Abstract
Objective
Glioblastoma stem-like cells (GSC) exhibit stem-like properties, are highly efficient at forming tumor xenografts, and resistant to many current therapies. Current molecular identifiers of GSCs are scarce and controversial. Here we describe differential cell surface gene expression profiling to identify GSC-specific markers.
Methods
Independent human GSC lines were isolated and maintained in standard neural stem cell media, and validated for self-renewal, multipotent differentiation, and tumor initiation properties. Candidate upregulated GSC-specific plasma membrane markers were identified via differential Affymetrix U133 Plus 2.0 gene expression profiling of GSCs, human neural stem cells (hNSC), normal brain tissue, and primary/recurrent GBM samples. Results were validated by comparative qRT-PCR and western analysis of GSC, hNSC, normal human astrocyte (NHA), U87 glioma, and patient matched serum-cultured GBM.
Results
A candidate GSC-specific signature of 19 upregulated known and novel plasma membrane-associated genes was identified. Preferential upregulation of 7 plasma-membrane-linked genes was validated by qPCR. Cadherin-19 has enhanced GSC-specific protein expression in minimally infiltrative GSC lines.
Conclusions
Gene expression profiling of GSCs has yielded cadherin-19 as an exciting new target for drug development and study of GBM tumorigenesis.
Keywords: cadherin-19, gene expression profiling, glioblastoma multiforme, glioblastoma stem-like cells
Introduction
Glioblastoma multiforme (GBM) is a highly malignant brain tumor with a median survival of 14.6 months 26. GBM often recurs despite maximal surgery, radiation, and chemotherapy. Glioblastoma stem-like cells (GSC) are hypothesized to initiate tumor recurrence and are resistant to current therapeutic approaches 2,3,7,16. Successful GBM therapies will need to address this recalcitrant tumor initiating population in combination with current strategies 10.
To date, isolation or enrichment of cancer stem-like cells has mainly incorporated strategies derived from normal stem cell biology. In hematopoietic 4,21, breast 1, and brain malignancies 23-25, normal stem cell markers were used to first identify stem-like cancer cells validated by their recapitulation of parental tumor pathology with serial implantation into immunodeficient mice. These approaches were effective in enriching for stem-like cancer cells; however, recent investigations have reported that some of the unlabeled cell populations also retain efficient tumor initiating properties 5,19. Moreover, the current markers cannot safely serve as drug targets since they are also expressed by normal adult self-renewing stem cells.
We used an unbiased gene expression profiling-based approach to identify novel GSC-specific plasma membrane markers. Two GSC lines were characterized using gene microarrays compared to human neural stem cells (hNSC), normal human brain, primary GBM, and recurrent GBM tissues. After filtering for plasma membrane transcripts, 19 GSC transcripts with multiple probe sets were found upregulated over normal controls and whole GBM tumor samples. Candidate genes were validated by qRT-PCR with two additional GSC lines, normal human astrocytes (NHA), U87, and serum cultured, patient-matched GBM lines 22T and 33T. Expression of cadherin-19 (CDH19) is restricted to minimally infiltrative GSCs, with no detectable protein in other GSC, GBM, or normal neural cell lines on immunoblotting. These findings suggest that CDH19 (a type II atypical cadherin specific to myelinating cells during development) 30, could serve as a feasible marker for GSC identification, isolation, and drug discovery.
Materials and Methods
GSC, GBM, and Control Cell Line Culture
All studies were performed with approval from the University of Wisconsin-Madison Institutional Review Board (IRB) (2012-0024) with informed consent obtained from patients, and with approval from the Institutional Animal Care and Use Committee (IACUC) (M02223). Glioblastoma stem-like cells (GSC) were isolated the following previously reported protocols 7,12,14,23,27, without the use of surface markers. Briefly, fresh GBM tissue was directly collected according to IRB-approved protocol after histological diagnosis using WHO criteria, weighed, coarsely minced with a scalpel blade, and subsequently chopped 2× at 200 μm using a tissue chopper (Sorvall TC-2 Smith-Farquahar). Chopped tissue was directly plated in suspension, and cultured in passaging medium: 70% Dulbecco modified Eagle medium-high glucose, 30% Ham's F12, 1× B27 supplement, 5 μg/mL heparin, penicillin-streptomycin-amphotericin (PSA), supplemented with 20 ng/ml each of human recombinant epidermal growth factor (EGF) and bovine fibroblast growth factor (bFGF) 27. Sphere cultures were passaged approximately every 7 days by tissue chopping 2× at 100 μm. Individual patient-derived GSC lines 12.1, 22, 33, and 44 were cultured in suspension, and rigorously validated for self-renewal by neurosphere formation, expression of stem cell markers (i.e. AC/CD133), multipotency, tumor initiation, and serial implantation in non-obese diabetic severe combined immunodeficient (NOD-SCID) mice (Harlan Sprague-Dawley) 8,30. Standard serum conditions were used to maintain patient-matched 22T and 33T GBM bulk tumor lines, U87, and normal human astrocytes (NHA) lines (DMEM, 10% fetal bovine serum, 1% antibiotics) (Invitrogen, Grand Island, NY). GSCs were compared to human neural stem cells (hNSC), a kind gift from Dr. Clive Svendsen (Cedars-Sinai Medical Center, Los Angeles, California), and maintained as previously described 27. Establishing and cryopreservation of cell cultures ranged from passages 1-10. Cells used for experiments ranged from passages 20 to 25.
Gene Expression Profiling
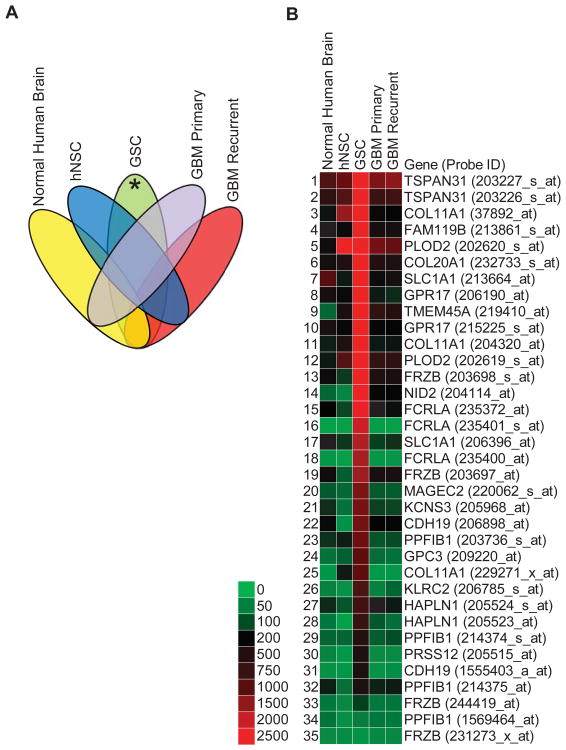
Pooled gene expression profiling of human GSC lines 12.1 and 22 (n=2) (NCBI GEO, GSM1253303 & GSM1253304, respectively) were compared to hNSCs M031 CTX (n=2) (NCBI GEO, GSM458064 & GSM458065), normal human brain (n=21), primary GBM tumors (n=21), and recurrent GBM tumors (n=22) (Table 1). Total RNA was extracted from GSCs with an RNeasy kit (Qiagen), then samples were sent to LC Sciences (Houston, TX) for final gene expression processing and analysis. In brief, total RNA was reverse transcribed with T7-Oligo(dT) primers. After cleanup of cDNA, cRNA was synthesized using in vitro transcription (3′ IVT). cRNA was biotinylated and fragmented before hybridization to an U133 plus 2.0 array (Affymetrix, Santa Clara, CA) that contained probes for over 47,000 transcripts. Hybridized cRNA was labeled with streptavidin-phycoerythrin for visualization. Hybridization images were collected using a laser scanner (GenePix 4000B, Molecular Devices, Sunnyvale, CA, USA) and digitized using Array-Pro image analysis software (Media Cybernetics, Bethesda, MD, USA). U133 plus 2.0 gene expression profiles for hNSCs were obtained from Dr. Clive Svendsen. Profiling data of gross tissue samples of normal human brain, primary GBM and recurrent GBM were obtained from the National Cancer Institute Repository of Molecular Brain Neoplasia Database (NCI REMBRANDT) (Table 1). All of the expression profiles were batch normalized by a robust multichip average (RMA) algorithm using Geospiza GeneSifter (PerkinElmer) online microarray database and analysis software. The data was then exported into Microsoft Office Excel 2010 and organized for GSC transcripts with raw intensity values 10-fold or higher over normal brain, hNSCs, primary GBM and recurrent GBM samples. The reverse sorting algorithm was done to obtain downregulated GSC transcripts (NCBI GEO GSE51822). This analysis provided a list of upregulated plasma membrane transcripts in GSCs (Fig. 1). Since each transcript has multiple probes on U133 plus 2.0 arrays, all the probes for an upregulated transcript are shown to demonstrate signal redundancy. The data is represented as a heat map of raw intensity values with the provided scale.
Table 1.
Gene expression profile file names downloaded from NCI REMBRANDT.
| # | Normal Human Brain (Non-Tumor) | GBM Primary | GBM Recurrent |
|---|---|---|---|
| 1 | NT_HF0088_U133P2 | 1_E09233_U133P2 | 2_E09138_U133P2 |
| 2 | NT_HF0120_U133P2 | 1_E09405_U133P2 | 2_E09139_U133P2 |
| 3 | NT_HF0131_U133P2 | 1_E09451_U133P2 | 2_E09167_U133P2 |
| 4 | NT_HF0137_U133P2 | 1_E09489_U133P2 | 2_E09192_U133P2 |
| 5 | NT_HF0151_U133P2 | 1_E09511_U133P2 | 2_E09231_U133P2 |
| 6 | NT_HF0163_U133P2 | 1_E09531_U133P2 | 2_E09483_U133P2 |
| 7 | NT_HF0171_U133P2 | 1_E09654_U133P2 | 2_E09546_U133P2 |
| 8 | NT_HF0178_U133P2 | 1_E09698_U133P2 | 2_E09601_U133P2 |
| 9 | NT_HF0211_U133P2 | 1_E09704_U133P2 | 2_E09602_U133P2 |
| 10 | NT_HF0232_U133P2 | 1_E09774_U133P2 | 2_E09606_U133P2 |
| 11 | NT_HF0295_U133P2 | 1_E09782_U133P2 | 2_E09610_U133P2 |
| 12 | NT_HF0303_U133P2 | 1_E09832_U133P2 | 2_E09624_U133P2 |
| 13 | NT_HF0377_U133P2 | 1_E09846_U133P2 | 2_E09647_U133P2 |
| 14 | NT_HF0383_U133P2 | 1_E09847_U133P2 | 2_E09649_U133P2 |
| 15 | NT_HF0467_U133P2 | 1_E09857_U133P2 | 2_E09670_U133P2 |
| 16 | NT_HF0512_U133P2 | 1_E09910_U133P2 | 2_E09787_U133P2 |
| 17 | NT_HF0523_U133P2 | 1_E09917_U133P2 | 2_E09791_U133P2 |
| 18 | NT_HF0526_U133P2 | 1_E09938_U133P2 | 2_E09802_U133P2 |
| 19 | NT_HF0533_U133P2 | 1_E09964_U133P2 | 2_E09852_U133P2 |
| 20 | NT_HF0593_U133P2 | 1_E09967_U133P2 | 2_E09868_U133P2 |
| 21 | NT_HF0616_U133P2 | 1_E09998_U133P2 | 2_E09930_U133P2 |
| 22 | NA | NA | 2_E09965_U133P2 |
Figure 1.
Nineteen upregulated plasma membrane GSC-specific transcripts. (A): Logic diagram showing how gene expression profiles were sorted to identify GSC-specific transcripts (*). Normal human brain (n=21), primary GBM (n=21), and recurrent GBM (n=22) gene expression profiles were obtained from NCI REMBRANDT (Table 1). GSCs (n=2) were isolated from two patients at University of Wisconsin. hNSC (n=2) were isolated from two human fetal brains and obtained courtesy of Dr. Clive Svendsen. (B): Heat map with accompanying color key of raw intensity values from gene expression profiles generated from Affymetrix U133 Plus 2.0 arrays and processed with Geospiza GeneSifter (PerkinElmer). Data was filtered for plasma membrane transcripts and gated for GSC values greater than 10-fold change above all other samples. Acronyms correspond to official gene symbols found online at the National Center for Biotechnology Information. Multiple probe sets shown to demonstrate internal reproducibility.
qRT-PCR Validation of mRNA Expression Profiles
Gene expression profiling was validated by qRT-PCR to confirm differential upregulation of GSC transcripts. RNA was isolated from the following cell lines using the RNeasy Kit (Qiagen): GSCs 12.1, 22, 33, and 44; hNSCs; NHAs; serum cultured GBM tumor lines 22T and 33T. Isolated RNA was reverse transcribed with the Omniscript Reverse Transcription Kit (Qiagen) to create a cDNA pool for each cell line. Nona-deoxyribonucleotide random primer mixture (Takara, Shiga, Japan) and human placental optizyme ribonuclease inhibitor (Fisher BioReagents) were used for reverse transcription as per manufacturer's instructions. Quantitative PCR was performed with the Express SYBR GreenER qPCR Supermix Universal Kit (Invitrogen). Reactions were scaled down to 20 μL, combined in an ABI Prism 96-well optical reaction plate, and loaded into an ABI Prism 7000 (Applied Biosystems). Primers were designed according to online tools at Integrated DNA Technologies (http://www.idtdna.com) and only chosen if the predicted amplicon contained two exon regions and a melting temperature near 60°C (Table 2). Intron spanning and gene specificity were confirmed by a NCBI BLAST search (http://blast.ncbi.nlm.gov). 18S RNA was used as the reference gene. hNSC cycle threshold or C(t) values were used as the calibrator for calculations. All other cell lines were set as the target. C(t) values were chosen during linear growth of the produced sigmoidal curves. Gene expression was quantified by the ΔΔC(t) method. In brief, the ΔC(t) value was calculated by subtracting the C(t) of the target with the reference values. The ΔΔC(t) was calculated by subtracting the ΔC(t) of the target with the calibrator. Finally, the formula, 2(x0005E)( - ΔΔC(t)), was used to report fold change differences over the calibrator value.
Table 2.
qRT-PCR primers for gene expression profile validation.
| Accession # | Gene ID | Forward (5′-3′) | Reverse (5′-3′) | Amplicon Size | Amplicon CDS Position (Exons) | Tm (For/Rev) |
|---|---|---|---|---|---|---|
| NR_003286 | 18S | GTTGGTGGAGCGATTTGTCTGGTT | TAGCATGCCAGAGTCTCGTTCGTT | 57 | 1345-1401 (N/A) | 59.6/59.9 |
|
NM_080630 NM_0800629 NM_001854 |
COL11A1 | AATGGAGCTGATGGACCACAAGGA | TCTCCAACACCACCAACTGAACCA | 62 | 3580-3641 (Exon 49-50) | 60.1/60.2 |
| NM_0208862 | COL20A1 | ACCTTGCAGATCTTCGAGCTCACT | TCCTCAATCACAAACTCCCTCCGA | 71 | 274-344 (Exon 4-5) | 59.8/59.4 |
| NM_021153 | CDH19 | AGTCATCACATCGGCCAGCTAAGA | TACTTCCAGCTCCAGCTCCCAAA | 88 | 178-265 (Exon 2-3) | 59.7/59.9 |
|
NM_015433.2 NM_206914.1 |
FAM119B | TGCTGACCATCACGCAGAACTTTG | CCTTCTTGCCTCGGAAATCCACAT | 120 | 116-235 (Exon 1-2) | 59.7/59.1 |
| NM_032738 | FCRLA | CAGCCACTGAGGACAACCAAGTTT | AAGCACCCTGCACTCTGATCTCTA | 66 | 776-841 (Exon 4-5) | 59.4/59.3 |
| NM_001463 | FRZB | TGCCTCTGCCCTCCACTTAATGTT | TACCGAGTCGATCCTTCCACTTCT | 124 | 727-825 (Exon 4-5) | 60.3/58.7 |
| NM_004484 | GPC3 | AACCAGCTCCTGAGAACCATGTCT | TCATCATCACCGCAGTCTCCACTT | 98 | 1408-1505 (Exon 6-7) | 59.7/59.8 |
| NM_005291 | GPR17 | AAACGGAGTTGGTGGGCTGGAT | GCCACTTCAAGGCCATTCATGCTT | 98 | 7-104 (Exon 2-3) | 61.1/60.2 |
| NM_001884 | HAPLN1 | CTGTTGTGGTAGCACTGGACTTAC | CCCAGTCGTGGAAAGTAAGGGAATAC | 58 | 446-503 (Exon 3-4) | 57.1/58.6 |
| NM_002252 | KCNS3 | CCATGAAGTTGGGCTTCTGCTTCT | GGCTGGATGTGTGGTCATCTTTCT | 98 | 963-1060 (N/A) | 59.3/58.9 |
| NM_002260 | KLRC2 | GCCAGCATTTTACCTTCCTCA | ACTGCACAGTTAAGTTCAGCAT | 131 | 493-623 (Exon 5-6) | 60.3/60.2 |
| NM_016249 | MAGEC2 | TGCCAGACAGTGAGTCCTCTTTCA | ACAGGCTCCTCTGCTTCGTATTTG | 97 | 389-385 (N/A) | 59.5/58.7 |
| NM_007361 | NID2 | GCCACAGCAGCATTGATGTTTCCT | TTGGTGTGAGGATCCAAGGTGGAA | 85 | 1013-1097 (Exon 4-5) | 60.1/60.0 |
| NM_00935 NM_182943 |
PLOD2 | TGGACCCACCAAGATTCTCCTGAA | AGTGCAGCCATTATCCTGTGTCCA | 76 | 765-840 (Exon 7-8) | 59.6/60.2 |
|
NM_003622 NM_17744 |
PPFIBP1 | TGCCAGATCCCAGATTCAACAGCA | TCCCTGGTAGGTGTCCATTTGTCA | 79 | 214-292 (Exon 4-5) | 60.4/59.5 |
| NM_003619 | PRSS12 | TTCTGGACTGGGCCTTATTCCCAT | TTTCTTCATCTCCTCGGCAACGGA | 62 | 672-733 (Exon 2-3) | 59.8/60.0 |
| NM_004170 | SLC1A1 | GAAGCAGTGGCAGCGGTGTTTATT | ATGTGGCCGTGATACTGATGGTGA | 88 | 1120-1207 (Exon 10-11) | 60.0/59.9 |
| NM_018004 | TMEM45A | TGGAGCTATTGCGGTCAAGTCTCA | TCCAATCTGAAAGAACCAGCTCCC | 59 | 536-594 (Exon 4-5) | 59.8/58.7 |
| NM_005981 | TSPAN31 | TGCTGGTGAGCTTGTTGCTCATTG | ATGATGTGGATGCTGGACACCAGA | 76 | 62-137 (Exon 1-2) | 60.0/60.0 |
Immunoblotting Analysis
Immunoblotting was performed as described 30. Cells were lysed using cell extraction buffer (Invitrogen, FNN0011) containing protease inhibitor cocktail (Sigma Aldrich, P8340). Total protein was quantified using an EZQ Protein Quantification assay (Invitrogen, R33200). 30 μg of protein were resuspended in 2X reducing sample buffer (Invitrogen, LC2676), electrophoresed on 10-20% tris-glycine gels (Invitrogen), transferred using a semi-dry transfer system (Bio-Rad) to polyvinylidene difluoride membranes (PVDF) (Millipore), and probed with Ms-pAb-anti-CDH19 (Abnova, H00028513-B01P) at 1:250, identified at ∼114k kDa. Rb-pAb-anti-β-actin (Abcam, ab8227) at 1:2000 was used as loading control. Immunocomplexes were detected using luminescence Supersignal West Pico Substrate (Thermo Scientific) per manufacturer instructions.
Results
Differential plasma membrane transcripts in GSCs
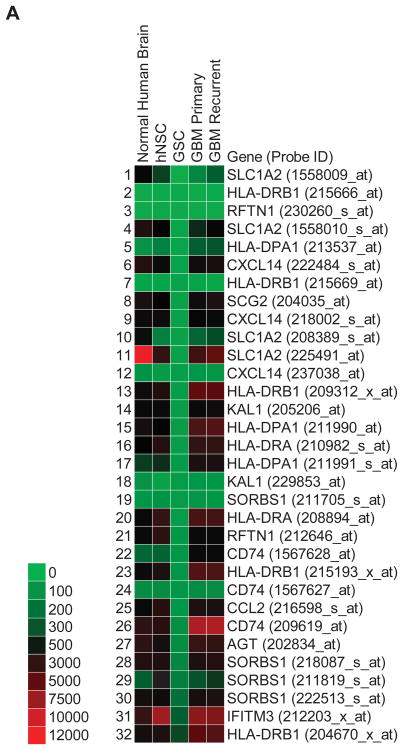
Gene expression profiles were sorted for differentially upregulated (greater than 10-fold) GSC plasma membrane transcripts compared to normal human (non-tumor) brain, human neural stem cells (hNSC), and primary GBM and recurrent GBM (Fig. 1A). 19 distinct transcripts with multiple redundant probe sets were found to be specifically upregulated in GSCs (Fig. 1B). As expected, PROM1 (i.e. CD133) expression in GSCs was not meaningfully upregulated (2.2 fold) over hNSCs, thus demonstrating the validity of our approach. Additionally, a set of downregulated GSC transcripts was generated that include reduced expression of three different human leukocyte antigens (HLA-DRB1, HLA-DPA1, and HLA-DRA) in GSCs (Fig.2).
Figure 2.
Thirteen downregulated plasma membrane GSC-specific transcripts. Heat map with accompanying color key of raw intensity values from gene expression profiles generated from Affymetrix U133 Plus 2.0 arrays and processed with Geospiza GeneSifter (PerkinElmer). Data was filtered for plasma membrane transcripts and gated for GSC values less than 10-fold change below all other samples. Acronyms correspond to official gene symbols found online at the National Center for Biotechnology Information. Multiple probe sets shown to demonstrate internal reproducibility.
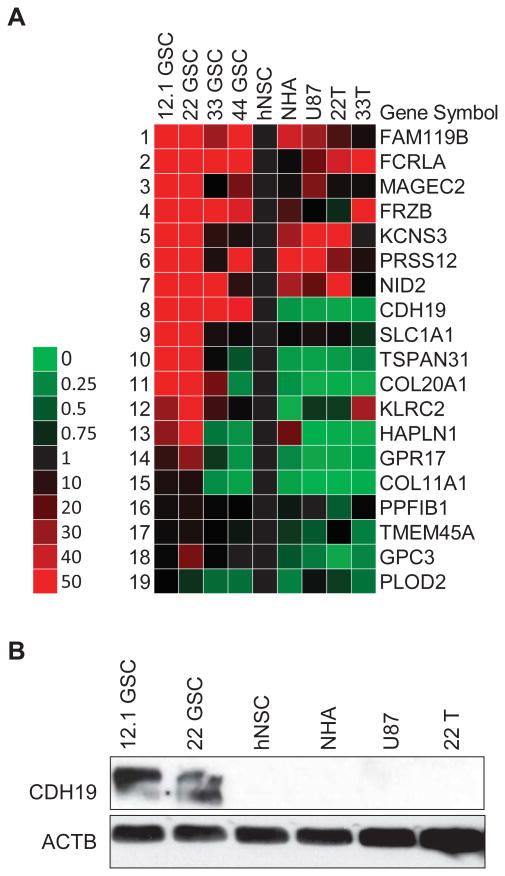
Cadherin-19 is upregulated in minimally infiltrative GSCs
qPCR primers were designed for the signature of 19 upregulated GSC plasma membrane transcripts to validate the mRNA expression profiles (Table 2). Additional GSC lines 33 and 44 were added to this analysis along with serum-cultured normal human astrocytes (NHA), U87 glioma cell line, and patient-matched GBM lines 22T and 33T. GBM lines are representative of the non-stem-like cancer cell population. Cadherin-19 (CDH19) demonstrated elevated transcript levels across all GSC samples compared to hNSCs, NHA, U87, 22T, and 33T (Fig. 3A). SLCA1, TSPAN31, COL20A1, GPR17, and GPC3 also maintained similar expression profiles in qPCR compared to the mRNA microarrays; however, their upregulation did not uniformly extend to GSC lines 33 and 44. Western blotting confirmed 12.1 and 22 GSC-specific expression of CDH19 (Fig. 3B). These two GSC lines express oligodendrocyte and neural progenitor markers and were previously profiled as minimally infiltrative in xenograft studies 30. GSC lines 33 and 44 did not express CDH19 at the protein level (data not shown).
Figure 3.
Cadherin-19 is specifically expressed in minimally infiltrative GSCs. (A): Heat map of qRT-PCR validation of all candidate GSC cell surface markers. Additional GSC lines, 33 & 44 GSCs, were evaluated compared to hNSCs, normal human astrocytes (NHA), U87 glioma cells, and patient matched, serum-cultured GBM tumor cells 22T and 33T. Data is representative of two experimental repetitions normalized to hNSC, and reported as a fold change value with accompanying color key. (B): Western blot of cadherin-19 (CDH19) specifically expressed in 12.1 and 22 GSCs. Ms-pAb-anti-CDH19 (Abnova, H00028513-B01P) at 1:250, identified at ∼114k kDa. Rb-pAb-anti-β-actin (Abcam, ab8227) at 1:2000 was used as a loading control.
Cadherin-19 expression in normal human tissues and GBM tumors
In human tissues, CDH19 transcript (probe ID: 206898_at) is expressed meaningfully in only two tissues, olfactory bulb and dorsal root ganglion (Fig. 4), but their respective raw intensity levels, ∼700 and ∼500, are lower than the ∼2,000 we observe in GSCs (Fig. 1B). Interestingly, patients with tumors expressing elevated levels (greater than 2 fold, n=27) of CDH19 in the NCI REMBRANDT database had a significantly higher survival probability compared to those expressing intermediate levels (p=0.03, Log Rank Test) (Fig. 5). Although, inclusion of down regulated samples provided no statistically significant increase in survival probability (p=0.07, Log Rank Test), it still offered a robust trend towards higher survival probability.
Figure 4.
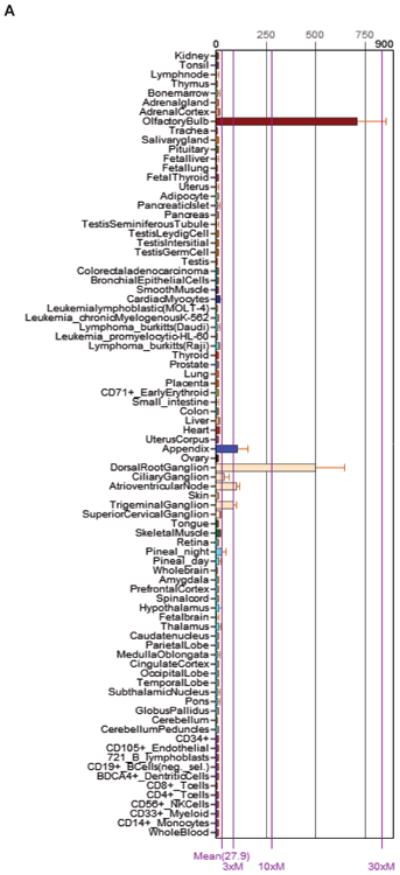
Cadherin-19 transcript found in olfactory bulb and dorsal root ganglia from human tissue in BioGPS. Compiled from gene expression profiles (probe ID: 206898_at). Accessed June 5, 2013 (http://biogps.org).
Figure 5.
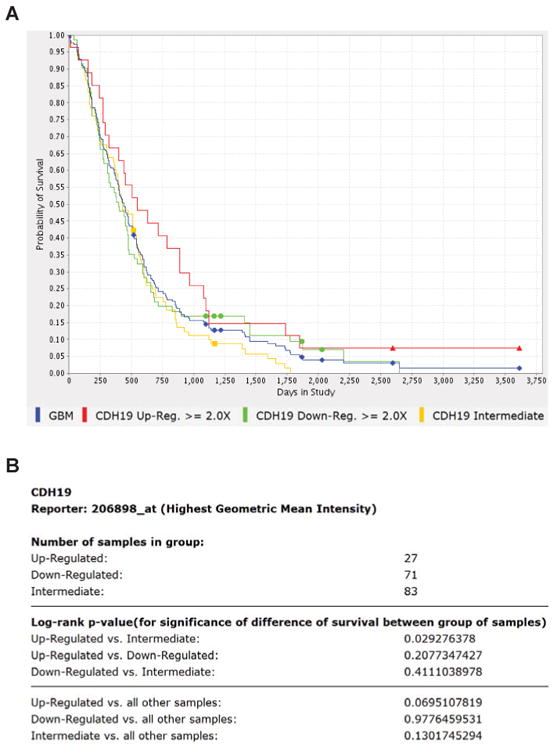
Cadherin-19 upregulation in GBM tissue from NCI REMBRANDT shows higher survival probability. (A): Kaplan-Meir survival probability curve for CDH19 (probe ID: 206898_at, highest geometric mean intensity). Upregulation and downregulation was designated at a 2-fold difference in expression. (B): Statistics generated using a log-rank test. Upregulated vs. intermediate group is statistically significant for difference in survival (p=0.03). Accessed June 5, 2013 (https://caintegrator.nci.nih.gov/rembrandt).
Discussion
We report the identification of cadherin-19 (CDH19) as a marker for minimally infiltrative GSCs through gene expression profiling, qRT-PCR, immunoblot analysis correlated to animal tumor xenograft studies. CDH19 expression is restricted to cells responsible for myelination in the developing nervous systems of chickens and rats and maintains low expression in the adult human olfactory bulb and dorsal root ganglia 15,28. Our understanding of atypical type II cadherin biology remains sparse 18, yet the little we know about CDH19 prompts further investigation into its role in GBM tumorigenesis.
Similar to type I cadherins, crystallographic studies determined that type II cadherins mediate homodimeric adhesion through their EC1 domain, yet structural differences suggest their inability to cross-interact with type I cadherins, thereby conferring confined functionality within tissues 18. CDH19 is expressed developmentally in chicken Schwann cells and oligodendrocytes 15, and in rat Schwann cell precursors and cranial ganglia 28. Restricted cellular expression of type-II cadherins in the developing nervous system and their functional isolation from other cadherin family members make them ideal potential markers and targets for GSC identification, isolation, and drug discovery.
CDH19-restricted expression in minimally infiltrative GSCs and its potential role in enhancing survival probability in GBM patients may not be a coincidence. GSC lines 12.1 and 22 morphologically resemble oligodendrocyte progenitor cells (OPC) and express OPC markers such as 2′, 3′-cyclic-nucleotide 3′-phosphodiesterase (CNP) – a prognostically favorable immunohistochemical marker for GBM 30. Likewise, CDH19 upregulation was prognostically favorable in NCI REMBRANDT against a subset of samples (Fig. 5). Since CDH19 expression is limited to the GSCs, pooled mRNA transcripts from a whole tumor sample are likely diluted for this analytical marker. Immunohistochemical labeling seems to improve GSC resolution, as we observed with pockets of CNP expression in a clinically-annotated GBM tissue microarray 30, yet CNP was not prognostically favorable the samples of NCI REMBRANDT.
Recent literature revealed a potential tumorigenic role for type II cadherins. Transcriptional regulation of CDH19 and CDH12 was reportedly targeted by monocyte chemotactic protein-1 induced protein (MCPIP), which promoted capillary-like tube formation in human umbilical vein endothelial cells (HUVEC) 17. MCPIP knockdown via small interfering RNA suppressed angiogenesis-related genes VEGF and HIF1-alpha, CDH19, and CDH12, and reduced capillary-like tube formation. Pathologically, vascular endothelial proliferation is observed in OPC-like minimally infiltrative GSC xenografts, but not seen in tumor xenografts from high infiltrative GSC that express abundant astrocyte progenitor cell markers 30. Perhaps CDH19-related upregulation of angiogenic signaling enables certain GSCs to grow into focal tumors instead of invading the normal brain parenchyma. Conversely, another type II cadherin was found expressed as proteins in GBM and GSCs: cadherin-11 (CDH11). Knockdown of CDH11 in serum-cultured U87 and LN-229 GBM tumor cells reduced migration in a scratch wound assay, and shortened survival in a flank xenograft model 13. Thus, each cadherin family member exerts different properties, and must be studied independently to appreciate the extent of their involvement in GBM tumorigenesis.
In addition to CDH19, other differentially expressed transcripts that associate with cancer and oligodendrocyte biology were revealed in our studies. Glypican-3 (GPC3) transcript was upregulated in minimally infiltrative GSCs and confirmed by qRT-PCR; it was also described as a bonafide marker and drug target for hepatocellular carcinoma 9, and highly correlated with CD90+ liver cancer stem-like cells 11. This is the first report to establish its relationship with GBM. Likewise, upregulation of oligodendrocyte-specific G protein-coupled receptor 17 (GPR17) mRNA was validated in GSC lines 12.1 and 22, described as an intrinsic timer of myelination 6, thereby strengthening the OPC-like resemblance of these GSCs.
Three downregulated transcripts of interest were human leukocyte antigen (HLA) class II genes, HLA-DRB1, HLA-DPA1, and HLA-DRA, also known as major histocompatibility complex (MHC) proteins. Typically in these gene expression profiles, we observe low levels in human brain, higher levels in GBM tumors, and downregulated expression in GSCs. Most astrocytomas are infiltrated with immune-effector cells, primarily consisting of cytotoxic T cells (CTL) and macrophages 20, presumably due to expression of HLA/MHC class II genes, as we have observed. This response can be activated by cytokine-based immunotherapy that target HLA/MHC class II molecules 22, however, their eventual failure against GBM may be due to the absence or low expression of HLA/MHC class II molecules in GSCs responsible for initiating and maintaining tumor growth.
Conclusions
Our data and analysis have shown that CDH19 may be a suitable marker and drug target for minimally infiltrative GSCs. Its low expression in developing neuroectodermal tissue, specific upregulation in GSCs, and potential angiogenic role in tumorigenesis prompt further development of research tools for study. Currently, no antibodies exist for live cell surface labeling of CDH19 that would help reveal possible associations with GSC tumor initiation. Since CDH19 is typically a homodimerizing plasma membrane protein, purified CDH19 conjugated with corresponding visualization chemistries could potentially be used as a research tool, however, potential heteromeric cadherin-nectin complexes may complicate this analysis 29. Future experiments are planned to address CDH19's potential involvement in GSC tumor initiation, angiogenesis, migration, and proliferation. Moreover, characterization of CDH19 in lower grade gliomas and oligodendrogliomas may provide more insights to glioma biology.
Acknowledgments
We thank Priya Ezhilan, Daniel Treisman, Jonathan D. Ebben, and Frank Hospod for expert technical assistance. Expert advice was generously provided by C. Svendsen, R. Vemuganti, and S. Zhang. We appreciate support from NIH T32GM007507, UL1RR025011, RC4AA020476, NCI HHSN261201000130C, P30CA014520 grants, the Wisconsin Partnership Program core grant support of the Center for Stem Cell and Regenerative Medicine, the University of Wisconsin (Graduate School, School of Medicine and Public Health and Dept. of Neurological Surgery), the HEADRUSH Brain Tumor Research Professorship and Roger Loff Memorial Fund for GBM Research.
List of Abbreviations
- CNP
2′, 3′-cyclic-nucleotide 3′-phosphodiesterase
- bFGF
bovine fibroblast growth factor
- CDH11
cadherin-11
- CDH12
cadherin-12
- CDH19
cadherin-19
- CTL
cytotoxic T cells
- GPR17
G protein-coupled receptor 17
- GBM
glioblastoma multiforme
- GSC
glioblastoma stem-like cells
- GPC3
glypican-3
- HLA
human leukocyte antigens
- hNSC
human neural stem cells
- EGF
human recombinant epidermal growth factor
- HUVEC
human umbilical vein endothelial cells
- MHC
major histocompatibility complex
- MCPIP
monocyte chemotactic protein-1 induced protein
- NCI REMBRANDT
National Cancer Institute Repository of Molecular Brain Neoplasia Database
- NOD-SCID
non-obese diabetic severe combined immunodeficient
- NHA
normal human astrocyte
- OPC
oligodendrocyte progenitor cells
- PSA
penicillin-streptomycin-amphotericin
- PVDF
polyvinylidene difluoride membranes
- RMA
robust multichip average
Footnotes
Contributing Authors: Michael Zorniak, Ph.D., The Scripps Research Institute, The Skaggs Institute for Chemical Biology, Departments of Chemistry and Cell and Molecular Biology, 10550 North Torrey Pines Road, BCC-526, La Jolla, CA 92037, USA, zorniak@scripps.edu
Paul A. Clark, Ph.D., University of Wisconsin School of Medicine and Public Health, Department of Neurological Surgery, CSC K4/879, 600 Highland Ave, Madison, WI 53792-8660, Phone: 608-262-7629, Fax: 608-263-1728, p.clark@neurosurgery.wisc.edu
Institutional Review Board (IRB) Assurance Number: 2012-0024
Institutional Animal Care and Use Committee (IACUC) Assurance Number: M02223
Database Repository and Accession Numbers: 12.1 GSC (NCBI GEO, GSM1253303)
22 GSC (NCBI GEO, GSM1253304)
hNSCs M031 CTX (NCBI GEO, GSM458064 & GSM458065)
Normal Human Brain, GBM Primary, GBM Recurrent (NCI REMBRANDT, Table 1)
Disclosure of Potential Conflicts of Interest: We do not report any conflicts of interest or competing interests.
Authors' Contributions: MZ, PAC, JSK designed all experiments; MZ and PAC collected, assembled, and analyzed data; PAC collected cell lines and arranged gene expression profiling; MZ performed qRT-PCR and western blotting; JSK provided financial support, study materials and supervised this study; MZ wrote the manuscript with comments and editing from all authors; all authors read and approved the final version of the manuscript.
References
- 1.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 3.Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 5.Chen R, Nishimura MC, Bumbaca SM, Kharbanda S, Forrest WF, Kasman IM, et al. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell. 2010;17:362–375. doi: 10.1016/j.ccr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Wu H, Wang S, Koito H, Li J, Ye F, et al. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat Neurosci. 2009;12:1398–1406. doi: 10.1038/nn.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark PA, Iida M, Treisman DM, Kalluri H, Ezhilan S, Zorniak M, et al. Activation of multiple ERBB family receptors mediates glioblastoma cancer stem-like cell resistance to EGFR-targeted inhibition. Neoplasia. 2012;14:420–428. doi: 10.1596/neo.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark PA, Treisman DM, Ebben J, Kuo JS. Developmental signaling pathways in brain tumor-derived stem-like cells. Dev Dyn. 2007;236:3297–3308. doi: 10.1002/dvdy.21381. [DOI] [PubMed] [Google Scholar]
- 9.Feng M, Gao W, Wang R, Chen W, Man YG, Figg WD, et al. Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2013;110:E1083–1091. doi: 10.1073/pnas.1217868110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Ho DW, Yang ZF, Yi K, Lam CT, Ng MN, Yu WC, et al. Gene expression profiling of liver cancer stem cells by RNA-sequencing. PLoS One. 2012;7:e37159. doi: 10.1371/journal.pone.0037159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 13.Kaur H, Phillips-Mason PJ, Burden-Gulley SM, Kerstetter-Fogle AE, Basilion JP, Sloan AE, et al. Cadherin-11, a marker of the mesenchymal phenotype, regulates glioblastoma cell migration and survival in vivo. Mol Cancer Res. 2012;10:293–304. doi: 10.1158/1541-7786.MCR-11-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Lin J, Luo J, Redies C. Cadherin-19 expression is restricted to myelin-forming cells in the chicken embryo. Neuroscience. 2010;165:168–178. doi: 10.1016/j.neuroscience.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP) J Biol Chem. 2008;283:14542–14551. doi: 10.1074/jbc.M802139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel SD, Ciatto C, Chen CP, Bahna F, Rajebhosale M, Arkus N, et al. Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell. 2006;124:1255–1268. doi: 10.1016/j.cell.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 19.Patru C, Romao L, Varlet P, Coulombel L, Raponi E, Cadusseau J, et al. CD133, CD15/SSEA-1, CD34 or side populations do not resume tumor-initiating properties of long-term cultured cancer stem cells from human malignant glio-neuronal tumors. BMC Cancer. 2010;10:66. doi: 10.1186/1471-2407-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plautz GE, Miller DW, Barnett GH, Stevens GH, Maffett S, Kim J, et al. T cell adoptive immunotherapy of newly diagnosed gliomas. Clin Cancer Res. 2000;6:2209–2218. [PubMed] [Google Scholar]
- 21.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 23.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 24.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 25.Son MJ, Woolard K, Nam DH, Lee J, Fine HA. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440–452. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 27.Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, et al. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi M, Osumi N. Identification of a novel type II classical cadherin: rat cadherin19 is expressed in the cranial ganglia and Schwann cell precursors during development. Dev Dyn. 2005;232:200–208. doi: 10.1002/dvdy.20209. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka Y, Nakanishi H, Kakunaga S, Okabe N, Kawakatsu T, Shimizu K, et al. Role of nectin in formation of E-cadherin-based adherens junctions in keratinocytes: analysis with the N-cadherin dominant negative mutant. Mol Biol Cell. 2003;14:1597–1609. doi: 10.1091/mbc.E02-10-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zorniak M, Clark PA, Leeper HE, Tipping MD, Francis DM, Kozak KR, et al. Differential expression of 2′,3′-cyclic-nucleotide 3′-phosphodiesterase and neural lineage markers correlate with glioblastoma xenograft infiltration and patient survival. Clin Cancer Res. 2012;18:3628–3636. doi: 10.1158/1078-0432.CCR-12-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Web Resources
- 1.Geospiza GeneSifter. [Accessed September 30, 2011];Online Microarray Database and Analysis Software. https://login.genesifter.net.
- 2.National Cancer Institute (NCI) Repository for Molecular Brain Neoplasia Data (REMBRANDT) [Accessed June 5, 2013];Database. http://rembrandt-db.nci.nih.gov.
- 3.BioGPS. [Accessed June 5, 2013];Customizable Gene Annotation Portal. http://biogps.org/#goto=welcome.