Abstract
C-arm cone-beam computed tomography (CBCT) can be used to visualize tumor-feeding vessels and parenchymal staining during transcatheter arterial chemoembolization (TACE). To capture these two phases, all current commercially available CBCT systems necessitate two separate contrast-enhanced scans. In this feasibility study, we report initial results of novel software that enhanced our current CBCT system to capture these two phases using only one contrast injection. Novelty of this work is the addition of software that enabled the acquisition of two sequential, back-to-back CBCT scans (dual-phase CBCT, DPCBCT) so both tumor feeding vessels and parenchyma are captured using only one contrast injection. To illustrate our initial experience, DPCBCT was used for TACE treatments involving lipiodol, drug-eluting beads, and Yttrium-90 radioembolizing microspheres. For each case, the DPCBCT images were compared to pre-intervention contrast-enhanced MR/CT. DPCBCT is feasible for TACE treatments and the preliminary results show positive correlation with pre-intervention conventional CT and MR. In addition, the degree of embolization can be monitored. DPCBCT is a promising technology that provides comprehensive visualization of tumor-feeding vessels and parenchymal staining using a single injection of contrast. DPCBCT could potentially be used during TACE to verify catheter position and monitor the embolization effect.
Keywords: Emoblization, imaging, 3-D, technology for surgical and interventional radiology
Introduction
Transcatheter arterial chemoembolization (TACE) has been shown to significantly improve patient survival from liver cancer (1–3). Visualization of tumor-feeding blood vessels and parenchymal space are important to determine catheter position and injection parameters for chemoembolization drug delivery, and can ultimately impact on treatment outcomes. The development of C-arm cone-beam computed tomography (CBCT) has greatly aided in the visualization of tumors (4–7). Like CT hepatic arteriography and CT arterial portography, the timing of contrast agent injection and image capture directly impacts on tumor conspicuity. In addition, acquiring images during early and late arterial blood flow phases has been shown to provide superior liver tumor conspicuity than imaging a single phase alone (8). Imaging these dual phases requires two contrast injections on current commercially available CBCT systems. In this work, we describe a novel dual-phase CBCT system that requires only a single contrast injection. We illustrate our initial experience with this technique where dual-phase CBCT was used for tumor visualization during three types of intra-arterial liver tumor treatments: TACE with lipiodol, with drug-eluting beads, and radioembolization with Yttrium-90 microspheres.
Material and methods
The study was approved by our institutional review board, complied with the Health Insurance Portability and Accountability Act, and patient informed consent was obtained. The imaging was performed using a commercially available angiographic system (Allura Xper FD20, Philips Healthcare, Best, the Netherlands). This system was equipped with the XperCT option, enabling C-arm cone-beam CT acquisition and volumetric image reconstruction (Feldkamp back projection (9)). For each CBCT scan, the area of interest was positioned in the system isocenter, and over approximately ten seconds, 312 projection images (30 frames per second) were acquired with the motorized C-arm covering a 200° clockwise arc at 20° per sec rotation speed. As the images were being acquired, the projections were transferred via fiber optic to the reconstruction computer to produce volumetric data. The image transfer and reconstruction were automatically done and typically take approximately 60–90 seconds. This processing time and the need to reset the C-arm to its starting rotation position make the XperCT suitable for singular CBCT scans that capture either the early or late arterial phase with a single contrast injection. If both phases are to be acquired, two separate scans using two total contrast injections are required. The novelty of this work is the addition of software that enables the acquisition of two sequential, back-to-back CBCT scans so both early and delayed arterial phases are captured using only one contrast injection. The Philips Healthcare software prototype was written in C++ and modifies the standard image acquisition procedure in three ways:
Image acquisition can occur with both clockwise and counter-clockwise C-arm rotations,
image reconstruction of both scans occurs after the completion of both scans, and
both reconstructed datasets are displayed side-by-side and also allow for simultaneous cine viewing.
The first modification eliminates the need to reset the C-arm to its starting rotation position, saving approximately ten seconds. The second modification further saves time (approximately 60–90 seconds) by reordering the workflow. The approximately total 70–100 seconds saved create the window for the second (delayed arterial) scan to capture parenchymal enhancement using the contrast injected from the first phase. The third modification allows for direct visual comparison between the two phases or between pre-and post-treatment images. This is an improvement over the standard software where only one scan can be displayed at a time.
In this feasibility study, the two scans were triggered at 3 and 28 sec following a selective single injection of undiluted contrast medium through a 3-French micro-catheter placed into the vessel expected to feed the tumor. The same contrast injection protocol was applied to all cases (amount: 20 ml, rate: 2 ml/sec, Oxilan 300 mg I/mL, Guerbet LLC, Bloomington, IN, USA). Representative dual-phase CBCT images of ultrafluid lipiodol (79-year-old female; Lipiodol, Therapex, E-Z-EM, Montreal, Canada), drug-eluting beads (59-year-old-male; DC Bead, Biocompatibles, Surrey, United Kingdom), and radiosphere (58-year-old-male; Yttrium-90 radioactive microspheres, TheraSpheres, MDS Nordion, Ottawa, Canada) based TACE were selected to show tumor-feeding vessels and parenchymal conspicuity for a range of treatments. In addition, pre-intervention contrast-enhanced CT (arterial and venous phase timing) or MR images acquired at 20 and 70 seconds after contrast injection, similarly enhancing the early and late arterial phases as the dual-phase CBCT, were also shown at approximately the same slice level for comparison.
X-ray dose measurements were made in a porcine model to understand the amount of radiation imparted by an additional CBCT scan in dual-phase CBCT. One cannot simply double the dose from a single CBCT to obtain dose measurements in dual-phase CBCT because the C-arm movement geometry (clock-wise and counter-clockwise rotations) is different. This was done in an animal so that multiple dual-phase scans could be made with statistical power, and because there are no radiation exposure concerns unlike with humans. Animal care and use committee approval was obtained. X-ray dose and exposure measurements were made under the same x-ray imaging conditions on a 31.8 kg pig. Three measurements of dosimetry were done in the centreline of the X-ray path over the liver. For the dose measurements, two dosimeters (Model DMC 2000 XB, MGP Instruments, Smyrna, Georgia USA) were used with one placed on top (entrance dose) of the pig and the other below (exit dose). The absorbed dose was calculated as entrance dose minus exit dose. For the exposure measurements, the ionization chamber (Model 451-P, Fluke Biomedical, Everett, WA, USA) was placed on the imaging table adjacent to the right side of the pig.
Results
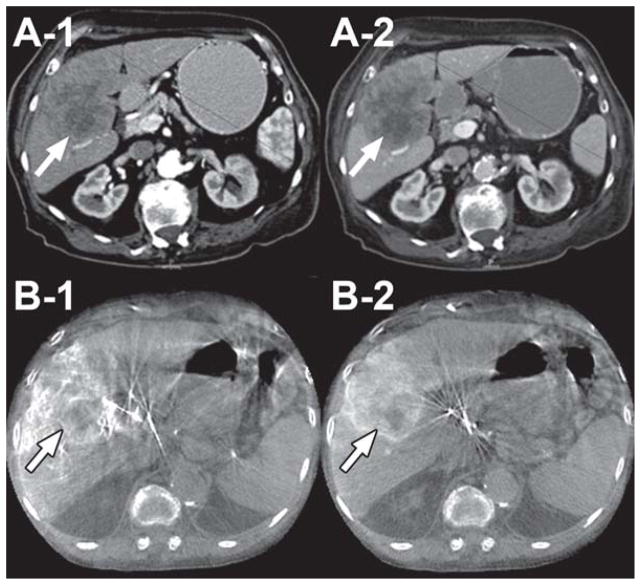
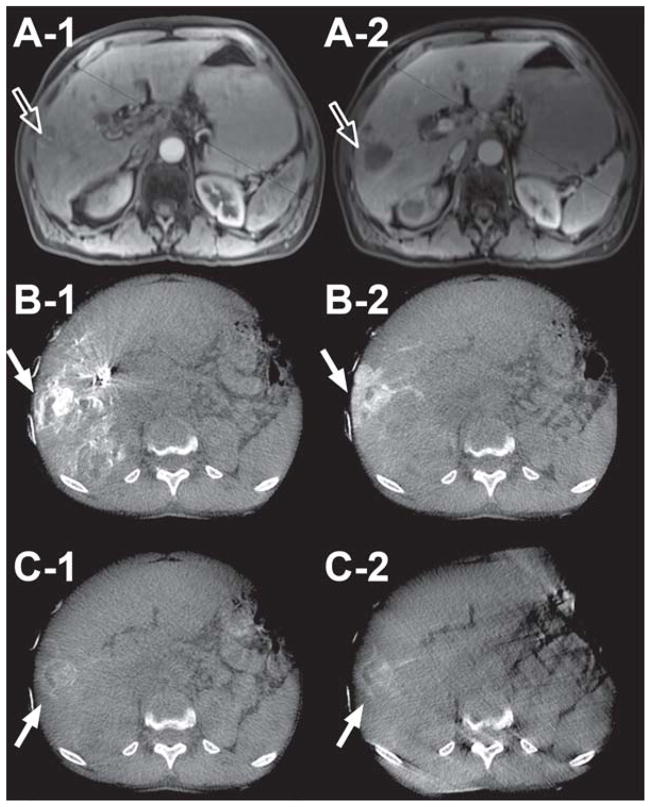
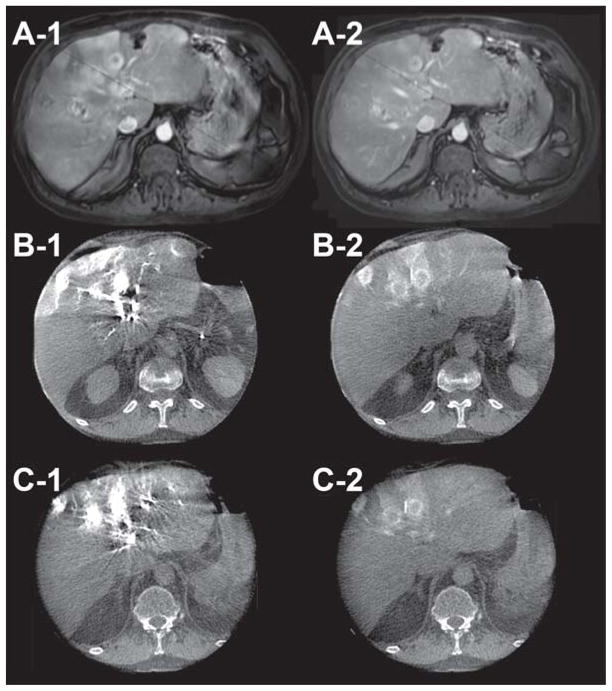
Dual-phase CBCT imaging was technically successful. Feeding vessels and tumor parenchyma were visualized using the same time delay and injection protocol for all of the successfully imaged patients. The value of this technique is illustrated in the following cases. The first case is a patient with poorly differentiated liver carcinoma, treated with lipiodol. In Figure 1, A-1 and A-2 are the arterial and venous phase contrast-enhanced diagnostic CT images, respectively. B-1 and B-2 are the pre-lipiodol treatment early and late arterial dual-phase CBCT images, respectively. The reported hepatic arteriography with the use of dual-phase CBCT technology showed the superiority of the late arterial phase image (B-2) in visualizing peripheral enhancement of the tumor compared to both the early arterial phase scan of the dual-phase (B-1) and the pre-TACE venous phase of the contrast-enhanced conventional diagnostic CT (A-2). The second case is a patient treated with drug-eluting beads. Figure 2 illustrates the ability of the dual-phase CBCT to identify the tumor-feeding vessels and to show parenchymal conspicuity. In Figure 2, A-1 and A-2 are pre-intervention-contrast enhanced MR images at 20 and 70 sec post-contrast injection, respectively. B-1, -2 and C-1, -2 are pre-and post-drug eluting bead administration, respectively, of early (B-1, C-1) and late (B-2, C-2) arterial dual-phase CBCT images. The pre-treatment, early arterial axial CBCT image (B-1) showed complete nodular enhancement of the hepatocellular carcinoma (HCC) lesions, indicating highly vascularized tumor tissue. After treatment, peripheral enhancement is only seen around the HCC nodule (C-1, -2). In addition, there was a lack of feeding vessel enhancement, suggesting complete embolization of the feeding arteries and successful therapy. The third case is a patient with multiple metastatic pancreatic neuroendocrine tumors. A-1 and A-2 in Figure 3 show pre-intervention contrast-enhanced MR images at 20 and 70 sec post-contrast injection, respectively. B-1, -2 and C-1, -2 are pre- and post-radioembolization with Y-90 microspheres, respectively, of early (B-1, C-1) and late (B-2, C-2) arterial dual-phase CBCT images. Dual-phase CBCT early (B-1) and late (B-2) arterial phase pre-treatment images showed better visual characterization of the number and volume of tumors, along with improved enhancement compared to pre-intervention contrast-enhanced MR images (A-1, A-2). After radioembolization, the late arterial phase (C-2) dual-phase CBCT showed decreased but persistent tumor perfusion, indicating that the embolic effect from radioembolization was minimal compared to pre-treatment (B-2) and allows for retreatment if indicated.
Figure 1.
70-year-old female with poorly differentiated carcinoma (Child-Pugh score = 7/Class B, no ascites, no cirrhosis) in liver segments 5 and 6 (arrows). A-1 and A-2 are arterial and venous phase contrast-enhanced CT images, respectively. B-1 and B-2 are early and late arterial dual-phase CBCT images, respectively, immediately before lipiodol treatment. Note how the late arterial phase image (B-2) shows very clear tumor parenchymal space, border, and matching with the CT image (A-2).
Figure 2.
59-year-old male patient with HCC (Child-Pugh score = 6/Class A, no ascites, cirrhosis present) in liver segments 2, 4a, 7, and 8 (arrow outlines). A-1 and A-2 are pre-intervention contrast enhanced MR images at 20 and 70 sec post-contrast injection, respectively. B-1, -2 and C-1, -2 are pre- and post-drug eluting bead administration, respectively, of early (B-1 & C-1) and late (B-2 & C-2) arterial dual-phase CBCT images. Note the change in tumor enhancement and lack of feeding vessel enhancement before and after bead embolization for both early and late arterial images (solid arrows) in the axial slices, indicating successful embolization.
Figure 3.
58-year-old male with metastatic pancreatic neuroendocrine tumors (Child-Pugh score = 5/Class A, no ascites, no cirrhosis) in liver segments 4, 6, and 8 who had not received any previous systemic therapy. A-1 and A-2 are pre-intervention contrast-enhanced MR images at 20 and 70 sec post-contrast injection, respectively. B-1, -2 and C-1, -2 are pre- and post-radioembolization with Y-90 microspheres (TheraSpheres), respectively, of early (B-1, C-1) and late (B-2, C-2) arterial dual-phase CBCT images. Note the similarity of tumor conspicuity and perfusion between the pre- and post-radioembolization in the dual-phase CBCT images (B-1 to C-1 and B-2 to C-2). This indicates minimal embolization and permits retreatment if indicated.
For the X-ray dosimetry conducted on the porcine model, the entrance dose was 141.7 ± 0.6 mrem, exit dose was 64.7 ± 0.6 mrem, absorbed dose was 77.0 ± 0.0 mrem, and exposure was 17.7 ± 2.1 mR per CBCT scan (total of 566.8 mrem, 258.8 mrem, 308.0 mrem, and 70.8 mR for entrance dose, exit dose, absorbed dose, and exposure, respectively for two dual-phase CBCT scans), similar to what was obtained in other work (10).
Discussion
C-arm CT technology allows for the acquisition of a three-dimensional (3D) dataset generated from one rotational run with the use of cone-beam CT principles. It has been shown that CBCT imaging can provide more information than DSA alone for trans-catheter arterial chemoembolization (4–8). Similarly, early and on-going clinical experience has demonstrated that CBCT using dual-phase imaging can provide better information than DSA for TACE and, occasionally, conventional CT or MR about the number and distribution of tumors because of the hyperacute nature of the arterial contrast medium bolus in enhancing hepatomas which by nature are predominately supplied by the hepatic artery as opposed to the parenchyma/portal vein for healthy tissue. The dual-phase CBCT scan uses an intra-arterial contrast injection whereas conventional CT and MR use a peripheral injection. This results in more effective and safer interventions (4–6). The cases illustrated in this work demonstrate several potential clinical advantages of dual-phase CBCT. The novelty of the technology presented in this article is the possibility for tumor imaging at both early (tumor feeding vessels enhancement) and delayed arterial (tumor parenchymal enhancement) phases using only one intra-arterial injection of contrast medium. This improvement reduces the use of iodinated contrast medium by 50% for the CBCT component of the interventional procedure while allowing for visualization of lipiodol-targeted regions and visualization of changes in temporal enhancement before and after drug-eluting beads and radiospheres treatment. Furthermore, the simultaneous display of both phases side-by-side (as shown in the Figures) and with cine through the slices allows for direct visual comparison between the two phases or between pre- and post-treatment images. The former visual comparison (early vs. late) could provide an idea of the vascular density of the tumor in relation to the feeding vessel while the second comparison (pre- vs. post-) could provide an idea of the enhancement change due to embolization. The X-ray effective dose from CBCT imaging is significantly less than a typical abdomen scan using diagnostic multi-detector CT (675 mrem), even when four CBCT scans at total of 308 mrem (2 dual-phase acquisitions: One pre- and one post-embolization) are performed (10).
Our technique continues to evolve as we gain more experience with this novel CBCT application. For example, three kinds of adjustments are under study for helping to minimize image artifacts, and to optimize the contrast injection and imaging protocol:
Reducing the scan time while emphasizing patient compliance for breath-holds (respiratory motion results in image streak artifacts),
improving post-acquisition image processing to provide motion compensation and metal artifact correction, and
using diluted contrast media to potentially reduce artifacts in the early arterial phase.
Studies are underway to evaluate this technique in a larger population and to optimize image acquisition and injection timing relative to catheter location and tumor type. In conclusion, dual-phase CBCT is feasible and has the potential for routine use during TACE to visualize tumor characteristics and monitor embolization outcome.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Llovet J, Burroughs A, Bruix J. Hepatocellular Carcinoma. The Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–9. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Ngan H, Lai CL, Fan ST, Lai ECS, Yuen WK, Tso WK. Treatment of inoperable hepatocellular carcinoma by transcatheter arterial chemoembolization using an emulsion of cisplatin in iodized oil and gelfoam. Clin Radiol. 1993;47:315–20. doi: 10.1016/s0009-9260(05)81446-1. [DOI] [PubMed] [Google Scholar]
- 4.Wallace MJ. C-arm computed tomography for guiding hepatic vascular interventions. Tech Vasc Interv Radiol. 2007;10:79–86. doi: 10.1053/j.tvir.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Wallace MJ, Kuo MD, Glaiberman C, Binkert CA, Orth RC, Soulez G. Three-dimensional C-arm cone-beam CT: applications in the interventional suite. J Vasc Interv Radiol. 2008;19:799–813. doi: 10.1016/j.jvir.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Miyayama S, Yamashiro M, Okuda M, Yoshie Y, Sugimori N, Igarashi S, et al. Usefulness of cone-beam computed tomography during ultraselective transcatheter arterial chemoembolization for small hepatocellular carcinomas that cannot be demonstrated on angiography. Cardiovasc Intervent Radiol. 2009;32:255–64. doi: 10.1007/s00270-008-9468-4. [DOI] [PubMed] [Google Scholar]
- 7.Miyayama S, Matsui O, Yamashiro M, Ryu Y, Takata H, Takeda T, et al. Detection of hepatocellular carcinoma by CT during arterial portography using a cone-beam CT technology: comparison with conventional CTAP. Abdom Imaging. 2009;34:502–6. doi: 10.1007/s00261-007-9254-9. [DOI] [PubMed] [Google Scholar]
- 8.Meyer BC, Frericks BB, Voges M, Borchert M, Martus P, Justiz J, et al. Visualization of hypervascular liver lesions During TACE: comparison of angiographic C-arm CT and MDCT. AJR Am J Roentgenol. 2008;190:W263–9. doi: 10.2214/AJR.07.2695. [DOI] [PubMed] [Google Scholar]
- 9.Feldkamp LA, Davis LC, Kress JW. Practical cone-beam algorithm. J Opt Soc Am. 1984;A1:612–19. [Google Scholar]
- 10.Racadio J, Yoshizumi T, Toncheva G, Stueve D, Anderson-Evans C, Frush D. Radiation dosimetry evaluation of C-arm cone beam CT for pediatric interventional radiology procedures : a comparison with MDCT. RSNA; Chicago, IL, USA: 2008. [Google Scholar]