Abstract
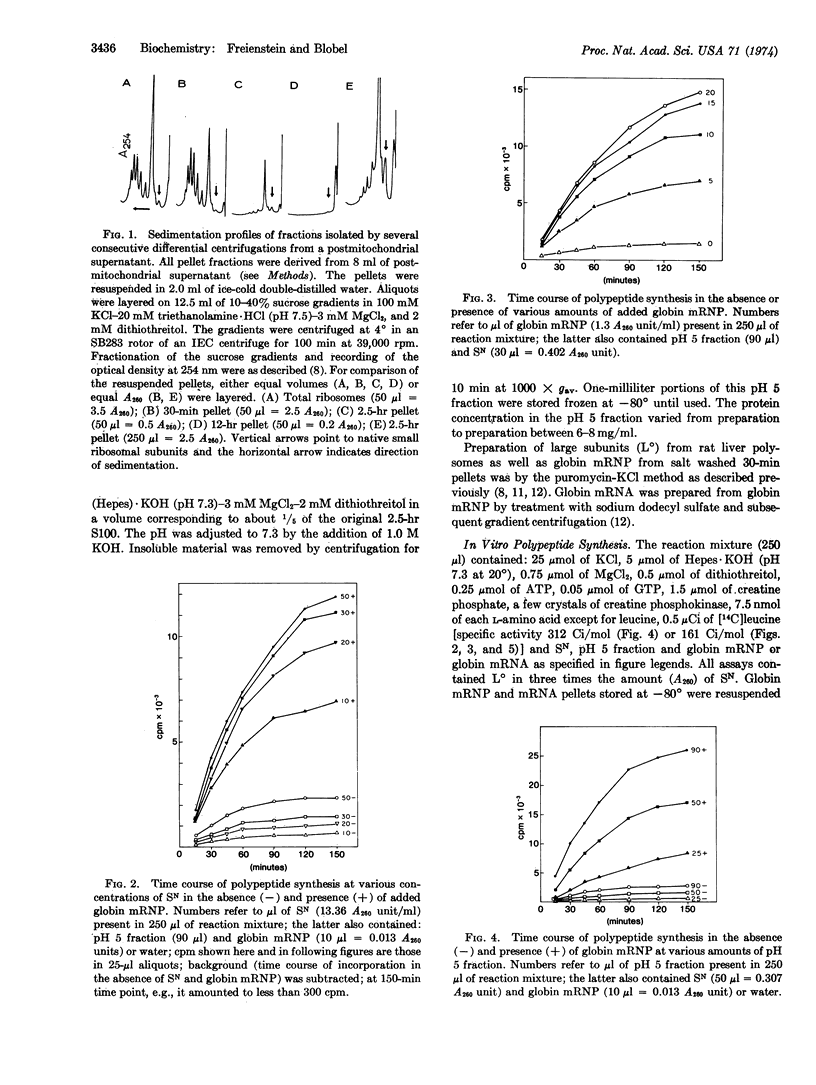
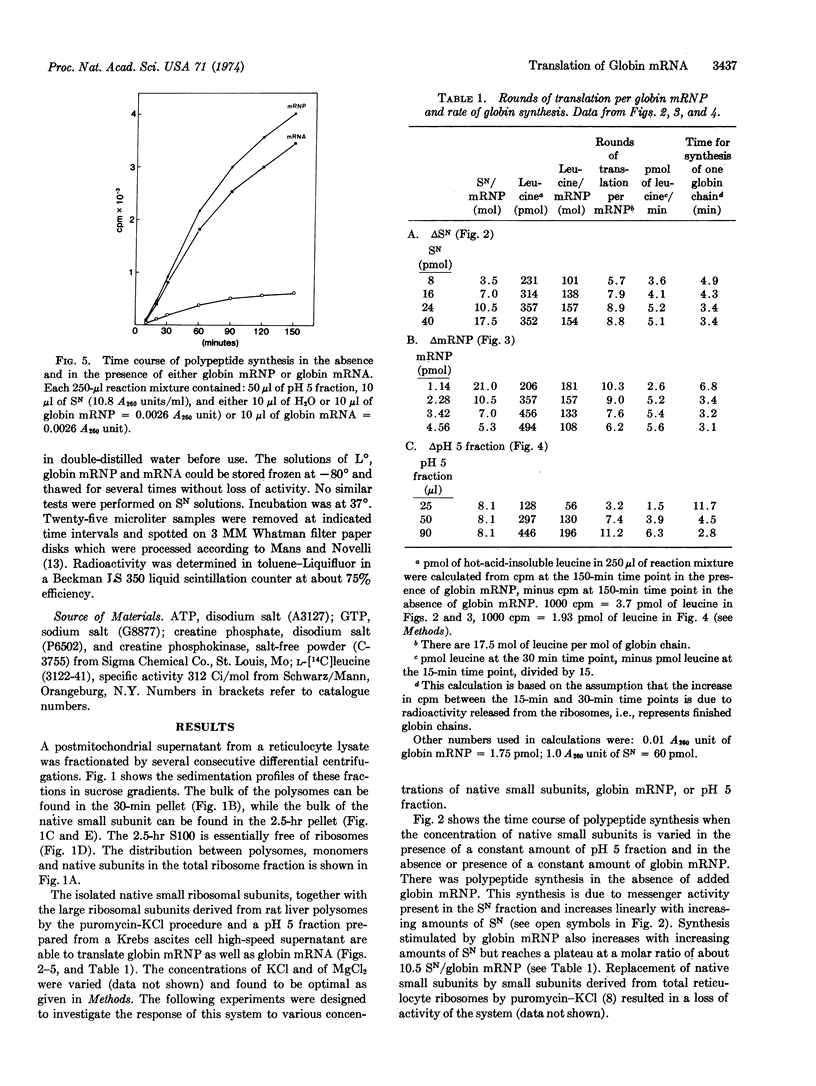
A highly active in vitro system for the translation of globin mRNA, resulting in more than 10 rounds of translation, is described. The reconstituted system consists of native small ribosomal subunits of rabbit reticulocytes (as a source of initiation factors as well as small ribosomal subunits), large subunits derived from rat liver polysomes by the puromycin-KCl procedure, and a pH 5 fraction obtained from a Krebs ascites cell high speed supernatant. In this system no differences were found between globin messenger ribonucleoprotein and globin mRNA.
Keywords: rabbit reticulocytes, rat liver polysomes, Krebs ascites pH 5 supernatant
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Howard G. A., Herbert E. The ribosome cycle in a reconstituted cell-free system from reticulocytes. Cold Spring Harb Symp Quant Biol. 1969;34:547–554. doi: 10.1101/sqb.1969.034.01.062. [DOI] [PubMed] [Google Scholar]
- BORSOOK H., DEASY C. L., HAAGENSMIT A. J., KEIGHLEY G., LOWY P. H. Incorporation in vitro of labeled amino acids into proteins of rabbit reticulocytes. J Biol Chem. 1952 May;196(2):669–694. [PubMed] [Google Scholar]
- Bishop J. O. Initiation of haemoglobin polypeptide chains. Biochim Biophys Acta. 1966 Apr 18;119(1):130–145. doi: 10.1016/0005-2787(66)90045-1. [DOI] [PubMed] [Google Scholar]
- Blobel G. A protein of molecular weight 78,000 bound to the polyadenylate region of eukaryotic messenger RNAs. Proc Natl Acad Sci U S A. 1973 Mar;70(3):924–928. doi: 10.1073/pnas.70.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. Protein tightly bound to globin mRNA. Biochem Biophys Res Commun. 1972 Apr 14;47(1):88–95. doi: 10.1016/s0006-291x(72)80014-7. [DOI] [PubMed] [Google Scholar]
- Blobel G. Release, identification, and isolation of messenger RNA from mammalian ribosomes. Proc Natl Acad Sci U S A. 1971 Apr;68(4):832–835. doi: 10.1073/pnas.68.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnbrough C., Hunt T., Jackson R. J. A complex between met-tRNA F and native 40S subunits in reticulocyte lysates and its disappearance during incubation with double-stranded RNA. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1556–1564. doi: 10.1016/0006-291x(72)90891-1. [DOI] [PubMed] [Google Scholar]
- Darnbrough C., Legon S., Hunt T., Jackson R. J. Initiation of protein synthesis: evidence for messenger RNA-independent binding of methionyl-transfer RNA to the 40 S ribosomal subunit. J Mol Biol. 1973 May 25;76(3):379–403. doi: 10.1016/0022-2836(73)90511-1. [DOI] [PubMed] [Google Scholar]
- Eisenstadt J. M., Brawerman G. The role of the native subribosomal particles of Escherichia coli in polypeptide chain initiation. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1560–1565. doi: 10.1073/pnas.58.4.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvey A. K., Staehelin T. Structure and function of mammalian ribosomes. I. Isolation and characterization of active liver ribosomal subunits. J Mol Biol. 1970 Oct 14;53(1):1–19. doi: 10.1016/0022-2836(70)90042-2. [DOI] [PubMed] [Google Scholar]
- Hirsch C. A., Cox M. A., van Venrooij W. J., Henshaw E. C. The ribosome cycle in mammalian protein synthesis. II. Association of the native smaller ribosomal subunit with protein factors. J Biol Chem. 1973 Jun 25;248(12):4377–4385. [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- KNOPF P. M., LAMFROM H. CHANGES IN THE RIBOSOME DISTRIBUTION DURING INCUBATION OF RABBIT RETICULOCYTES IN VITRO. Biochim Biophys Acta. 1965 Mar 15;95:398–407. doi: 10.1016/0005-2787(65)90186-3. [DOI] [PubMed] [Google Scholar]
- Lubsen N. H., Davis B. D. Use of purified polysomes from rabbit reticulocytes in a specific test for initiation factors. Proc Natl Acad Sci U S A. 1974 Jan;71(1):68–72. doi: 10.1073/pnas.71.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti-Rosina R., Eisenstadt A., Eisenstadt J. M. Isolation of protein initiation factors from 30S ribosomal subunits. Nature. 1969 Jan 25;221(5178):363–365. doi: 10.1038/221363a0. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]




