Abstract
The contribution of plasma prekallikrein (PK) to vascular remodeling is becoming increasingly recognized. Plasma PK is activated when the zymogen PK is digested to an active enzyme by activated factor XII (FXII). Here, we present our findings that vascular smooth muscle cells (VSMC) activate plasma PK in the absence of FXII. Extracted plasma membrane and cytosolic fractions of VSMCs activate PK, but the rate of PK activation was greater by the membrane fraction. FXII neutralizing antibody did not affect PK activation by extracted proteins of VSMCs. VSMC PKA was inhibited by the serine protease inhibitors such as aprotinin, phenylmethylsulfonyl fluoride, leupeptin and CTI with CI50 of 0.78 µM, 1 mM, 3.13 µM and 40 nM on the cultured cells, respectively. No inhibition of PK activation by cysteine, aspartic acid, and metalloprotease inhibitors was observed. This is the first report of the presence of an intrinsic PKA in VSMC. Considering that VSMCs are normally separated from the circulating blood by endothelial cells, direct PK activation by VSMCs may play a role in disease states like diabetes, hyperlipidemia or hypertension where endothelial layer is damaged.
Introduction
Atherosclerosis is the leading cause of death in diabetes, and a major source of morbidity and mortality. Early atherosclerotic lesions are characterized by endothelial dysfunction, impaired endothelium-dependent relaxation of blood vessels, accumulation of inflammatory cells, VSMC proliferation and migration and extracellular matrix deposition in the vessel wall (1–3). The role of impaired endothelium-dependent vasodilation and the mechanisms underlying its dysfunction in vascular disease remain unknown. However, evidence indicates that abnormalities in endothelial synthesis and release of nitric oxide may contribute to vascular complications (4).
The localization of kinin receptors within the vascular wall and their activation by bradykinin (BK) implies a role for this system in the regulation of vascular tone and ultrastructure. Two forms of the kallikrein-kinin system (KKS) exist, one in tissue and the other in plasma. Tissue kallikrein, mainly expressed in the pancreas and salivary glands but also in other tissues such as kidney, vasculature (VSMC) and brain, acts on low and high molecular weight kininogen substrate to release Lysyl-BK (5). The plasma KKS, which includes factor XII, prekallikrein and high molecular weight kininogen (HMWK), has been linked to the activation of the intrinsic pathway of blood clotting. Plasma PK circulates in an inactive form, complexed with HMWK (6). BK, the principal effector of the KKS system, can be generated both systemically and locally within the vessel wall (7–10). Thus, BK can act in a paracrine or autocrine manner to influence vascular function.
The relevance and significance of kinin-mediated vascular growth and dysfunction is greater if there is accelerated kinin generation in populations at risk for vascular disease. Increased circulating levels of KKS components in patients at risk for vascular disease would provide evidence for heightened system activity and may point to a potential role in vascular disease. We have previously shown that type 1 diabetic patients at risk for developing macrovascular disease (those with altered glomerular hemodynamics who are at high risk for subsequent nephropathy) show increased renal kallikrein and kinin production (11). Furthermore, we demonstrated that circulating levels of plasma PK are increased in type 1 diabetic patients who are hypertensive. This increase in plasma PK levels was associated with an increase in albumin excretion rate (12). These observations together with our recent discoveries that BK promotes VSMC remodeling, provide evidence for the involvement of the plasma KKS as a modulator of vascular disease risk in diabetes (13–17).
In normal plasma, prekallikrein circulates as a bimolecular complex with HMWK (18). Recent studies have identified a binding site or receptor for kininogen on endothelial cells (19). This kininogen binding site was later identified to be a multiprotein kininogen receptor that consists of cytokeratin 1, urokinase plasminogen activator receptor and gC1qR (20). Once kininogen is bound to endothelial cells, it serves as a binding site for prekallikrein. Binding of prekallikrein to endothelial cells results in its activation to kallikrein via propylcarboxypeptidase (PRCP) (21,22). The generation of active kallikrein on endothelial cells then cleaves its receptor and substrate, HMWK, to release BK, which in turn stimulates the release of modulators of vessel wall function and ultrastructure such as nitric oxide and prostacyclin (22).
Here, we describe a novel mechanism of plasma PK activation by VSMC. Unlike endothelial cells, activation of plasma PK by VSMC occurs irrespective of HMWK binding to the surfaces of VSMC. Furthermore, our data reveal that the plasma PK activator in VSMC is not PRCP, the plasma PK activator identified on endothelial cells. Understanding the processes of activation of plasma prekallikrein may provide insights into the mechanisms through which plasma PK regulates the vasculature and hence lead to novel strategies for intervention.
Methods
Cell Culture
Rat aortic VSMC from male Sprague-Dawley rats (Charles-River, Laboratories, Wilmington, MA) were prepared as previously described (13). A 2-cm segment of artery cleaned of fat and adventitia was incubated in 1 mg/ml collagenase for 3h at room temperature. The artery was then cut into small sections and fixed to a culture flask for explantation in minimal essential media (MEM) containing 10% fetal calf serum (FCS), 1% non-essential amino acids, 100 mU/ml penicillin and 100 µg/ml Streptomycin. Cells were incubated at 37°C in a humidified atmosphere of 95% air −5% CO2. Medium was changed every 3–4 days and cells were passaged every 6–8 days by harvesting with trypsin-EDTA. VSMC were identified by the following criteria. They stained positive for intracellular cytoskeletal fibrils of actin and smooth muscle cell specific myosin and negative for factor VIII antigens. VSMC isolated by this procedure were homogeneous and were used in all studies between passages 2–6. The studies were done in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996) and approved by the MUSC Institutional Animal Care and Use Committee.
Human aortic vascular smooth muscle cells (HAVSMs), human aortic endothelial cells (HAECs) and human umbilical vein endothelial cells (HUVECs) were purchased from BioWhittaker (Walkersville, MD). They are subcultured in gelatin coated dishes using endothelial basal medium-2 (EBM-2) with SingleQuots (Catalog No. CC4176) from BioWhittaker (Walkersville, MD), containing 2% FBS and 1% AAS. All cells were used from the third passage.
Extraction of Cellular Proteins
VSMCs were subcultured in the 100mm culture dishes until 80% confluent. After washing with ice cold PBS, the cells were harvested with 150µl of isolation buffer (10mM Tris-HCL, 250mM sucrose, pH 7.5) on ice. The cell suspensions were further homogenized using 27-gauge needle and 1mm-syringe. No detergent was used in this procedure. Cell homogenates were centrifuged for 10min at 40°C and the supernatants were ultracentrifuged at 45,000 rpm for 60min at 4°C (Beckman Optima TM TLX ultracentrifuge, TLX-45 Fixed Angle Rotor). The supernatants were collected as the cytosolic fraction of the cellular proteins and kept at −80°C until used. The pellets from ultracentrifugation were resuspended and homogenized in 50µl of lysis buffer (50 mmol/LTris-HCl, 150 mmol/L NaCl, 0.1% SDS, 0.02% Sodium azide,1% NP 40 (IGEPAL), 0.5% sodium deoxycholate, 1mmol/L PMSF, and protease inhibitor cocktail, pH 8.0) on the ice. The homogenates were centrifuged at 2,000 rpm for 15min at 4°C. The supernatants were then centrifuged at 30,000 rpm for 30 min at 4°C. The supernatants were collected for as the plasma membrane fraction of cellular proteins and kept at −80°C until used. The protein concentration of each fraction of the cellular protein was measured using bicinchoninic acid (BCA) method.
Western Blotting of Plasma Prekallikrein
VSMCs cultured in 6-well plates to 80% confluence, were incubated with plasma prekallikrein and/or factor XII and plasma prekallikrein for various times. Conditioned media from the cells was collected and subjected to SDS-PAGE (12%), under reducing conditions and transferred onto nitrocellulose membrane (BioRad, Hercules, CA) with semi-dry transfer method (Biorad, Hercules, CA) at 20V for 50 min. The membranes were immunoblotted with anti-plasma prekallikrein polyclonal antibody (1:1,000 dilution, Enzyme Research Laboratories, South Bend, IN) overnight at 4°C followed by incubating the membranes in a secondary antibody conjugated to horse radish peroxidase (HRP). The immunoreactive bands were visualized using the chemiluminiscence reagent Renaissance (New England Nuclear Life Science Products, Boston, MA), according to the procedure described by the supplier. Membranes were exposed to Kodak LS film and bands were measured by densitometry and quantified by Scion Image program (Scion Corporation, Frederick, MA).
Plasma Prekallikrein Activation Assay
1~2×104 VSMCs were subcultured on Falcon® 96-well sterile polystyrene tissue culture plates (Becton Dickinson, Franklin Lakes, NJ) for one to two days for the cells to become greater than 95% confluent. Cells were starved by incubating in 200 µl of serum-free EMEM for 24 hrs. For endothelial cells, 2×104 HUVECs and HAECs were subcultured on the same 96-well tissue culture polystyrene plates with or without gelatin coating. Assays were performed in Hepes buffer (10 mM Hepes, 137 mM NaCl, 4 mM KCl, 11 mM D-glucose, 0.5 mg/ml RIA grade BSA, pH 7.4) at room temperature using 0.4 mM of S-2302 (H-D-Prolyl-L-Phenylalanyl-L-arginine-p-nitroaniline dihydrochloride), a relatively specific chromogenic substrate for plasma kallikrein (Chromogenix, Milano, Italy). After washing the cells with 200µl of warm Hepes buffer 3× at room temperature, the cells were incubated with 3.58 nM human plasma PK (Enzyme Research Laboratories, South Bend, IN) in Hepes buffer. 2.40 nM human plasma FXII (Enzyme Research Laboratories, South Bend, IN) and 4.28 nM human plasma HMWK (Enzyme Research Laboratories, South Bend, IN) were used in controls. These concentrations were chosen by applying the ratios among the plasma concentrations of the three components of KKS, FXII and HMWK; PK 50 µg/mL (670 nM), FXII 30 µg/mL (375 nM) and HK 80 µg/mL (560 nM). Proper activation of PK on the cultured VSMCs was obtained with these concentrations. The optical density (OD) (absorbance at 405 nm) of p-nitroaniline was measured at 2 min intervals using a SpectraMax 340PC (Molecular Devices, Sunnyvale, CA) to follow the kinetics of PK activation. Vmax was defined as the maximum slope of the kinetic display of optical density versus time and milli-optical density per minute (mOD/min) was applied in our experiment. In detail, Vmax represents a steepest slope of a segment of 11 contiguous points during the reaction time given; the slopes were calculated by applying linear regression of line segments consisting of time points 1 to 11, 2 to 12, 3 to 13 and so forth. The ODmax of our experiment was 0.8 at 405 nm when we used 0.4 mM of S-2302. To characterize the nature of PK activator on VSMCs, PK activation was done in the presence or absence of various protease inhibitors such as corn trypsin inhibitor (CTI), aprotinin, phenylmethylsulfonyl fluoride (PMSF), leupeptin, cystatin, pepstatin, ethylenediaminetetraacetic acid (EDTA) and ethylenle glycol-bis(β-aminoethylether)-N,N,N’,N’-tetraacetic acid (EGTA). To test the effect of CTI and anti-FXII antibody on the activation of PK on the cells or cell-free systems, CTI and anti-FXII antibody were pretreated with cultured VSMCs or extracted proteins from VSMCs for 30 min at room temperature.
Statistical Analysis
Data were expressed as mean±SE and analyzed by analysis of variance for repeated measures. For comparisons involving more than two experimental groups, one way ANOVA using the Tukey-Kramer multiple comparison tests were employed to determine statistical significance using SAS (Statistical Analysis Systems). The rates of PK activation between groups were compared by using Vmax from kinetic data. The differences of mean Vmax between groups were analyzed using the Student’s t test. Significance was considered if P<0.05.
Results
Direct Activation of Plasma PK by VSMC
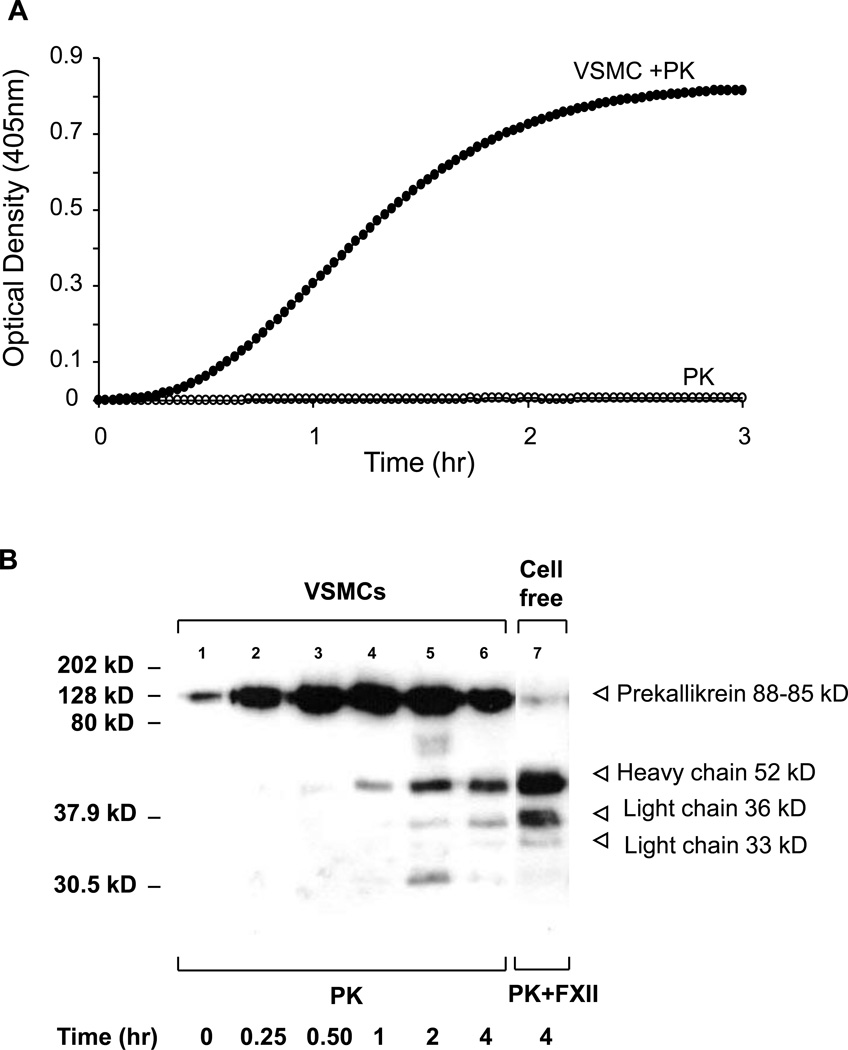
We initiated studies to explore the possible mechanisms of plasma PK activation by VSMC. VSMCs cultured on gelatin coated 96-well plates were serum starved by incubating them in 200 µl of serum-free EMEM for 24 hrs. The cells were washed at least 3 times with HEPES buffer (10 mM HEPES, 137 mM NaCl, 4 mM KCl, 11 mM D-glucose, 0.5 mg/ml RIA grade BSA, pH 7.4) followed by the addition of 0.4 mM of S-2302 and 3.58 nM human plasma PK (ERL, South Bend, IN). The optical density (OD) (absorbance at 405 nm) of p-nitroaniline was measured at 2 min interval to follow the kinetics of plasma PK activation. The results shown in Figure 1A demonstrate the conversion of plasma PK to active kallikrein upon its contact with VSMC as evidenced by its ability to cleave S-2302. In contrast, the chromogenic substrate S-2302 was not cleaved by plasma PK in a cell free system, thus excluding the possibility of PK autoactivation.
Figure 1. Prekallikrein activation by cultured VSMCs in the absence of FXII.
A: VSMCs were subcultured in the 96-well microplate. Optical densities (OD) were recorded at 405 nm at 2-min intervals for 3 hrs using SpectraMax® 340 PC laser microplate reader. No activation of PK was present by using 3.85 nM of PK and 0.4 mM of kallikrein substrate S-2302® in the cell-free system (open circle). When the same amount of PK was added to VSMCs, activation of PK was detected in the absence of FXII (closed circle). The graphs are from a representatives set of 10 experiments. B, Immunoblot analysis of conditioning media of VSMCs incubated with 3.58 nM of prekallikrein shows the conversion of PK into various digestion fragments including those corresponding to heavy chain (52 kD) and light chains (36 and 33 kD) of the PK as a function of time. PK digestion fragments by FXII in cell-free system are shown for comparison.
Activation of plasma PK by VSMC was also determined by western blot analysis. VSMC were incubated with a known concentration of plasma PK (3.58 nM) for various times. The appearance of cleaved fragments of PK in conditioned media is indicative of the conversion of prekallikrein to kallikrein. Figure 1B demonstrates the detection of cleaved fragments of PK in the conditioned media as early as 1h that correspond to one heavy chain (52kD) and two light chains (36 and 33 kDs). As a positive control, PK activated with FXII in cell free system for 4h, resulted in a similar pattern of PK cleaved fragments as that generated by VSMC.
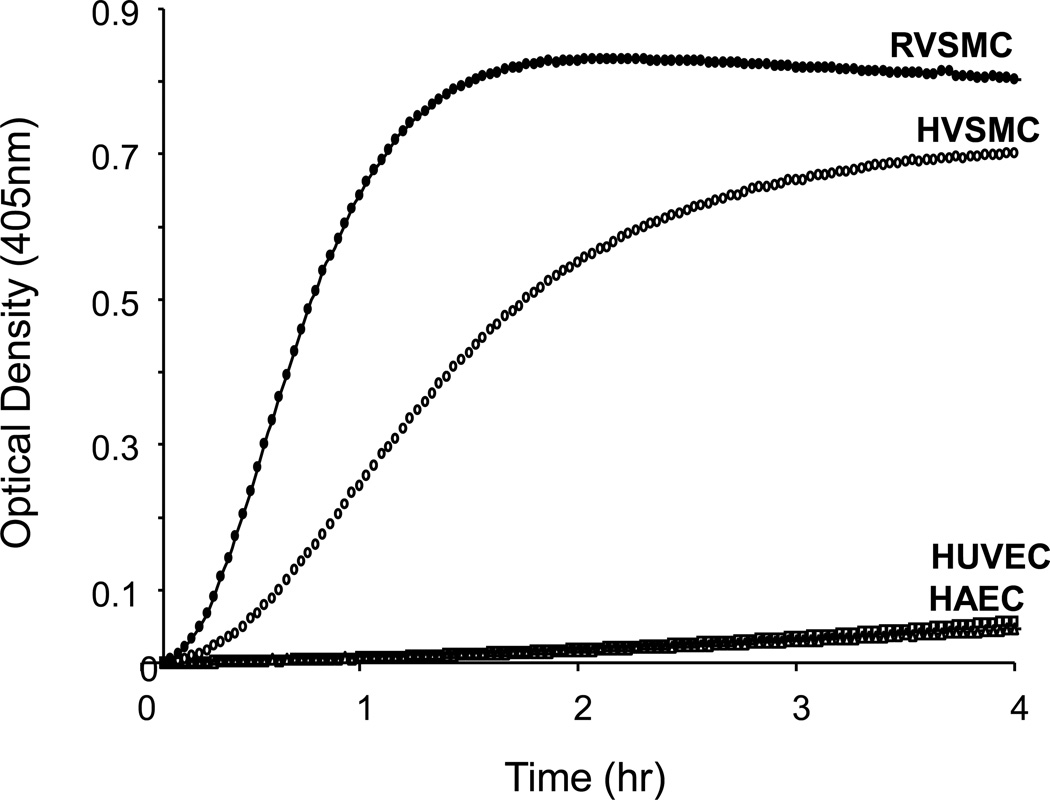
The rate of plasma PK activation between VSMC and endothelial cells was compared in the absence of added HMWK. The results shown in Figure 2, demonstrate that both human aortic VSMC and rat VSMC can directly activate plasma PK to active kallikrein. In contrast, no activation of plasma PK occurred, when plasma PK was added to human aortic endothelial cells (HAEC) and/or human umbilical vein endothelial cells (HUVEC).
Figure 2. Comparison of PK activation by endothelial and VSMC.
Rat aortic VSMCs, human aortic VSMCs (HVSMCs), human umbilical vein endothelial cells (HUVECs) and human aortic endothelial cells (HAECs) were subcultured in different wells and incubated with 3.58 nM of PK and 0.4 mM of S-2302. Optical densities were recorded at 405 nm at every 2 min for 2 hrs. While HUVECs and HAECs did not show PK activation, rat VSMCs (closed circle) and HVSMCs (open circle) showed remarkable activation of PK in the absence of FXII. Graphs are from a representative set of 3 experiments.
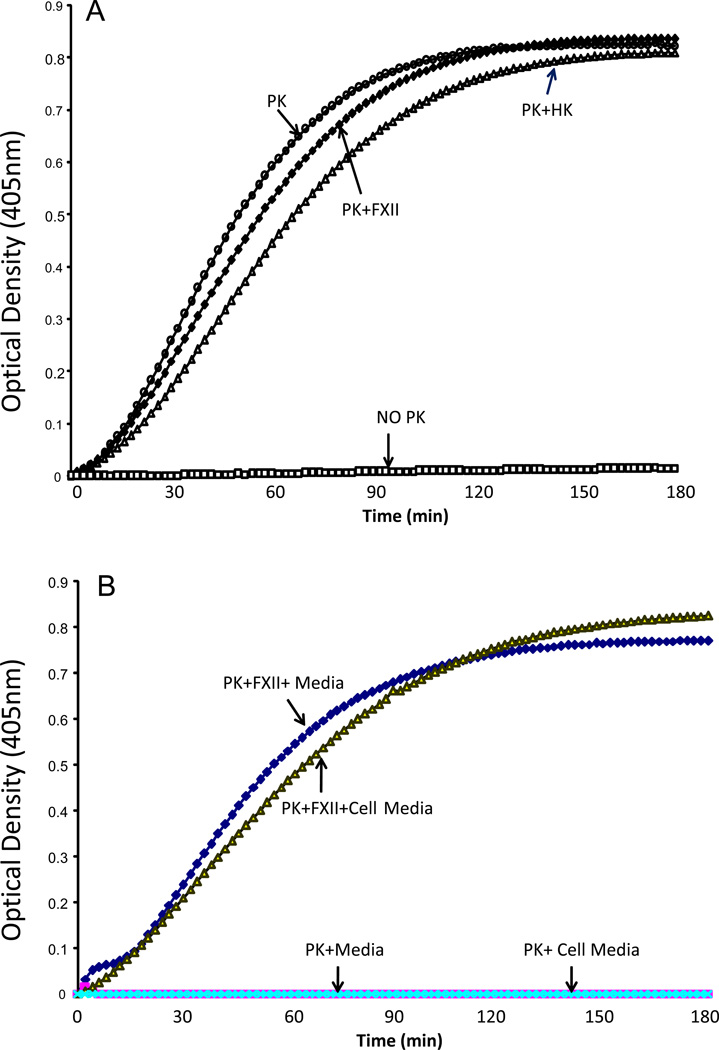
To assess whether VSMC can hydrolyze S2302 in the absence of PK, rat aortic VSMCs were incubated with 0.4 mM of S-2302 in the presence and absence of 3.58 nM of PK either alone or in the presence of FXII or HMWK. The results in Figure 3A show that cleavage of S2302 by VSMC was observed only in the presence of PK, PK+FXII and PK+HMWK. No cleavage of S2302 was observed by VSMC in the absence of PK (NO PK). We next determined whether VSMC release factors into the conditioned media that may result in the hydrolysis of S2302. Conditioned media collected 24 h after incubation with VSMC was incubated with 0.4 mM of S-2302 and 3.58 nM of PK in the presence and absence of FXII. The results in Figure 3B show that no hydrolysis of S2302 by PK was observed in conditioned media (PK+Cell Media) collected from VSMC and in PK+ Media. Hydrolysis of S2302 by PK was observed only in the presence of FXII.
Figure 3.
(A) Cleavage of S2303 by PK in VSMC. Rat aortic VSMCs were incubated with 0.4 mM of S-2302 in the presence and absence of 3.58 nM of PK alone and in the presence of FXII and/or HK. Optical densities were recorded at 405 nm at every 2 min for 3 hrs. Cleavage of S2302 by VSMC was observed only in the presence of PK, PK+FXII and PK+HK. No cleavage of S2302 was observed by VSMC in the absence of PK (NO PK). Graphs are from a representative set of 4 experiments. (B). Effect of cell culture media on PK activation. 0.4 mM of S-2302 and 3.58 nM of PK were incubated with conditioned media collected 24 h after incubation with VSMC in the presence and absence of FXII. No hydrolysis of S2302 by PK was observed in conditioned media (PK+Cell Media) collected from VSMC and in PK+ Media. Hydrolysis of S2302 by PK was observed only in the presence of FXII. Graphs are from a representative set of 4 experiments.
Activation of Plasma PK by Cellular Proteins Extracted From VSMCs
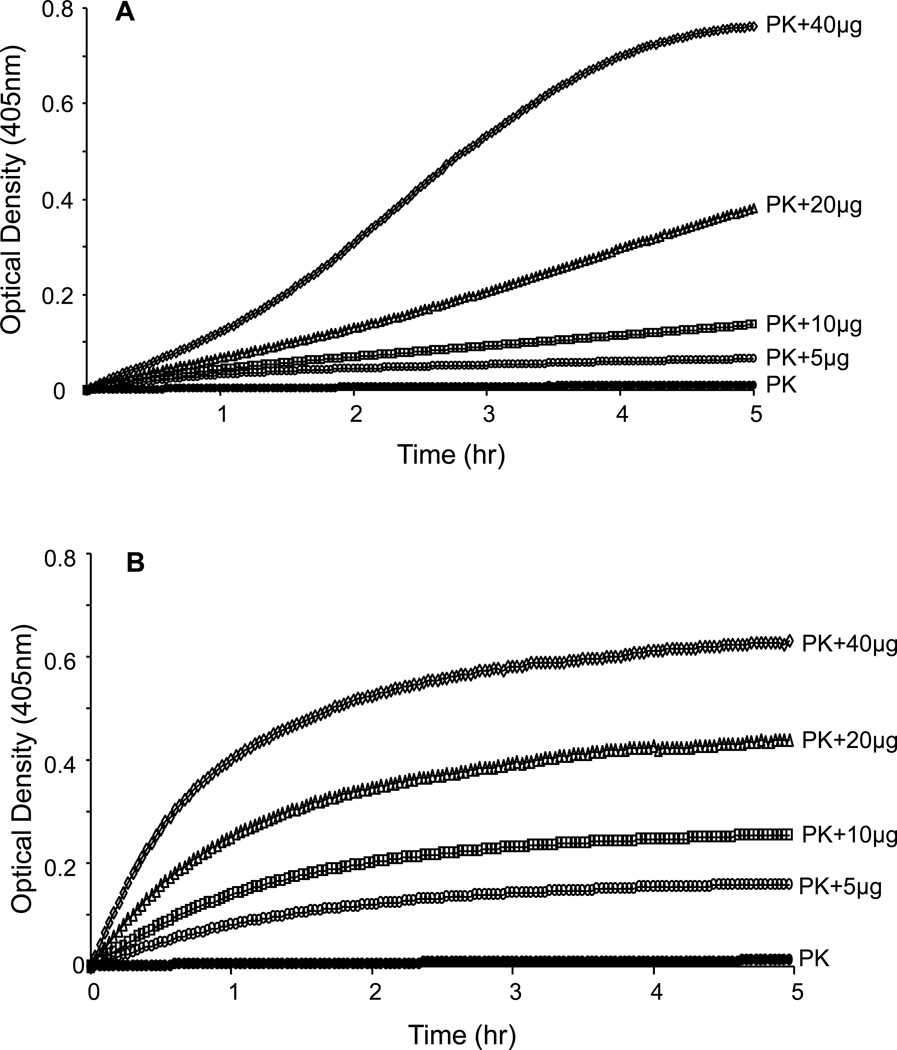
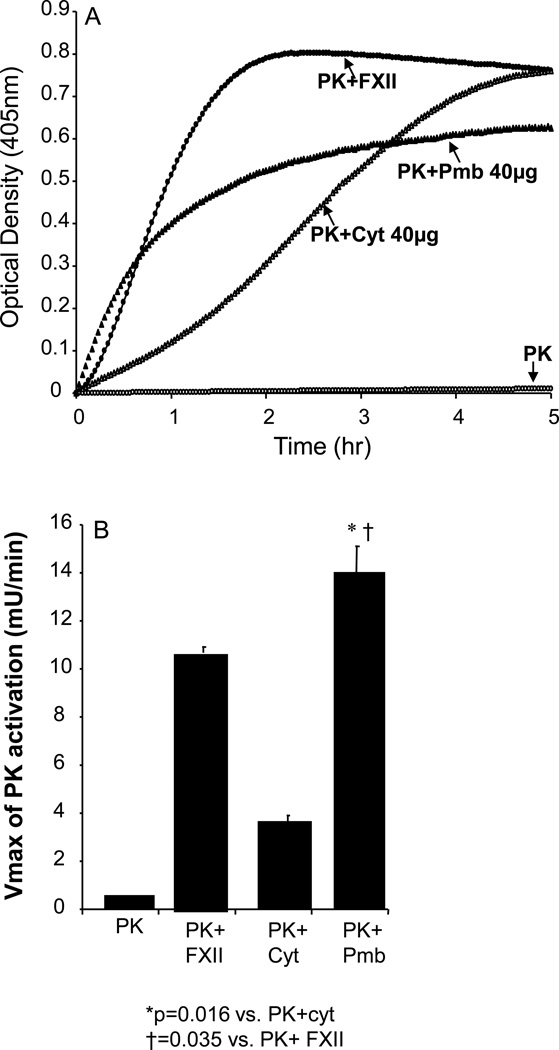
We next determined whether the putative plasma PK activator we discovered on the surfaces of VSMC is mainly associated with the plasma membrane or with the cytosol. We extracted the cellular protein from cultured VSMCs without using any detergent, which might affect the activity of enzymes involved in activation of the KKS, and separated each fraction using ultracentrifugation. The results shown in Figure 4A&B demonstrated that incubation of plasma PK (3.58nM) and 0.4 mM S-2302 with either cytosolic or plasma membrane fractions resulted in a concentration dependent increase in plasma PK chromogenic activity. The higher the protein concentration the faster the rate of conversation of plasma prekallikrein to active kallikrein
Figure 4. Dose-response prekallikrein activation by cytosolic and plasma membrane fractions.
A: Dose response of cytosolic fractions (5, 10, 20 and 40 µg) on PK activation (3.58 nM of PK, closed circles). B: Dose response of plasma membrane fraction (Pmb) on PK activation in a cell free system. The results are representative of 4 separate experiments.
The next series of studies compared Vmax between the membrane and cytosolic fractions. The results in Figure 5A demonstrate that both the cytosolic and plasma membrane fraction showed activation of plasma PK when 40µg protein of each fraction was used. As a control, the results were compared to activation of plasma PK by FXII in a cell free system. The Vmax of the PK activation using 40µg of plasma membrane protein was significantly higher (P<0.016) than that for cytosolic fraction of the same protein concentration (Figure 5B). Furthermore, the Vmax of 40µg of plasma membrane fraction was significantly higher (P<0.035) than that observed for FXII (2.40 nM) activation of plasma PK in this experiment. The Vmax of the reaction was significantly greater for plasma membrane at any given protein concentration, indicating that the rate of conversion of prekallikrein to kallikrein is higher in plasma membrane fractions than the cytosolic fractions, indicating that the concentration of PK activator is higher (as a fraction of total protein) in plasma membrane than in cytosol. No activation of plasma PK was observed in a cell-free system (Figure 5A).
Figure 5. Prekallikrein activation by extracted proteins from VSMCs in cell-free system.
A: 40 µg of cytosolic (Cyt) and/or plasma membrane (Pmb) protein fractions extracted from VSMC were used to assess activation of PK in cell-free system. Optical densities were read at 405 nm at 2-min intervals for 5 hr. Autoactivation of PK was not observed in the absence of cellular protein (open circle). Both cytosolic fraction (open triangle) and plasma membrane fraction (closed triangle) activated PK. B: The rate of PK activation by plasma membrane fraction was faster than that by cytosolic protein based on the maximum rate of the kinetic profile (Vmax). The Vmax of plasma membrane was even higher than that by factor XII (FXII) (closed circle in A). The results are averages from 3 experiments, each with triplicates.
Characterization of the Plasma PK Activator on VSMC
Role of Factor XII in Plasma PK Activation
To exclude the possibility that activation of plasma PK by VSMC may be due to contamination by FXII that is present as part of the FBS used to culture VSMC, we determined whether activation of plasma PK is sensitive to inhibition with corn trypsin inhibitor (CTI), a select inhibitor of factor XII.
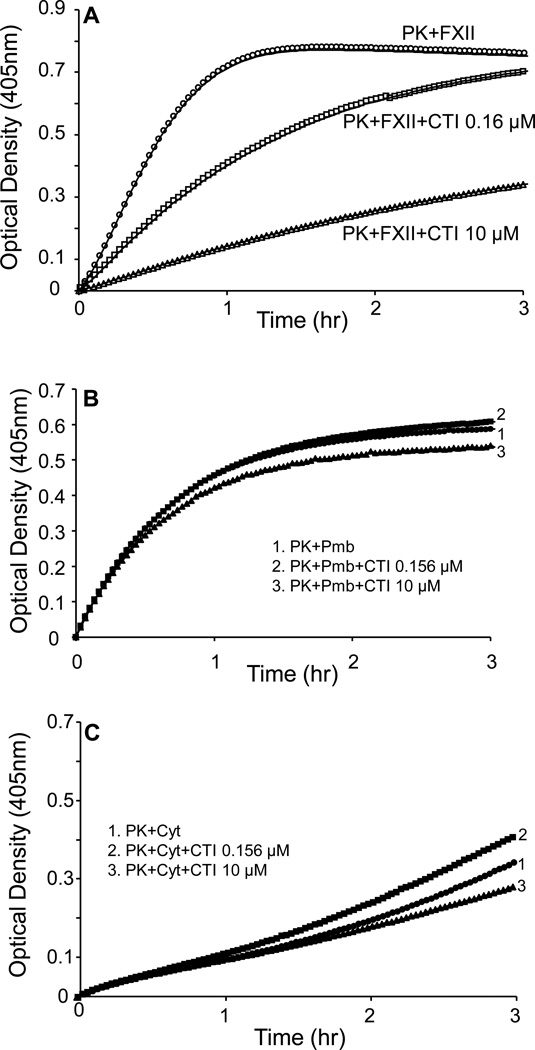
Our first experiments determined the inhibitory profile in our assay conditions of CTI on plasma PK activation by FXII in a cell free system. The inhibitory profile of CTI on plasma PK activation by FXII was assessed by performing a concentration-response curve using various concentrations of CTI (0.5nM–10µM). A concentration dependent inhibition of plasma PK activation by FXII was observed in response to CTI treatment. The IC50 was between 0.312 µM and 0.156 µM, with peak inhibition of about 80% by 10µM of CTI (Figure 6A). Based on this finding we elected to use two concentrations of CTI (0.156µM and 10µM) to assess whether these concentrations would inhibit plasma PK activation to response to proteins extracted from the plasma membrane or cytosol of VSMC.
Figure 6. Inhibitory profile of PK activation BY CTI in cell-free system.
A, PK activation by FXII in the absence of CTI is shown. Using 0.156 µM and 10 µM, CTI inhibited activation of PK by factor XII (FXII) in dose-dependent manner (p=0.0161 and p=0.000176 for 0.156 µM and 10 µM, respectively, n=3). B, 40 µg of plasma membrane fraction (Pmb) of VSMC activated PK was not inhibited by 0.156 µM and 10 µM of CTI (p=0.996 and p=0.906 for 0.156 µM and 10 µM, respectively, n=3). C, Cytosolic fraction also activated PK, which did not show a significant inhibition by 0.156 µM and 10 µM of CTI (p=0.165 and p=0.765 for 0.156 µM and 10 µM, respectively, n=3).
Plasma membrane and cytosolic protein extracts (40µg) were incubated in the presence or absence of 0.156 µM or 10 µM of CTI for 30 min prior to performing the activation assay. The results shown in Figure 6A, demonstrate that activation of plasma PK by FXII was significantly inhibited in the presence of CTI. Both concentrations of CTI (0.156µM and 10µM) significantly inhibited plasma PK activation by FXII (54% and 80% inhibition in Vmax, P<0.05 and P<0.0001, respectively). Incubation of plasma PK with either the plasma membrane or cytosolic fraction resulted in significant activation of plasma PK (Figure 6B & 6C). However, no inhibition of PK activation was observed by either concentration of CTI (0.156µM and 10µM) when plasma membrane and cytosolic fractions were used to activate PK (11% increase and 2.4% decrease in Vmax, respectively, P=0.08 and P=0.66, respectively, Figure 6B &6C).
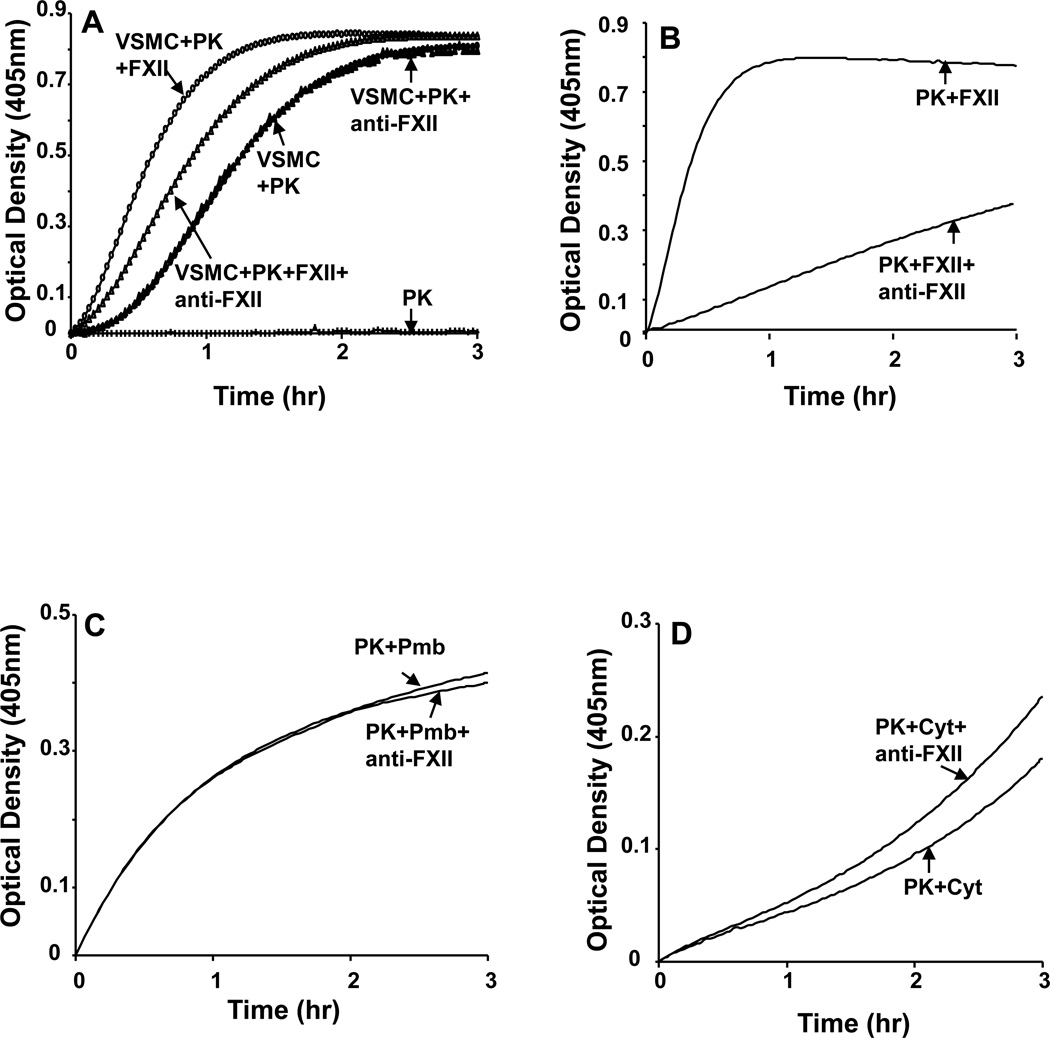
To explore further the involvement of FXII in plasma PK activation by VSMC, plasma PK activation by VSMC alone and/or by FXII and VSMC was determined in the presence and absence of neutralizing anti-FXII antibodies (100µg/mL). The results shown in Figure 7A demonstrate that, VSMC alone and/or VSMC+FXII resulted in plasma PK activation. However, in the presence of anti-FXII antibodies, the activation of plasma PK that was observed in response to FXII and VSMCs was significantly reduced compared to FXII treated VSMC (34% inhibition in Vmax, P<0.001). On the other hand, pretreatment of VSMC with anti-FXII antibodies had no significant effect on plasma PK activation by VSMC alone (9% inhibition in Vmax, P=0.254, Figure 7A).
Figure 7. The effect of anti-FXII antibody on the activation of PK.
A: VSMCs incubated with anti-FXII antibody for 30 min at room temperature. Anti-FXII (100µg/ml) antibody inhibited PK activation by FXII (p=0.00674). However, activation of PK by the VSMCs in the absence of FXII was not inhibited by 100µg/mL of anti-FXII (closed circles and closed triangles). B, Anti-FXII inhibited PK activation by FXII (p=0.0144). C: Anti-FXII did not inhibit PK activation by plasma membrane fraction (Pmb, 40 µg) of VSMCs. D, Anti-FXII did not inhibit PK activation by cytosolic fraction (Cyt, 40µg) of VSMCs. The graphs are representative sets from 4 separate experiments.
To assess whether the concentration of anti-FXII antibody we used was effective in neutralizing the effects of FXII, we measured the rate of plasma PK activation by FXII in a cell free system in the presence and absence of anti-FXII antibody (100µg/mL). The results in Figure 7B show that the increase in plasma PK activation in response to FXII was significantly inhibited in the presence of neutralizing anti-FXII antibodies (88% inhibition in Vmax, P<0.0001).
The effect of neutralizing anti-FXII antibodies on plasma PK activation in response to plasma membrane and cytosolic fractions extraction from VSMC was also assessed. The results shown in Figure 7C&D demonstrate that anti-FXII antibodies had no significant effect on plasma PK activation in response to either the membrane fraction or the cytosolic fraction (3% increase each in Vmax; P=0.578). Taken together, these findings rule out a role for the involvement of FXII in activation of plasma PK by VSMC.
Effect of Protease Inhibitors on VSMC Plasma PK Activator
To characterize the plasma PK activator in VSMC, the inhibitory profile of the plasma PK activator was assessed by using a panel of protease inhibitors. In these studies plasma PK (3.58nM) was added to VSMC cultured on 96 well-plates in the presence and absence of high concentrations of i) serine protease inhibitors (aprotinin 0–100 µM; phenylmethylsulfonyl chloride 0–10mM; leupeptin 0–100 µM) and antipain 0–100µM); ii) cysteine protease inhibitor (cystatin 0–100 µg/ml); iii) aspartic acid protease inhibitor (pepstatin 0–1.56µg/ml); iv) metalloprotease inhibitors (bestatin 0–250 µg/ml) and v) calcium-dependent proteases (EDTA-Na2 0–3.125 mM, EGTA 0–12.5mM). The amount of kallikrein activity formed in the presence of the above inhibitors was determined by the hydrolysis of 0.4 mM S-2302 and compared with an uninhibited control. In addition to assessing the effect of these inhibitors on the chromogenic activity of PK, we also determined whether these inhibitors would block the conversion of PK to active kallikrein. This was assessed by SDS-PAGE, an event that is independent of kallikrein amidolytic activity.
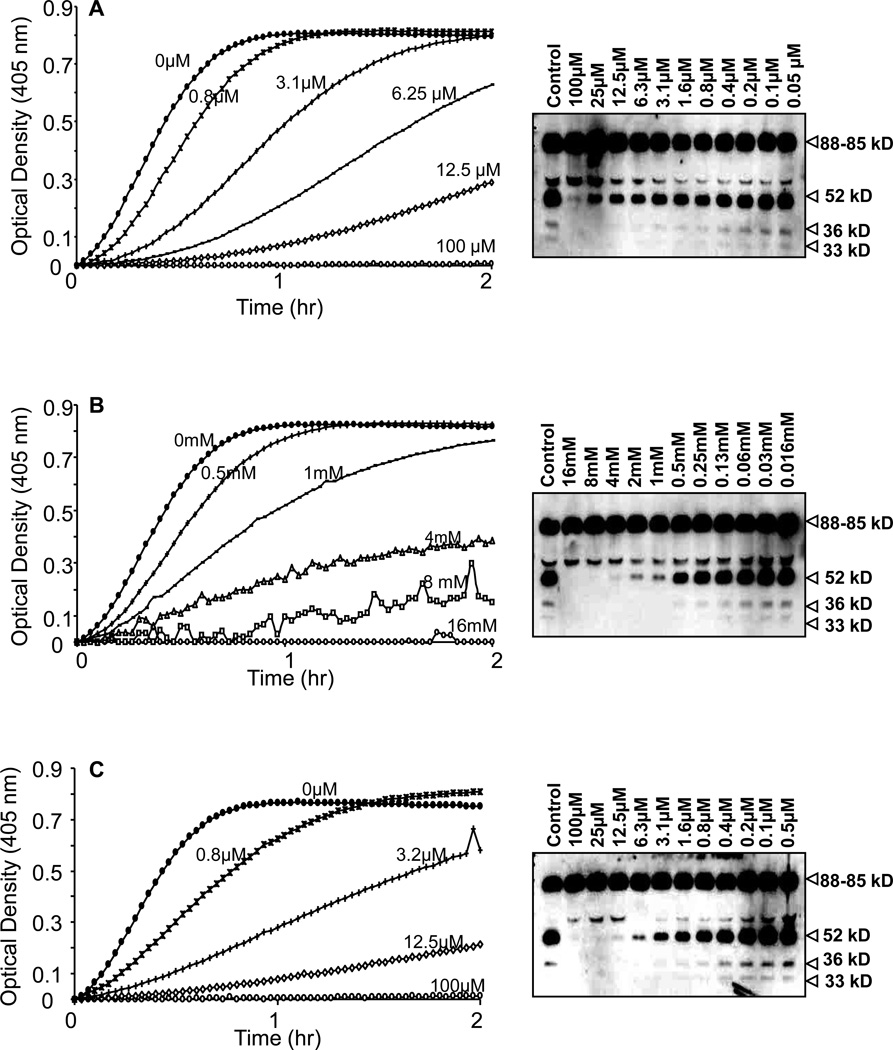
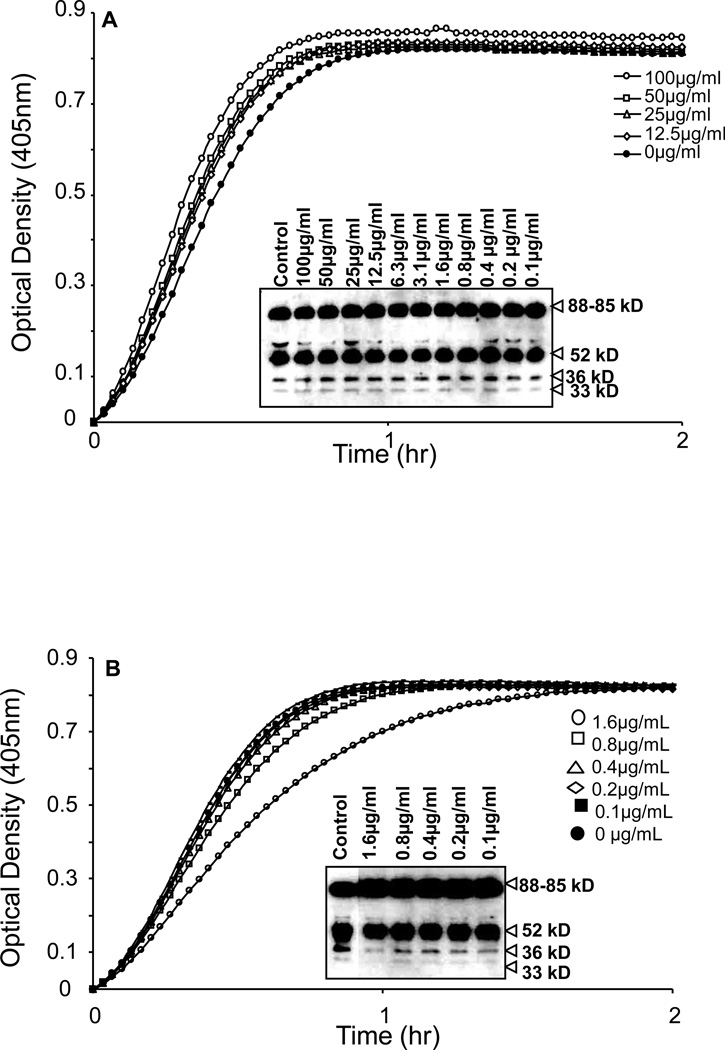
The results shown in Figure 8 demonstrated that aprotinin (Figure 8A), PMSF (Figure 8B) and leupeptin (Figure 8C), inhibited the chromogenic activity of PK in a concentration dependent manner with an IC50 0f 0.78µM, 1mM and 3.12µM, respectively. We next determined whether aprotinin, PMSF and leupeptin would block the conversion of PK to kallikrein as assessed by SDS-PAGE, an event that is independent of kallikrein amidolytic activity. PK migrated as a thick band between 85–88kD (Figure 8A–C). Incubation of PK with VSMC for 4h resulted in the generation of multiple breakdown products of PK that migrated at 52 and 36 and 33KD. However, in the presence of increasing concentrations of aprotinin, PMSF and leupeptin, no breakdown products of PK were observed, indicating that the PK activating enzyme(s) associated with VSMC is sensitive to inhibition with aprotinin, PMSF and leupeptin.
Figure 8. Inhibition profile of PK activation on surfaces of VSMCs by serine protease inhibitors.
A–C, PK activation by VSMCs was dose-dependently inhibited by aprotinin (A), phenylmethylsulfonyl fluoride (PMSF) (B) and leupeptin (C). Each graph of PK activation and the bands obtained by Western blotting represent the effect of serial dilution of protease inhibitors used (n=3).
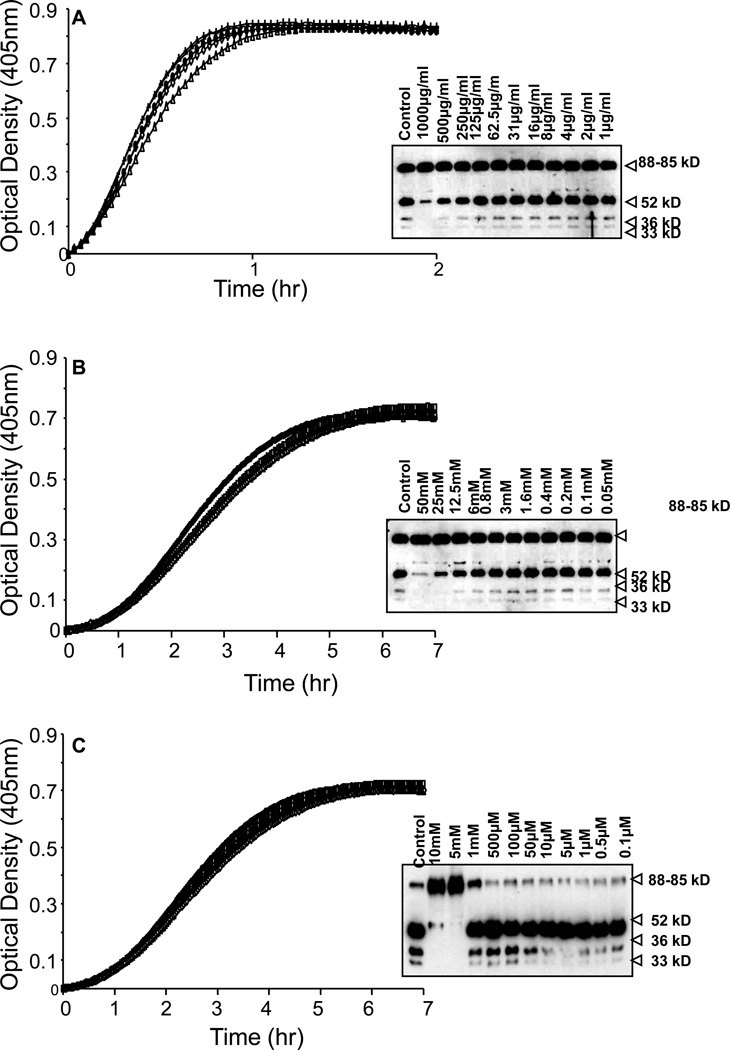
The cysteine protease inhibitor cystatin (Figure 9A) and an aspartic acid protease inhibitor pepstatin (Figure 9B) did not inhibit the chromogenic activity of plasma PK, nor did they block the conversion of plasma PK to kallikrein as assessed by SDS-PAGE. Furthermore, the metalloprotease inhibitor bestatin (Figure 10A) and the calcium-dependent proteases, EDTA-Na2 (Figure 10B) and EGTA (Figure 10C) also had no significant effect on the chromogenic activity of plasma PK or the conversion of plasma PK to kallikrein as assessed by SDS-PAGE.
Figure 9. Inhibitory profile of PK activation by cysteine and aspartic acid protease inhibitors.
A, PK activation by VSMCs was not inhibited by cystatin, a cysteine protease inhibitor. B, PK activation by VSMCs was not inhibited by pepstatin, an aspartic acid protease inhibitor (n=3 experiments).
Figure 10. Inhibition profile of PK activation by metalloprotease inhibitors.
A–C, PK activation by VSMCs was not inhibited by bestatin (A), EDTA-Na2 (B) and EGTA (C). Activation assays and western blot analysis showed similar results (n=3 experiments).
Effect of Angiotensin II and Bradykinin
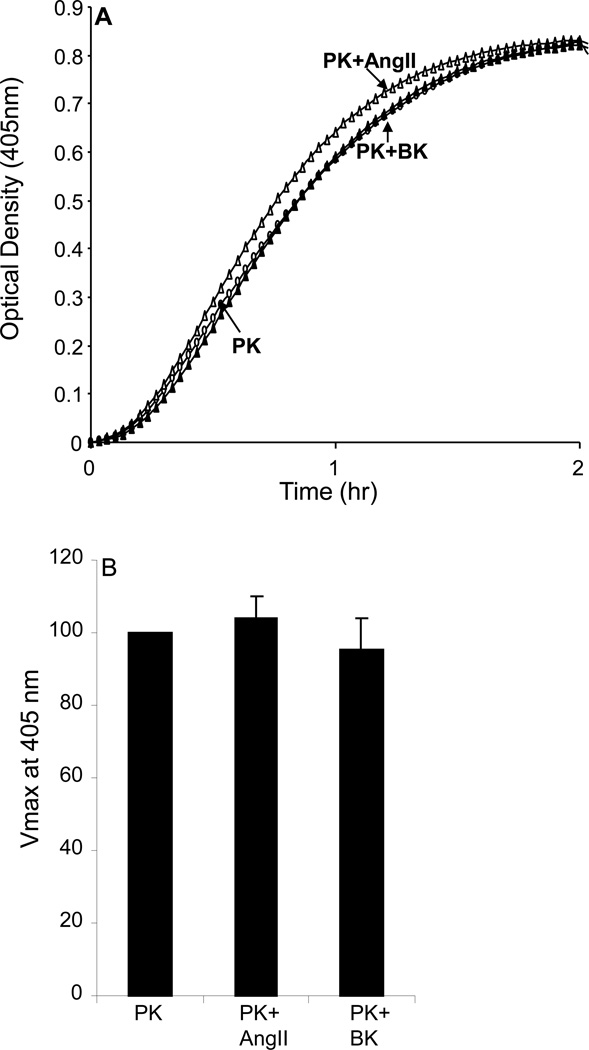
We next evaluated whether the putative plasma PK activator in VSMC is the endothelial cell plasma PK activator propylcarboxypeptidase (PRCP). Plasma PK (3.58nM) was added to VSMC in the presence and absence of 10 µM angiotensin II (Ang II) and/or 100 µM of BK. Both Ang II and BK are substrates for PRCP that inhibit PRCP activity (20). The amount of kallikrein activity formed in the presence of Ang II and BK was determined by the hydrolysis of 0.4 mM S-2302 and compared with an uninhibited control. The results shown in Figure 11 demonstrated that neither AngII nor BK inhibited the chromogenic activity of PK, thus ruling out PRCP as the putative PK activator in VSMC (4.3% increase and 4.7% decrease in Vmax, respectively; P=0.547 and P=0.651, Figure 11B).
Figure 11. Effect of angiotensin II (AngII) and bradykinin (BK) on prekallikrein (PK) activation by VSMCs.
PK activation was done in the absence (open circle) or presence of AngII (10 µM, open triangle) and BK (100 µM, closed triangle) for 2 hrs at room temperature. No inhibition of PK activation by VSMCs was observed by incubating PK in the presence of AngII or BK. The graphs were representative of 3 separate experiments.
Discussion
The pathophysiological significance of plasma PK independent of its role in blood coagulation is just beginning to be realized. Plasma PK has recently been shown to be associated with high blood pressure and albuminuria in type 1 diabetic patients, to modulate thrombosis risk and to promote vascular remodeling (12,18). The plasma PK gene KLKB1 is encoded by a single gene that is mapped to the q34–q35 region of chromosome 4 and is composed of 15 exons and 14 introns that correspond to the coding regions of the heavy and light chains of plasma kallikrein. The heavy chain consists of four tandem repeat sequences called “apple” domains (named A1–A4) that are responsible for binding of PK to HMWK. The catalytic domain of PK is in the light chain (23). Plasma PK is predominately synthesized in the liver and secreted into the circulation where it circulates as a bimolecular complex bound to its substrate HMWK (5). Under physiological conditions, the assembly of the plasma PK-HMWK complex on the surfaces of endothelial cells is facilitated by the binding of domains 3, 4 and 5 of HMWK to a multiprotein receptor complex that consists of cytokeratin 1 (CK1), the receptor for globular head of the C1q (gC1qR) and the urokinase plasminogen activator receptor (u-PAR) (19–21). Once assembled on the surfaces of endothelial cells, PRCP, which is bound to the complex, activates plasma PK to activate kallikrein, which in turn cleaves HMWK to release BK (22). The generated, BK can act on its B2-receptors in an autocrine and or paracrine manner to initiate a multitude of cellular signals that influence vascular structure and tone. For instance, activation of B2 receptors on endothelial cells by BK results in the generation of nitric oxide, which promotes vasodilatation of the vessel wall and inhibits proliferation of the underlying VSMC (24).
However, when the endothelium is denuded or injured, as a result of the diabetic state, plasma PK will be in direct contact with VSMC leading to its activation and initiation of its signal transduction mechanisms. Furthermore, VSMC comprise the main cellular component in atherosclerotic lesions and their number and activity largely determines the thickness of the intima. In this regard, circulating levels of plasma PK are in constant contact with the atherosclerotic plaque leading to its activation by VSMC and initiation of PK signaling pathways that may ultimately alter the pathophysiology of the atherosclerotic plaque.
However, whereas HMWK plays a pivotal role in PK activation by HSP90 or PRCP, HMWK was not essential for activation of PK on surfaces of VSMCs in our experiments (20,25). When HMWK was added together with PK, the rate of PK activation was slightly lower than PK alone. This finding is in contrast to a previous report by Fernando et al, showing that addition of PK and HMWK to VSMC did not result in PK activation as evidenced by hydrolysis of S2302 (26). In addition, anti-factor FXII antibody, did not inhibit the activation of PK by VSMCs themselves or extracted proteins from the VSMCs. Additionally, using extracted VSMC proteins, CTI did not show a dramatic inhibition as is seen with CTI’s inhibition of PK activation by FXII. Thus, the direct activation of PK on VSMCs cannot be attributed to contaminating FXII arising from some trace amounts of remaining FXII that might be present in FBS used in the culture media.
The direct activation of PK by PK activator of VSMC seen in this study was inhibited by other serine protease inhibitors such as aprotinin, phenylmethylsulfonyl fluoride (PMSF), and leupeptin. No inhibition of PK activation was observed in response to inhibitors of cysteine and aspartic acid proteases and metalloproteases. Angiotensin II (AngII) and BK are known substrates for PRCP and both inhibit PK activation by PRCP in the endothelial cells (20,25). However, when we examined AngII and BK, they did not inhibit the activation of PK by VSMCs. This suggests that the activator of VSMCs in this experiment may be different from PRCP, a known endothelial PK activator.
Furthermore, the pattern of kinetics of PK activation by the cytosolic fraction of extracted protein was quite different from that by plasma membrane fractions. The rate of PK activation measured using Vmax was much faster with the plasma membrane fraction than with the cytosolic fraction. In the early phase, PK activation by plasma membrane fraction was even faster than that by FXII on the same plate. This suggests that the PK activator is not FXII and that it constitutes a larger fraction of plasma membrane protein that cytosolic protein.
A possible alternate pathway for activation of PK by vascular cells, a number of studies in cell free systems implicated a role for proteoglycans in the autoactivation of PK and factor XII (27–29). However, a number of recent studies using endothelial cells have shown that negatively charged proteoglycans inhibit the binding of HMWK to endothelial surfaces and limit the liberation of BK from kininogen cleavage (30). Furthermore, heparin was shown not to affect the activation of PK on endothelial cells (31). These results are in agreement with our studies showing that when PK was added to either HAEC or to HUVECS, no activation of PK was observed, thus ruling out a potential role of proteoglycans in PK activation. Since VSMC synthesize and secrete proteoglycans, we tested whether the culture medium collected from the cell layer of cultured VSMC will stimulate PK activation (32,33). No PK activation was observed when incubated with culture media collected from VSMC. PK activation was observed only in the presence of Factor XII, thus ruling out a potential role for cell surface proteoglycans in PK activation by VSMC. However, the data presented in the current manuscript do not rule out a possible role of activation of PK by proteoglycans that are not rapidly secreted by VSMC.
In summary, the findings of this manuscript point to a novel mechanism of plasma PK activation by VSMC. Unlike endothelial cells, activation of plasma PK by VSMC occurs irrespective of HMWK binding to the surfaces of VSMC. Future studies will aim to purify, characterize and identify the nature of this putative plasma PK activator present in VSMC.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health Grants HL077192 and HL087986 (AAJ).
References
- 1.Ross R. Atherosclerosis: An inflammatory disease. NEJM. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Clowes AW, Karnovsky MJ. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977;265:625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- 3.Jackson CL, Schwartz SM. Pharmacology of smooth muscle cell replication. Hypertension. 1992;20:713–736. doi: 10.1161/01.hyp.20.6.713. [DOI] [PubMed] [Google Scholar]
- 4.Luscher TF, Tanner FC, Tschudi MR, Noll G. Endothelial dysfunction in coronary artery disease. Annu Rev Med. 1993;44:395–418. doi: 10.1146/annurev.me.44.020193.002143. [DOI] [PubMed] [Google Scholar]
- 5.Shaw Jl, Diamandis EP. Distribution of 15 human kallikreins in tissues and biological fluids. Clin Chem. 2007;53:1423–1432. doi: 10.1373/clinchem.2007.088104. [DOI] [PubMed] [Google Scholar]
- 6.Colman RW, Schamier AH. Contact System: A vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive and proinflammatory attributes. Blood. 1997;90:3819–3843. [PubMed] [Google Scholar]
- 7.Margolius HS. Kallikrein and Kinins. Some unanswered questions about system characteristics and roles in human disease. Hypertension. 1995;26:221–229. doi: 10.1161/01.hyp.26.2.221. [DOI] [PubMed] [Google Scholar]
- 8.Mombouli JV, Vanhoutte PM. Kinins and endothelial control of vascular smooth muscle. Ann Rev Pharmacol Toxicol. 1995;35:679–705. doi: 10.1146/annurev.pa.35.040195.003335. [DOI] [PubMed] [Google Scholar]
- 9.Saed G, Carretero OA, MacDonald RJ, Scicli GA. Kallikrein messenger RNA in arteries and viens. Circ Res. 1990;67:510–516. doi: 10.1161/01.res.67.2.510. [DOI] [PubMed] [Google Scholar]
- 10.Sakakibara T, Hintze TH, Nasjletti A. Determinants of kinin release in isolated rat hindquarters. Am J Physiol. 1998;274:R120–R125. doi: 10.1152/ajpregu.1998.274.1.R120. [DOI] [PubMed] [Google Scholar]
- 11.Harvey JN, Edmundson AW, Jaffa AA, Martin LL, Mayfield RK. Renal excretion of kallikrein and eicosanoids in patients with type I diabetes: relationship to glomerular and tubular function. Diabetolgia. 1992;35:857–862. doi: 10.1007/BF00399932. [DOI] [PubMed] [Google Scholar]
- 12.Jaffa AA, Durazo-Arvizu R, Zheng D, Lackland DT, Srikanth S, Garvey WT, Schmaier AH the DCCT/EDIC Study Group. Plasma Prekallikrein: A Marker for hypertension and nephropathy in type 1 diabetes. Diabetes. 2003;52:1215–1221. doi: 10.2337/diabetes.52.5.1215. [DOI] [PubMed] [Google Scholar]
- 13.Velarde V, Ullian ME, Morinelli TA, Mayfield RK, Jaffa AA. Mechanisms of MAPK activation by bradykinin in vascular smooth muscle cells. Am J Physiol. 1999;277:C253–C261. doi: 10.1152/ajpcell.1999.277.2.C253. [DOI] [PubMed] [Google Scholar]
- 14.Naidu P, Velarde V, Kappler C, Young RC, Mayfield RK, Jaffa AA. Calcium and calmodulin mediate bradykinin induced MAPK phosphorylation and c-fos induction in vascular cells. Am J Physiol. 1999;277:H1061–H1068. doi: 10.1152/ajpheart.1999.277.3.H1061. [DOI] [PubMed] [Google Scholar]
- 15.Greene EL, Velarde V, Jaffa AA. Role of reactive oxygen species in bradykinin induced MAPK and c-fos induction in vascular cells. Hypertension. 2000;35:942–947. doi: 10.1161/01.hyp.35.4.942. [DOI] [PubMed] [Google Scholar]
- 16.Douillet CD, Velarde V, Christopher JT, Mayfield RK, Trojanowska ME, Jaffa AA. Mechanisms by which bradykinin promotes fibrosis in vascular smooth muscle cells: role of TGF-β and MAPK. Am J Physiol. 2000;279:H2829–H2837. doi: 10.1152/ajpheart.2000.279.6.H2829. [DOI] [PubMed] [Google Scholar]
- 17.Webb J, Tan Y, Jaffa MA, Jaffa AA. Evidence for Prostacyclin and cAMP Upregulation by Bradykinin and Insulin-Like Growth Factor 1 in vascular smooth muscle cells. Journal of Receptors and Signal Transduction. 2010;30(2):61–71. doi: 10.3109/10799890903563768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmaier AH. Assembly, activation and physiologic influence of plasma kallikrein/kinin system. International Immunopharmacology. 2008;8:161–165. doi: 10.1016/j.intimp.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motta G, Rojkjaer R, Hasan A, Cines D, Schmaier AH. High molecular weight kininogen regulates prekallikrein assembly and activation on endothelial cells: A novel mechanism for contact activation. Blood. 1998;91:516–528. [PubMed] [Google Scholar]
- 20.Shariat-Madar Z, Mahdi F, Schmaier AH. Identification and characterization of prolylcarboxypeptidase as an endothelial cell prekallikrein activator. J Biol Chem. 2002;277:17962–17969. doi: 10.1074/jbc.M106101200. [DOI] [PubMed] [Google Scholar]
- 21.Shariat-Madar Z, Mahdi F, Schmaier AH. Recombinant prolylcarboxypeptidase activates plasma prekallikrein. Blood. 2004;103:4554–4561. doi: 10.1182/blood-2003-07-2510. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Qiu Q, Mahdi F, Shariat-Madar Z, Rojkjer R, Schmaier AH. Assembly and activation of HP-HK complex on endothelial cells results in bradykinin liberation and NO formation. Am J Physiol. 2001;280:H1821–H1829. doi: 10.1152/ajpheart.2001.280.4.H1821. [DOI] [PubMed] [Google Scholar]
- 23.Yu H, Anderson PJ, Freedman BI, Rich SS, Bowden DW. Genomic structure of the human plasma prekallikrein gene, identification of allelic variants and analysis in end stage renal disease. Genomics. 2000;69:225–234. doi: 10.1006/geno.2000.6330. [DOI] [PubMed] [Google Scholar]
- 24.Briner VA, Tsai P, Schrier RW. Bradykinin: potential for vascular constriction in the presence of endothelial injury. Am J Physiol. 1993;264:F322–F327. doi: 10.1152/ajprenal.1993.264.2.F322. [DOI] [PubMed] [Google Scholar]
- 25.Joseph K, Tholanikunnel B, Kaplan A. Heat shock protein 90 catalyzes activation of the prekallikrein-kininogen complex in the absence of factor XII. Proc Natl Acad Sci USA. 2002;99:896–900. doi: 10.1073/pnas.022626899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernando AN, Fernando LP, Fukuda Y, Kaplan AP. Assembly, activation, and signaling by kinin-forming proteins on human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;289:H251–H257. doi: 10.1152/ajpheart.00206.2004. [DOI] [PubMed] [Google Scholar]
- 27.Hojima Y, Cochrane CG, Wiggins RC, Austin KF, Stevens RL. In vitro activation of the contact (Hageman Factor) system of plasma by heparin and chondroitin sulfate E. Blood. 1984;63:1453–1459. [PubMed] [Google Scholar]
- 28.Tankersley DL, Finlayson JS. Kinetics of activation and autoactivation of human factor XII. Biochemistry. 1984;23:273–279. doi: 10.1021/bi00297a016. [DOI] [PubMed] [Google Scholar]
- 29.Tans G, Rosing J, Berrettini M, Lammle B, Griffin JH. Autoactivation of human plasma prekallikrein. J Biol Chem. 1987;262:11308–11314. [PubMed] [Google Scholar]
- 30.Renne T, Schuh K, Muller-Esterl W. Local bradykinin formation is controlled by glycosaminoglycans. J immunology. 2005;175:3377–3385. doi: 10.4049/jimmunol.175.5.3377. [DOI] [PubMed] [Google Scholar]
- 31.Gozzo AJ, Motta G, Cruz-Silva I, Nunnes V, Barros NMT, Carmona AK, Sampaio MU, Michelacci YMC, Shimamoto K, Nader HB, Araujo MS. Heparin affects the interaction of kininogen on endothelial cells. Biochimie. 2001;93:1839–1845. doi: 10.1016/j.biochi.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 32.De Dois ST, Frontanilla KV, Nigro J, Ballinger ML, Ivey ME, Cawson EA, Little PJ. Regulation of the atherogenic properties of vascular smooth muscle proteoglycans by anti-hyperglycemic agents. J Diabetes Complications. 2007;21:108–117. doi: 10.1016/j.jdiacomp.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Oohira A, Wight TN, Bornstein P. Sulfacted proteoglycans synthesized by vascular endothelial cells in culture. J Biol Chem. 1983;258:2014–2021. [PubMed] [Google Scholar]