Abstract
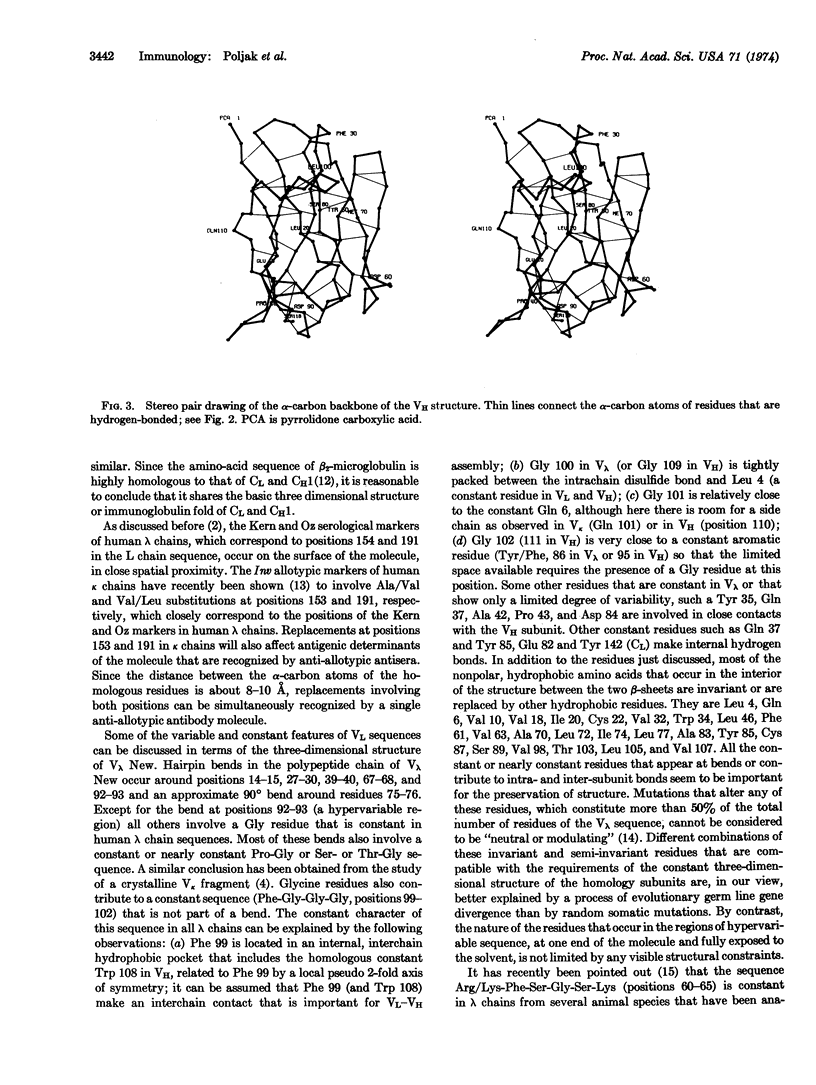
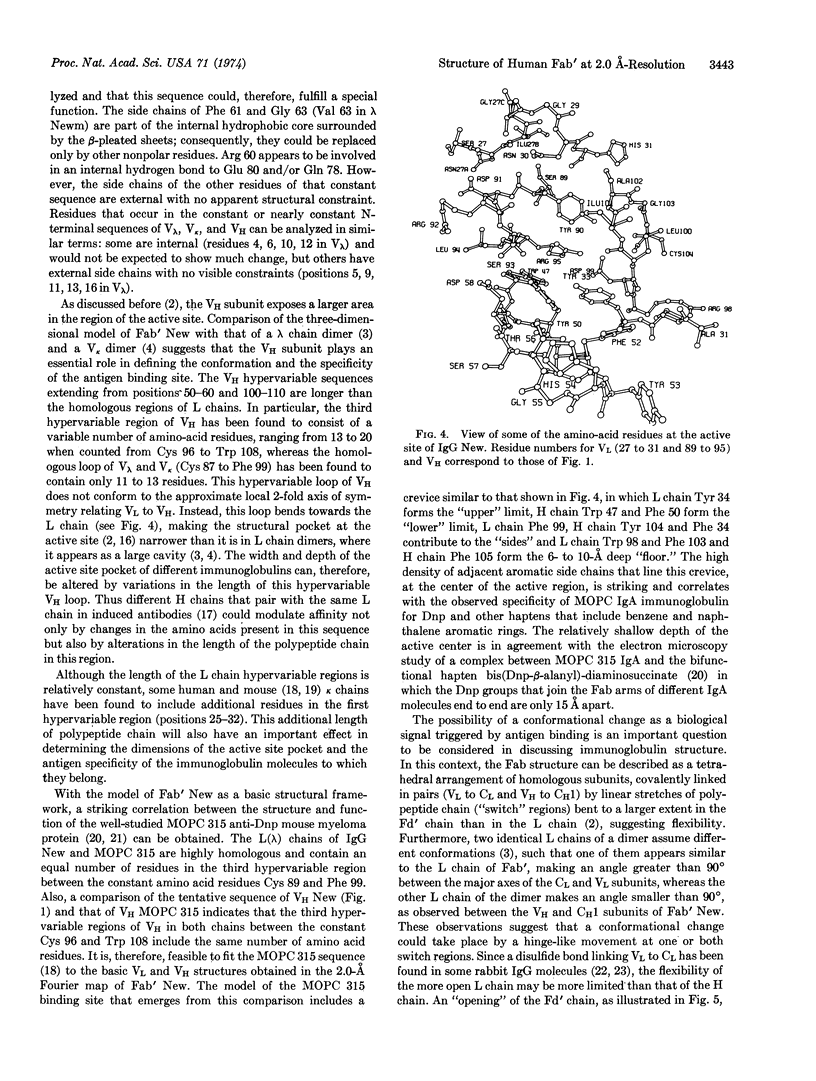
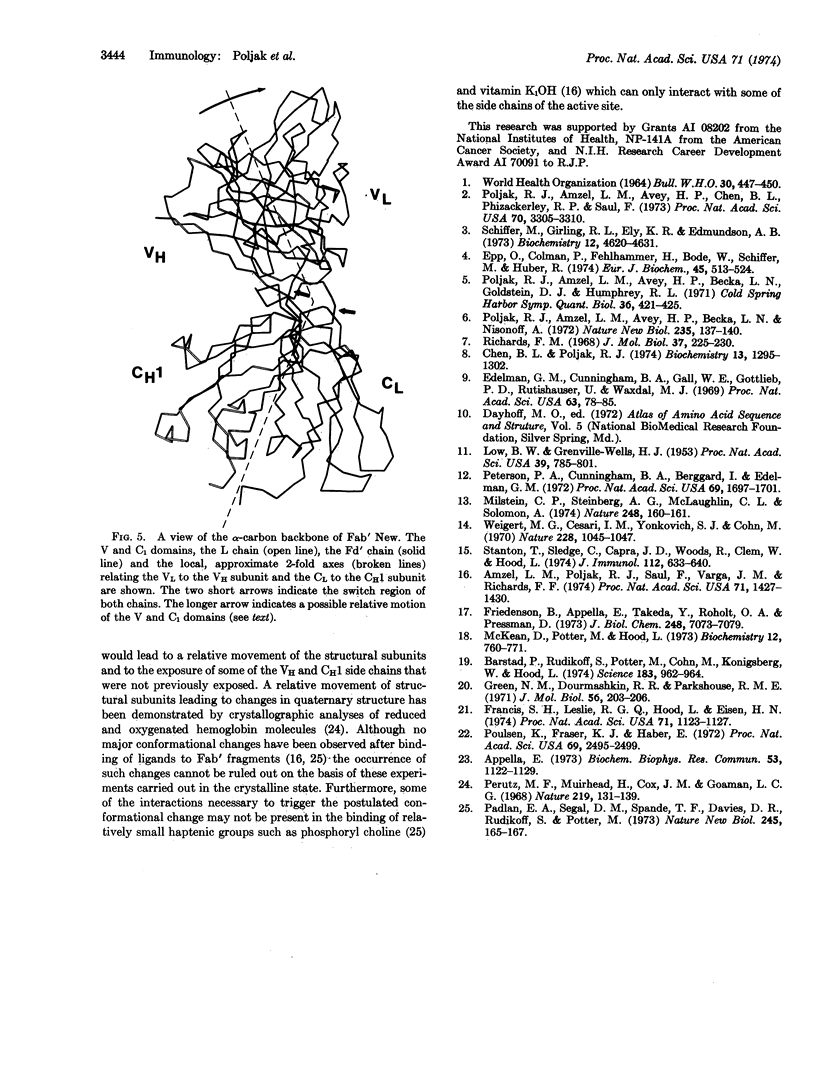
The structural analysis of the Fab′ fragment of human myeloma immunoglobulin IgGl(λ) New has been extended to a nominal resolution of 2.0 Å. Each of the structural subunits corresponding to the variable and to the constant homology regions of the light and heavy chains contains two irregular β-sheets which are roughly parallel to each other and surround a tightly packed interior of hydrophobic side chains. About 50-60% of the amino-acid residues are included in β-pleated sheets. Sequence alignments between the homology regions of Fab′ New obtained by comparison of their three-dimensional structures are given. Some of the sequence variations observed in light and heavy chains and the role of the regions of hypervariable sequence in defining the size and shape of the active site of different immunoglobulin molecules are discussed on the basis of the three-dimensional model of Fab′ New.
Keywords: β-sheets, sequence alignments, hypervariable regions, active site
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amzel L. M., Poljak R. J., Saul F., Varga J. M., Richards F. F. The three dimensional structure of a combining region-ligand complex of immunoglobulin NEW at 3.5-A resolution. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1427–1430. doi: 10.1073/pnas.71.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appella E., Roholt O. A., Chersi A., Radzimski G., Pressman D. Amino acid sequence of the light chain derived from a rabbit anti-p-azobenzoate antibody of restricted heterogeneity. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1122–1129. doi: 10.1016/0006-291x(73)90581-0. [DOI] [PubMed] [Google Scholar]
- Barstad P., Rudikoff S., Potter M., Cohn M., Konigsberg W., Hood L. Immunoglobulin structure: amino terminal sequences of mouse myeloma proteins that bind phosphorylcholine. Science. 1974 Mar 8;183(4128):962–966. doi: 10.1126/science.183.4128.962. [DOI] [PubMed] [Google Scholar]
- Chen B. L., Poljak R. J. Amino acid sequence of the (lambda) light chain of a human myeloma immunoglobulin (IgG New). Biochemistry. 1974 Mar 12;13(6):1295–1302. doi: 10.1021/bi00703a037. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp O., Colman P., Fehlhammer H., Bode W., Schiffer M., Huber R., Palm W. Crystal and molecular structure of a dimer composed of the variable portions of the Bence-Jones protein REI. Eur J Biochem. 1974 Jun 15;45(2):513–524. doi: 10.1111/j.1432-1033.1974.tb03576.x. [DOI] [PubMed] [Google Scholar]
- Francis S. H., Leslie R. G., Hood L., Eisen H. N. Amino-acid sequence of the variable region of the heavy (alpha) chain of a mouse myeloma protein with anti-hapten activity. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1123–1127. doi: 10.1073/pnas.71.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenson B., Appella E., Takeda Y., Roholt O. A., Pressman D. Immunoglobulin G antibodies from an individual rabbit in which several heavy chain variants are paired with one light chain sequence. J Biol Chem. 1973 Oct 25;248(20):7073–7079. [PubMed] [Google Scholar]
- Green N. M., Dourmashkin R. R., Parkhouse R. M. Electron microscopy of complexes between IgA (MOPC 315) and a bifunctional hapten. J Mol Biol. 1971 Feb 28;56(1):203–206. doi: 10.1016/0022-2836(71)90096-9. [DOI] [PubMed] [Google Scholar]
- Low B. W., Grenville-Wells H. J. Generalized Mathematical Relationships for Polypeptide Chain Helices: The Coordinates of the II Helix. Proc Natl Acad Sci U S A. 1953 Aug;39(8):785–801. doi: 10.1073/pnas.39.8.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean D., Potter M., Hood L. Mouse immunoglobulin chains. Pattern of sequence variation among kappa chains with limited sequence differences. Biochemistry. 1973 Feb;12(4):760–771. doi: 10.1021/bi00728a028. [DOI] [PubMed] [Google Scholar]
- Milstein C. P., Steinberg A. G., McLaughlin C. L., Solomon A. Amino acid sequence change associated with genetic marker Inv(2) of human immunoglobulin. Nature. 1974 Mar 8;248(5444):160–161. doi: 10.1038/248160a0. [DOI] [PubMed] [Google Scholar]
- Padlan E. A., Segal D. M., Spande T. F., Davies D. R., Rudikoff S., Potter M. Structure at 4.5 A resolution of a phosphorylcholine-binding fab. Nat New Biol. 1973 Oct 10;245(145):165–167. doi: 10.1038/newbio245165a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Peterson P. A., Cunningham B. A., Berggård I., Edelman G. M. 2 -Microglobulin--a free immunoglobulin domain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1697–1701. doi: 10.1073/pnas.69.7.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Becka L. N., Goldstein D. J., Humphrey R. L. X-ray crystallographic studies of the Fab and Fc fragments of human myeloma immunoglobulins. Cold Spring Harb Symp Quant Biol. 1972;36:421–425. doi: 10.1101/sqb.1972.036.01.054. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Becka L. N. Structure of Fab' New at 6 A resolution. Nat New Biol. 1972 Feb 2;235(57):137–140. doi: 10.1038/newbio235137a0. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Chen B. L., Phizackerley R. P., Saul F. Three-dimensional structure of the Fab' fragment of a human immunoglobulin at 2,8-A resolution. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3305–3310. doi: 10.1073/pnas.70.12.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen K., Fraser K. J., Haber E. An active derivative of rabbit antibody light chain composed of the constant and the variable domains held together only by a native disulfide bond. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2495–2499. doi: 10.1073/pnas.69.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards F. M. The matching of physical models to three-dimensional electron-density maps: a simple optical device. J Mol Biol. 1968 Oct 14;37(1):225–230. doi: 10.1016/0022-2836(68)90085-5. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Girling R. L., Ely K. R., Edmundson A. B. Structure of a lambda-type Bence-Jones protein at 3.5-A resolution. Biochemistry. 1973 Nov 6;12(23):4620–4631. doi: 10.1021/bi00747a013. [DOI] [PubMed] [Google Scholar]
- Stanton T., Sledge C., Capra J. D., Woods R., Clem W., Hood L. A sequence restriction in the variable region of immunoglobulin light chains from sharks, birds, and mammals. J Immunol. 1974 Feb;112(2):633–640. [PubMed] [Google Scholar]
- Weigert M. G., Cesari I. M., Yonkovich S. J., Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970 Dec 12;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]



