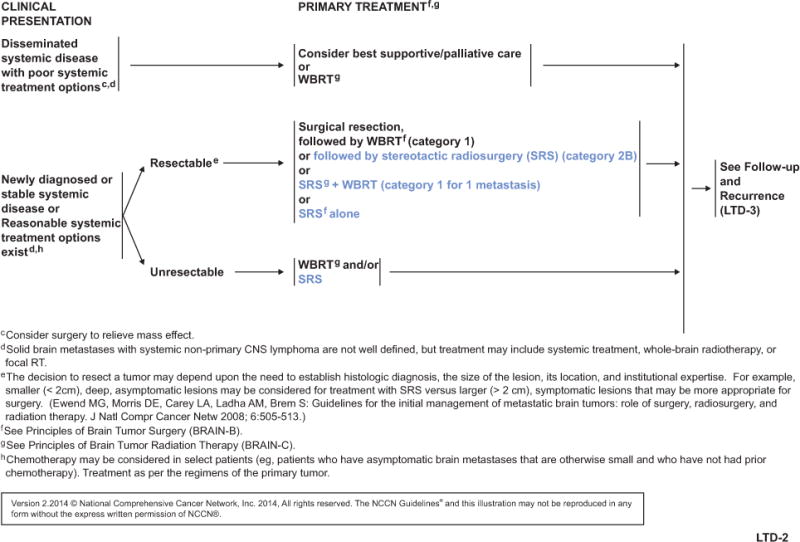
Historically, whole-brain radiation therapy (WBRT) and/or surgery were the mainstay of treatment for metastatic lesions in the brain. The advent of stereotactic radiosurgery (SRS) offered a minimally invasive option to treat unresectable lesions.5 Compared with WBRT, SRS-related cognitive changes are extremely rare. Although SRS was previously limited to cases with fewer than 3 brain lesions, its use is expanding to patients with more lesions but a low volume of disease to enhance quality of life.
NCCN convened a multidisciplinary panel of leading experts from NCCN Member Institutions to develop and continually update guidelines for the treatment of CNS cancers, including metastatic brain lesions. The latest full version of these guidelines, which include a complete list of updates, is available on the NCCN Web site (NCCN.org). These NCCN Guidelines Insights highlight recent revisions in the management of brain metastases using SRS.
Stereotactic Radiosurgery
SRS is a technique that delivers a high dose of extremely focused radiation to small lesions, most frequently in a single session, but it can also be delivered in 2 to 5 fractions. It is becoming a desirable option because of reduced side effects and morbidity compared with both surgery and WBRT.
SRS for Multiple Metastatic Lesions
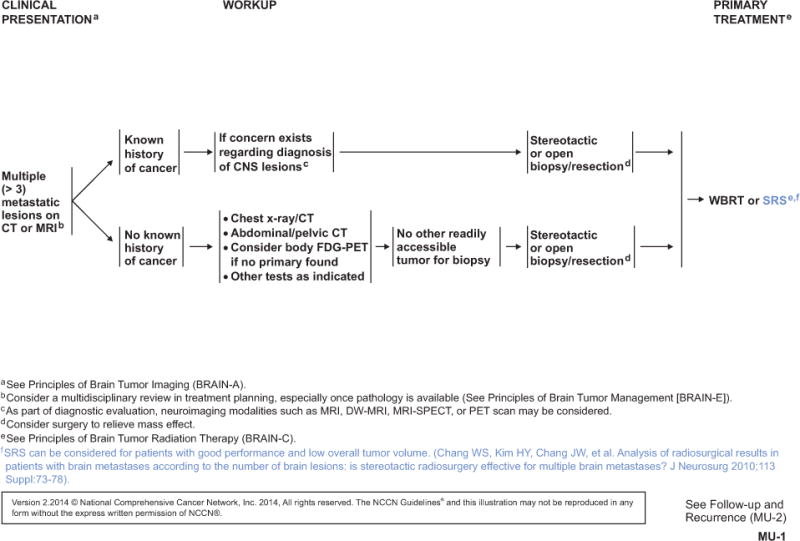
Accumulating evidence suggests that low disease volume is a better selection criterion for SRS than a low number of metastatic lesions. A multivariate analysis of 205 patients who received SRS for 4 or more brain metastases showed total treatment volume to be the most significant prognostic factor of survival, whereas the number of metastases did not reach significance.6 The same group conducted another analysis that identified a favorable subgroup of patients with a total treatment volume either less than 7 mL and fewer than 7 brain lesions. These patients had significantly prolonged median survival (13 months) compared with other patients (6 months; P<.00005).7 A cohort study revealed that patients with a total SRS-treated tumor volume of either less than 5 mL or 5 to 10 mL survived longer than those with a total treated volume greater than 10 mL.8 No survival difference was observed between patients with a single metastasis or multiple metastases. Another group analyzed patients divided by their number of brain lesions and found no difference in survival times or local control rates among the groups after SRS treatment.9 However, patients with more than 15 lesions had a higher risk of developing new lesions and distant disease progression.
Taken together, patients with multiple lesions but a low total volume of disease may be amenable to SRS. Additionally, patients with a favorable histology of the primary tumor (eg, breast cancer) or controlled primary tumors can often benefit from SRS regardless of the number of brain metastases present.10,11 Some brain metastases of radioresistant primary tumors, such as melanoma and renal cell carcinoma, have also been shown to achieve good local control with SRS.12 Other predictors of longer survival with SRS include younger age, good performance status, and primary tumor control.6,10,11,13
SRS Plus WBRT Versus WBRT Alone
The impact of SRS boost in addition to WBRT was evaluated in 2 published randomized controlled studies. The RTOG 9508 multi-institutional trial randomly assigned 333 patients with 1 to 3 brain metastases to WBRT plus SRS or WBRT alone.14 Despite the inclusion of larger tumors (3–4 cm) that are less favorable to SRS, the authors found a significant survival benefit in the combined arm (6.5 vs 4.9 months; P=.04) when treating a single metastasis; this benefit was not observed in patients with multiple (2 or 3) lesions. A much smaller trial of 27 patients with 2 to 4 lesions found no significant difference in survival, although SRS extended time to local failure (36 vs 6 months; P=.0005).15 Overall, no difference in overall survival was reported between the approaches in a meta-analysis of the 2 trials.16 However, the addition of SRS to WBRT significantly improved local control and performance status. SRS plus WBRT also prolonged the overall survival of patients with a single brain metastasis compared with those treated with WBRT alone (6.5 vs 4.9 months; P=.04).
SRS Plus WBRT Versus SRS Alone
In a randomized Japanese study of 132 patients with 1 to 4 metastatic brain tumors smaller than 3 cm, the addition of WBRT to SRS did not prolong median survival compared with SRS alone (7.5 vs 8.0 months, respectively).17 However, the 1-year brain recurrence rate was lowered in the WBRT plus SRS arm (47% vs 76%; P<.001). Another small randomized trial of 58 patients with 1 to 3 brain metastases was stopped early because of a significant decline in learning and memory function among the group receiving both SRS and WBRT compared with those receiving SRS alone (52% vs 24%).18 Analysis showed that SRS plus WBRT was associated with a better 1-year recurrence-free survival rate (73%) than SRS alone (27%). A third trial recruited 359 patients with 1 to 3 metastatic brain lesions who underwent surgery or SRS.19 They were randomized to either adjuvant WBRT or observation. Compared with the observation arm, intracranial relapse rates and neurologic mortality were lower in the WBRT arm, but overall survival and duration of functional independence were similar. A meta-analysis found no improvement in overall survival with the addition of WBRT to SRS.20
SRS Plus WBRT Versus Surgery Plus WBRT
Retrospective comparative studies showed that SRS plus WBRT resulted in equivalent, if not better, survival compared with surgery and WBRT.21–23 SRS also conferred a significant improvement in local control, especially for patients with radiosensitive tumors or solitary brain lesions. A prospective observational study of 1194 patients reported no difference in overall survival between patients with 2 to 4 metastatic brain lesions and those with 5 to 10 lesions treated with SRS alone (hazard ratio, 0.97; 95% CI, 0.81–1.18; P noninferiority <.0001).24 SRS alone compared with resection plus WBRT was evaluated in a randomized controlled trial by Muacevic et al,25 which was stopped prematurely because of poor accrual. In the final analysis based on 64 patients with solitary brain metastases, SRS alone was less invasive and resulted in equivalent survival and local control, but it was associated with a higher rate of distant relapse.
SRS for Recurrence
Several patient series have demonstrated local control rates greater than 70% with SRS in the recurrence setting for patients with good performance status and stable disease who have received prior WBRT.26–31
NCCN Recommendations
SRS has been an option for limited (1–3) metastatic brain lesions (see LTD-2, page 1519). The panel recently added SRS as a primary treatment option for multiple (>3) metastatic lesions, specifically in patients with good performance status and low overall tumor volume (see MU-1, page 1520). Maximum marginal doses of 24, 18, or 15 Gy according to tumor volume are recommended according to the RTOG 9005 protocol.32
Conclusions
These NCCN Guidelines Insights highlight important updates to the management of metastatic brain cancer with SRS in the NCCN Guidelines for Central Nervous System Cancers. The NCCN Guidelines are updated at least annually, and more often when new high-quality clinical data become available in the interim. The most up-to-date version of these continuously evolving guidelines is available online at NCCN.org. The recommendations in the NCCN Guidelines are based on evidence from clinical data, when available, and expert consensus of the NCCN panel. Independent medical judgment is required to apply these guidelines to individual patients to optimize care. The physician and patient have the responsibility to jointly explore and select the most appropriate option from among the available alternatives. When possible, consistent with NCCN philosophy, the panel strongly encourages patient/physician participation in prospective clinical trials.
NCCN Categories of Evidence and Consensus.
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B: Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate.
Category 3: Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
All recommendations are category 2A unless otherwise noted.
Clinical trials: NCCN believes that the best management for any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
Disclosure of Relevant Financial Relationships
Editor
Kerrin M. Green, MA, Assistant Managing Editor, JNCCN—Journal of the National Comprehensive Cancer Network, has disclosed that she has no relevant financial relationships.
CE Authors
Deborah J. Moonan, RN, BSN, Director, Continuing Education & Grants, NCCN, has disclosed that she has no relevant financial relationships.
Ann Gianola, MA, Manager, Continuing Education & Grants, NCCN, has disclosed that she has no relevant financial relationships.
Kristina M. Gregory, RN, MSN, OCN, Vice President, Clinical Information Operations, NCCN, has disclosed that she has no relevant financial relationships.
Rashmi Kumar, PhD, Senior Manager, Clinical Content, NCCN, has disclosed that she has no relevant financial relationships.
Individuals Who Provided Content Development and/or Authorship Assistance
Louis Burt Nabors, MD, Panel Chair, has disclosed that he has no relevant financial relationships.
Paul Brown, MD, Panel Member, has disclosed that he has no financial relationships.
Marc C. Chamberlain, MD, Panel Member, has disclosed that he has no financial relationships.
Nicole R. McMillian, MS, Guidelines Coordinator, NCCN, has disclosed that she has no relevant financial relationships.
Maria Ho, PhD, Oncology Scientist/Senior Medical Writer, NCCN, has disclosed that she has no relevant financial relationships.
Supported by educational grants from Eisai, Inc.; Millennium: The Takeda Oncology Company; Teva Pharmaceuticals; Bayer HealthCare Pharmaceuticals Inc.; Celgene Corporation; Endo Pharmaceuticals and HealthTronics; Genentech; and ARIAD Pharmaceuticals, Inc.
Footnotes
Please Note
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) are a statement of consensus of the authors regarding their views of currently accepted approaches to treatment. The NCCN Guidelines® Insights highlight important changes in the NCCN Guidelines® recommendations from previous versions. Colored markings in the algorithm show changes and the discussion aims to further understanding of these changes by summarizing salient portions of the panel’s discussion, including the literature reviewed.
The NCCN Guidelines Insights do not represent the full NCCN Guidelines; further, the National Comprehensive Cancer Network® (NCCN®) makes no representation or warranties of any kind regarding the content, use, or application of the NCCN Guidelines and NCCN Guidelines Insights and disclaims any responsibility for their applications or use in any way.
The full and most current version of these NCCN Guidelines are available at NCCN.org.
© National Comprehensive Cancer Network, Inc. 2014, All rights reserved. The NCCN Guidelines and the illustrations herein may not be reproduced in any form without the express written permission of NCCN.
References
- 1.Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 2.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 3.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22:3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 4.Eichler AF, Loeffiler JS. Multidisciplinary management of brain metastases. Oncologist. 2007;12:884–898. doi: 10.1634/theoncologist.12-7-884. [DOI] [PubMed] [Google Scholar]
- 5.Suh JH. Stereotactic radiosurgery for the management of brain metastases. N Engl J Med. 2010;362:1119–1127. doi: 10.1056/NEJMct0806951. [DOI] [PubMed] [Google Scholar]
- 6.Bhatnagar AK, Flickinger JC, Kondziolka D, Lunsford LD. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006;64:898–903. doi: 10.1016/j.ijrobp.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Bhatnagar AK, Kondziolka D, Lunsford LD, Flickinger JC. Recursive partitioning analysis of prognostic factors for patients with four or more intracranial metastases treated with radiosurgery. Technol Cancer Res Treat. 2007;6:153–160. doi: 10.1177/153303460700600301. [DOI] [PubMed] [Google Scholar]
- 8.Banfill KE, Bownes PJ, St Clair SE, et al. Stereotactic radiosurgery for the treatment of brain metastases: impact of cerebral disease burden on survival. Br J Neurosurg. 2012;26:674–678. doi: 10.3109/02688697.2012.690913. [DOI] [PubMed] [Google Scholar]
- 9.Chang WS, Kim HY, Chang JW, et al. Analysis of radiosurgical results in patients with brain metastases according to the number of brain lesions: is stereotactic radiosurgery effective for multiple brain metastases? J Neurosurg. 2010;113(Suppl):73–78. doi: 10.3171/2010.8.GKS10994. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson B, Hanssens P, Wolff R, et al. Thirty years’ experience with Gamma Knife surgery for metastases to the brain. J Neurosurg. 2009;111:449–457. doi: 10.3171/2008.10.JNS08214. [DOI] [PubMed] [Google Scholar]
- 11.Kased N, Binder DK, McDermott MW, et al. Gamma Knife radiosurgery for brain metastases from primary breast cancer. Int J Radiat Oncol Biol Phys. 2009;75:1132–1140. doi: 10.1016/j.ijrobp.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Hanson PW, Elaimy AL, Lamoreaux WT, et al. A concise review of the efficacy of stereotactic radiosurgery in the management of melanoma and renal cell carcinoma brain metastases. World J Surg Oncol. 2012;10:176. doi: 10.1186/1477-7819-10-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter GK, Suh JH, Reuther AM, et al. Treatment of five or more brain metastases with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:1394–1398. doi: 10.1016/j.ijrobp.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 15.Kondziolka D, Patel A, Lunsford LD, et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427–434. doi: 10.1016/s0360-3016(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 16.Patil CG, Pricola K, Sarmiento JM, et al. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database Syst Rev. 2012;9:CD006121. doi: 10.1002/14651858.CD006121.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 18.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 19.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118:2486–2493. doi: 10.1002/cncr.26515. [DOI] [PubMed] [Google Scholar]
- 21.O’Neill BP, Iturria NJ, Link MJ, et al. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55:1169–1176. doi: 10.1016/s0360-3016(02)04379-1. [DOI] [PubMed] [Google Scholar]
- 22.Rades D, Kueter JD, Veninga T, et al. Whole brain radiotherapy plus stereotactic radiosurgery (WBRT+SRS) versus surgery plus whole brain radiotherapy (OP+WBRT) for 1–3 brain metastases: results of a matched pair analysis. Eur J Cancer. 2009;45:400–404. doi: 10.1016/j.ejca.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 23.Schoggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien) 2000;142:621–626. doi: 10.1007/s007010070104. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 25.Muacevic A, Wowra B, Siefert A, et al. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol. 2008;87:299–307. doi: 10.1007/s11060-007-9510-4. [DOI] [PubMed] [Google Scholar]
- 26.Akyurek S, Chang EL, Mahajan A, et al. Stereotactic radiosurgical treatment of cerebral metastases arising from breast cancer. Am J Clin Oncol. 2007;30:310–314. doi: 10.1097/01.coc.0000258365.50975.f6. [DOI] [PubMed] [Google Scholar]
- 27.Loeffler JS, Kooy HM, Wen PY, et al. The treatment of recurrent brain metastases with stereotactic radiosurgery. J Clin Oncol. 1990;8:576–582. doi: 10.1200/JCO.1990.8.4.576. [DOI] [PubMed] [Google Scholar]
- 28.Noel G, Medioni J, Valery CA, et al. Three irradiation treatment options including radiosurgery for brain metastases from primary lung cancer. Lung Cancer. 2003;41:333–343. doi: 10.1016/s0169-5002(03)00236-8. [DOI] [PubMed] [Google Scholar]
- 29.Noel G, Proudhom MA, Valery CA, et al. Radiosurgery for re-irradiation of brain metastasis: results in 54 patients. Radiother Oncol. 2001;60:61–67. doi: 10.1016/s0167-8140(01)00359-0. [DOI] [PubMed] [Google Scholar]
- 30.Sheehan J, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for patients with recurrent small cell lung carcinoma metastatic to the brain: outcomes and prognostic factors. J Neurosurg. 2005;102(Suppl):247–254. doi: 10.3171/jns.2005.102.s_supplement.0247. [DOI] [PubMed] [Google Scholar]
- 31.Caballero JA, Sneed PK, Lamborn KR, et al. Prognostic factors for survival in patients treated with stereotactic radiosurgery for recurrent brain metastases after prior whole brain radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:303–309. doi: 10.1016/j.ijrobp.2011.06.1987. [DOI] [PubMed] [Google Scholar]
- 32.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]


