Abstract
A novel acetone extract from cottonseed oil sludge was firstly discovered against plant viruses including Tobacco mosaic virus (TMV), Rice stripe virus (RSV) and Southern rice black streaked dwarf virus (SRBSDV). Gossypol and β-sitosterol separated from the acetone extract were tested for their effects on anti-TMV and analysed by nuclear magnetic resonance (NMR) assay. In vivo and field trials in different geographic distributions and different host varieties declared that this extract mixture was more efficient than the commercial agent Ningnanmycin with a broad spectrum of anti-plant-viruses activity. No phytotoxic activity was observed in the treated plants and environmental toxicology showed that this new acetone extract was environmentally friendly, indicating that this acetone extract has potential application in the control of plant virus in the future.
Introduction
Viral diseases have long threatened sustainable development of agriculture [1, 2]. Over the last decades, efforts have been exerted to develop strategies for efficient control of them [3–5]. Biological and chemical methods have great potential for this purpose. Ningnanmycin is one of the most successfully registered anti-plant viral agent and displayed 56.0% in vivo curative effect at 500 μg mL-1 [3]. Ribavirin, another widely used plant viral inhibitor, has lower than 50% inhibitory effects at 500 μg mL-1 [3]. Lower cure rates (30–60%) and probably negative effects on environments of these antiviral agents limit their wider applications. Many efforts still have to be done to develop highly efficient and environmentally friendly antiviral agents.
Nowadays, natural-product-based antiviral agents are more popular than those produced by chemosynthesis. Since plants have evolved multiple mechanisms to resist pathogens by producing secondary metabolites with antimicrobial activities, natural products from plants have been proved to be a rich resource of antiviral agent. Moreover, nature-derived plant substances have less negative impact on the environment, as they are readily broken down and have short-lasting residual periods [6, 7]. With the increasing worldwide concern on virus diseases, exploitation of new nature-derived anti-viral agents received increasing attentions.
Tobacco mosaic virus (TMV), the first plant virus to be discovered, has been listed among the top 10 plant viruses in molecular plant pathology [2], and maintained as a model virus for more than 110 years [8]. Rice stripe virus (RSV) and Southern rice black streaked dwarf virus (SRBSDV) are two of the most damaging viruses in rice [9, 10]. In our preliminary study, we found that acetone extract from cottonseed oil sludge displayed moderate antiviral activity against plant viruses. The aim of this study was, therefore, to analyze the antiviral activity of the acetone extract compare its effects with Ningnanmycin, effective compounds were separated from the acetone extract. Phytotoxic activity and environmental toxicology of the acetone extract were also tested.
Materials and Methods
Materials
In order to improve the concentration of bioactive compounds in the acetone extract, cottonseed oil sludge was used as the raw material, instead of cottonseed. It was purchased from Jingyang Sanqu oil factory in shaanxi province. TMV isolates were provided by the State Key Laboratory of Crop Stress Biology for Arid Areas, Northwest A&F University in the form of virus infected plants of Nicotiana tabacum L. cv. K326. Nicotiana glutinosa was used as a local lesion host and cultivated in the insect-free greenhouse at 23–25°C. The experiments could be conducted when the plants grow to 5–6 leaves (about 8 weeks old). For the field trials, Rice (Oryza sativa L.) infected with RSV and SRBSDV all the year around was used to test the antiviral activities of the acetone extract. The experiment was carried out in most of the main rice producing regions in China (Table 1).
Table 1. Antiviral activity of the acetone extract against Rice stripe virus (RSV) under field conditions.
| Testing units and test site | Specific location | Contact Person | Host variety | Plot area for seeding | Compound | Dosage g ha-1 | Average disease index | Average control effect (%) | Significant difference analysis 5% |
|---|---|---|---|---|---|---|---|---|---|
| Zhangzhou Academy of Agricultural Sciences, Zhangzhou, Fujian | Zhangzhou City Longwen Area Chaoyang Town | Shunzhang Wu | Teyou Duoxi 1 | 20 m2 | Acetone extract | 300 | 2.46 | 51.01 | c |
| 450 | 1.66 | 63.83 | b | ||||||
| 600 | 1.24 | 75.06 | a | ||||||
| 8% Ningnanmycin | 750 | 1.69 | 66.32 | b | |||||
| Control | 4.89 | - | |||||||
| Institute of Plant Protection, Fujian Academy of Agricultural Sciences Longyan, Fujian | Longyan City Shanghang County Huyang Town Huyang Village | Furu Chen | Shanyou 63 | 30 m2 | Acetone extract | 300 | 3.21 | 51.8 | bA |
| 450 | 2.89 | 56.6 | abA | ||||||
| 600 | 2.59 | 61.11 | aA | ||||||
| 8% Ningnanmycin | 750 | 2.4 | 63.96 | aA | |||||
| Control | 6.66 | - | - | ||||||
| Institute of Plant Protection, Jiangsu Academy of Agricultural Sciences, Guanyun Jiangsu | Lianyungang City Guanyun County | Zhonghou Chen | Xindao 18 | 25 m2 | Acetone extract | 300 | 1.13 | 50.52 | b |
| 450 | 1.14 | 56.57 | a | ||||||
| 600 | 0.94 | 61.3 | a | ||||||
| 8% Ningnanmycin | 750 | 1.08 | 55.45 | ab | |||||
| Control | 2.77 | - | - | ||||||
| College of Agronomy, Jiangxi Agricultural University, Jiangxi | Jiujiang City Pengze County Qianshan Town Minjiaqiao Village | Jianmei Xiong | Liangyoupeijiu | 60 m2 | Acetone extract | 300 | 2.4 | 65.07 | a |
| 450 | 2.18 | 68.12 | a | ||||||
| 600 | 2.01 | 70.79 | a | ||||||
| 8% Ningnanmycin | 750 | 2.35 | 66.18 | a | |||||
| Control | 7.1 | - | - | ||||||
| College of Agronomy, Jiangxi Agricultural University, Jiangxi | Jiujiang City Pengze County Qianshan Town Minjiaqiao Village | Jianmei Xiong | Liangyou 6326 | 60 m2 | Acetone extract | 300 | 2.45 | 67.19 | a |
| 450 | 2.32 | 68.74 | a | ||||||
| 600 | 2.09 | 72.19 | a | ||||||
| 8% Ningnanmycin | 750 | 2.38 | 67.8 | a | |||||
| Control | 7.54 | - | - | ||||||
| Hunan plant Protection Institute, Hunan | Changsha City Changsha County Langli Town | Yong Liu | Jinyou 207 | 30 m2 | Acetone extract | 300 | 2.64 | 64.42 | b |
| 450 | 2.09 | 71.73 | ab | ||||||
| 600 | 1.48 | 80.23 | aA | ||||||
| 8% Ningnanmycin | 750 | 2.01 | 72.79 | ab | |||||
| Control | 7.46 | - | - | ||||||
| Hunan plant Protection Institute, Hunan | Changsha City Changsha County Langli Town | Yong Liu | Jinyou 207 | 30 m2 | Acetone extract | 300 | 3.38 | 63.66 | cB |
| 450 | 2.78 | 70.42 | b | ||||||
| 600 | 2.02 | 78.31 | aA | ||||||
| 8% Ningnanmycin | 750 | 2.71 | 71.07 | b | |||||
| Control | 9.49 | - | - |
Extract of the antiviral agent
The acetone extract was drawn by acetone from cottonseed oil sludge, which was the precipitate of cottonseed oil by adding sodium hydrate (15 baume degree). One kilogram of cottonseed oil sludge was dissolved in three liter of acetone. The mixture was agitated and kept at room temperature for 3 hour, and then filtrated. The residual filter was extracted again in the same way as described above and finally the two extracts were mixed together.
Separation of active compound
The 10 litre acetone extract from cottonseed oil sludge was evaporated and dissolved in water, and extracted with the same volume of petroleum ether, ethyl acetate and n-butanol, respectively. The ethyl acetate extracts and n-butanol extracts were isolated by means of silica gel (Qingdao Haiyang Chemical Co., Qingdao, China) and reverse-phase (RP-18) column chromatography (YMC-Triart C18) (YMC America, Inc. Pennsylvania, America), and were further purified by Sephedex LH-20 (40–70 μm) (Amersham Pharmacia Biotech AB, Uppsala, Sweden). Thin-layer chromatography was conducted on silica gel plates GF254 (Qingdao Haiyang Chemical Co., Qingdao, China). The products were unequivocally characterized by NMR AVANCE III (Switzerland), using Bruker Topspin software version 3.0 a.
Assays of antiviral activity in the greenhouse
By using the half-leaf method, we tested inhibition activities of the extract mixture and also the individual compounds gossypol and β-sitosterol, which were purchased from Sigma. Single or combination of gossypol and β-sitosterol were all dissolved with acetone and diluted with water at the concentrations of 100 and 500 μg mL-1. Ningnanmycin (8%) was diluted with water at the concentration of 500 μg mL-1 according to the manufacturer’s instructions. The extract mixture was dissolved with acetone and diluted with water at the concentrations of 100 and 500 μg mL-1.
TMV was purificated by using Gooding’s method [11] and Nicotiana tabacum L. cv. K326 inoculated with TMV was used. The upper leaves were selected, ground in phosphate buffer, and then filtered through a double-layer pledget. The filtrate was centrifuged at 10,000 g, treated twice with PEG, and centrifuged again. The whole experiment was carried out at 4°C. Absorbance values were estimated at 260 nm by using an ultraviolet spectrophotometer. TMV diluted to 6 ×10–3 mg mL-1 was used for the following experiment. Virus concentration was defined as the following formula.
Inactivation effect, protective effect and curative effect were tested as follows. Inactivation effect of acetone extract, β-sitosterol, gossypol and Ningnanmycin against TMV in vivo. The purified TMV (6 ×10–3 mg mL-1) was mixed with acetone extract, β-sitosterol, gossypol or Ningnanmycin solution at the same volume or concentration, respectively. After 30 min the mixture was rubbed on the left side of the leaves of tobacco (Nicotiana glutinosa), whereas the right side of the leaves was inoculated with the mixture of the solvent acetone and the purified TMV as control. The local lesion numbers were recorded 4–5 days after inoculation. Each experiment was repeated three times.
Protective effect of the acetone extract, β-sitosterol, gossypol and Ningnanmycin against TMV in vivo. The solution of the acetone extract, β-sitosterol, gossypol or Ningnanmycin was smeared on the left side respectively and the solvent acetone serving as control on the right side of Nicotiana glutinosa leaves of the same ages. After 12 h the leaves were then inoculated with the purified virus (TMV at 6 ×10–3 mg mL-1), which was previously scattered with silicon carbide. The leaves were then washed with water. The local lesion numbers were recorded 4–5 days after inoculation. Each experiment was repeated three times.
Curative effect of the acetone extract, β-sitosterol, gossypol and Ningnanmycin against TMV in vivo. Purified TMV at 6 ×10–3 mg mL-1 was inoculated on the whole leaves of tobacco (Nicotiana glutinosa) of the same age. Then the leaves were washed with water and dried. The tested solutions of the acetone extract, β-sitosterol, gossypol or Ningnanmycin were smeared on the left side, and the solvent acetone was rubbed on the right side as control. The local lesion numbers were then counted and recorded 4–5 days after inoculation. Each experiment was repeated three times.
The in vivo inhibition rates of the compounds were then calculated according to the following formula. Inhibition rate (%) = [(C-T)/C] × 100%, where C is average local lesion No. of control, and T is average local lesion No. of drug-treated tobacco leaves.
Analysis of inhibition effect of the acetone extract on TMV by Dot-ELISA. Leaves of N. tabacum cv. K326 were prepared according to the Leaf Disk Method. Growing leaves of 8-week-old tobacco were mechanically inoculated with equal volumes of purified TMV (30 μg mL-1). After 72 h, leaves that were smooth and thin and without major veins were cut off and floated in the different concentrations of the acetone extract (100 μg mL-1, 200 μg mL-1, 400 μg mL-1 and 800 μg mL-1), Ningnanmycin (200 μg mL-1) or the solvent acetone (positive control), respectively, and were kept in a culture chamber at 25°C for 48 h. Then leaves were collected and the Dot-ELISA assay was used to analyze the coat protein of TMV by using a commercial kit according to the manufacturer’s instructions (Neogen, Beijing, China).
Antiviral activity of the acetone extract in the field trials
The experiment was carried out by different institutes or universities in several outdoor experimental plots in the south of China (Tables 1 and 2), including Experimental Fields of Institute Zhangzhou Academy of Agricultural Sciences; Institute of Plant Protection, Fujian Academy of Agricultural Sciences; Institute of Plant Protection, Jiangsu Academy of Agricultural Sciences; College of Agronomy, Jiangxi Agricultural University; Hunan plant Protection Institute; Pesticides Administration of Jiangxi; College of Biosafety Science and Technology, Hunan Agricultural University. All the tested fields were private lands which belong to different institutes or universities and used especially for field test, specific locations and Contact person were shown in Tables 1 and 2. No specific permissions were required and no endangered or protected species were involved throughout the tested fields. In these fields, rice was naturally co-infected with Rice stripe virus (RSV) and Southern rice black streaked dwarf virus (SRBSDV) throughout the year. Twenty days after transplanting, the compounds (the extract mixture and Ningnanmycin) were sprayed twice with an interval of 14 days. Three dosages were used for the acetone mixture at 300, 450 and 600 g ha-1, compared with 8% Ningnanmycin at 750 g ha-1. Both of these compounds were diluted with 750 L water per hectare. Water alone was used as negative control. Each experiment was repeated four times and the area of each district was varied from 20–60 m2, depending on different geographical locations in the south of China (Tables 1 and 2). The survey was carried out following the national standard GB/T 17980. 19–2000 called ‘pesticide, guidelines for the field efficacy trials, fungicides against leaf diseases of rice’. In each plot, 50 plants for each of 5 points were selected in a diagonal line. Flag leaf, top second leaf and top third leaf were investigated in each plant and recorded as total leaf number, number of diseased leaves and disease grade. The classification of disease conditions were described as follows. Grade 0: no disease found in the whole plant; Grade 1: percentage of lesion size out of the total leaf area is lower than 1% and the length of lesion length is shorter than 0.3 cm; Grade 3: percentage of lesion size out of the total leaf area is between 2–5% and the lesion length is longer than 0.3 cm; Grade 5: percentage of lesion size out of the total leaf area is between 6–25%. Some lesions connect and are longer than 1 cm; Grade 7: percentage of lesion size out of the total leaf area is between 26–50%. Most of the lesions connect and are longer than 1 cm; Grade 9: percentage of lesion size out of the total leaf area is more than 50%. Lesions connect and large area of the leaf necroses. The disease index and control effects were calculated according to the following formulas.
Table 2. Antiviral activity of the acetone extract against Southern rice black streaked dwarf virus (SRBSDV) under field conditions.
| Testing units and test site | Specific location | Contact Person | Host variety | Plot area for seeding | Compound | Dosage g ha-1 | Average disease index | Average control effect | Significant difference analysis 5% |
|---|---|---|---|---|---|---|---|---|---|
| Zhangzhou Academy of Agricultural Sciences, Zhangzhou, Fujian | Zhangzhou City Longwen Area Chaoyang Town | Shunzhang Wu | Teyou Duoxi 1 | 20 m2 | Acetone extract | 300 | 2.11 | 43.67 | c |
| 450 | 1.59 | 57.33 | b | ||||||
| 600 | 1.18 | 67.85 | a | ||||||
| 8% Ningnanmycin | 750 | 1.31 | 63.90 | ab | |||||
| Control | 3.71 | - | - | ||||||
| Institute of Plant Protection, Fujian Academy of Agricultural Sciences Longyan, Fujian | Longyan City Shanghang County Huyang Town Huyang Village | Furu Chen | Teyou 175 | 30 m2 | Acetone extract | 300 | 5.5 | 55.09 | b |
| 450 | 4.0 | 67.34 | ab | ||||||
| 600 | 3.25 | 73.46 | a | ||||||
| 8% Ningnanmycin | 750 | 3.75 | 69.38 | a | |||||
| Control | 12.25 | - | - | ||||||
| Institute of Plant Protection, Jiangsu Academy of Agricultural Sciences, Guanyun Jiangsu | Lianyungang City Guanyun County | Zhonghou Chen | Xudao 3 | 25 m2 | Acetone extract | 300 | 0.8 | 48.61 | c |
| 450 | 0.72 | 55.64 | b | ||||||
| 600 | 0.68 | 62.16 | a | ||||||
| 8% Ningnanmycin | 750 | 0.7 | 56.16 | b | |||||
| Control | 1.85 | - | - | ||||||
| College of Agronomy, Jiangxi Agricultural University, Jiangxi | Ji’an City Wan’an County Luotang Town Fengjia Village | Jianmei Xiong | Liangyou 6326 | 60 m2 | Acetone extract | 300 | 2.86 | 67.68 | b |
| 450 | 2.57 | 70.97 | ab | ||||||
| 600 | 2.36 | 73.43 | a | ||||||
| 8% Ningnanmycin | 750 | 2.72 | 69.49 | ab | |||||
| Control | 8.91 | - | - | ||||||
| Pesticides Administration of Jiangxi, Jiangxi | Ji’an City Wan’an County Furong Town Guangming Village | Minghui Xiao | Rongyou 308 | 30 m2 | Acetone extract | 300 | 6.0 | 65.6 | c |
| 450 | 4.6 | 75.2 | b | ||||||
| 600 | 3.5 | 80.8 | a | ||||||
| 8% Ningnanmycin | 750 | 4.5 | 74.4 | b | |||||
| Control | 21.3 | - | - | ||||||
| Hunan plant Protection Institute, Hunan | Changsha City Changsha County Langli Town | Yong Liu | Jinyou 207 | 30 m2 | Acetone extract | 300 | 3.5 | 69.09 | cB |
| 450 | 2.75 | 78.82 | ab | ||||||
| 600 | 2.25 | 83.99 | aA | ||||||
| 8% Ningnanmycin | 750 | 3.25 | 74.85 | bc | |||||
| Control | 11.5 | - | - | ||||||
| College of Biosafety Science and Technology, Hunan Agricultural University, Hunan | Zhuzhou City Yanling County Luyuan Town Xitang Village | Tuyong Yi | Tianyou 998 | 30 m2 | Acetone extract | 300 | 3.8 | 57.0 | b |
| 450 | 3.0 | 65.9 | a | ||||||
| 600 | 2.8 | 68.5 | a | ||||||
| 8% Ningnanmycin | 750 | 3.1 | 64.3 | a | |||||
| Control | 8.8 | - | - |
DMRT method was applied to do variance analysis for determining the significance difference between every treatment.
Phytotoxic activity and environmental toxicology
For the study of phytotoxic activity, the acetone extract was diluted to 500 μg mL-1, sprayed on the leaves of the tested tobacco and rice, and regularly observed. Environmental toxicology was carried out by Institute of Plant Protection (IPP), Chinese Academy of Agricultural Sciences (CAAS) from 2012 to 2013.
Results
NMR analysis
Finally, two bioactive compounds were obtained from ethyl acetate extract. Both the compounds were identified by physical, chemical properties and organic spectroscopy (1H NMR, 13C NMR), as well as contrast with the standard compounds. Their structures were identified as Gossypol and β-sitosterol. NMR data were shown in Tables 3–5.
Table 3. 13C-NMR and 1H-NMR data of gossypol.
| Position C | δC | Position H | δH | DEPT | Couplings in HMBC |
|---|---|---|---|---|---|
| C-1,C-1’ | 151.32 | 1-OH, 1’-OH | 5.821, 5.862(s) | C | |
| C-2,C-2’ | 114.83 | C | C2, H4C2, 1-OH | ||
| C-3,C-3’ | 115.78 | C | C3, H4 C3, H14 | ||
| C-4,C-4’ | 118.52 | H4, H4’ | 7.901, 7.679(s) | CH | C4, H14 |
| C-5,C-5’ | 134.41 | C | C5, H4C5,6-OH, C5, H11C5, H12 | ||
| C-6,C-6’ | 143.5 | 6-OH, 6’-OH | 6.467, 6.312(s) | C | C6, 6-OH, C6H11, C6, 7-OH |
| C-7,C-7’ | 156.91 | 7-OH, 7’-OH’ | 15.522, 14.753(s) | C | C7, 7-OH, C7, 6-OH, C7, H15 |
| C-8,C-8’ | 111.28 | C | C8, H15C8, 7-OH | ||
| C-9,C-9’ | 151.04 | C | C9, H4C9, 1-OH | ||
| C-10,C-10’ | 130.08 | C | C10, H4C10, H11C10, H13 | ||
| C-11,C-11’ | 27.89 | H11, H11’ | 3.913, 3.815(m) | CH | C11, H12 |
| C-12,C-13 | 20.26 | H12, H13 | 1.567, 1.543(d) | CH3 | C12, H11 |
| C-12’,C-13’ | 19.92 | H12’, H13’ | 1.661,1.632(d) | CH3 | C13, H11 |
| C-14,C-14’ | 20.14 | H14, H14’ | 2.126, 1.139(s) | CH3 | C14, H4 |
| C-15,C-15’ | 200.24 | H15, H15’ | 11.237, 10.768(s) | CH | C15, 7-OH |
Table 5. 13C-NMR data of β-sitosterol.
| Position C | δC | Position C | δC |
|---|---|---|---|
| C-1 | 36.91 | C-16 | 29.25 |
| C-2 | 30.26 | C-17 | 56.14 |
| C-3 | 71.67 | C-18 | 11.96 |
| C-4 | 43.19 | C-19 | 19.76 |
| C-5 | 104.68 | C-20 | 35.91 |
| C-6 | 121.71 | C-21 | 19.40 |
| C-7 | 31.82 | C-22 | 34.03 |
| C-8 | 31.89 | C-23 | 27.32 |
| C-9 | 50.21 | C-24 | 45.35 |
| C-10 | 36.43 | C-25 | 29.06 |
| C-11 | 22.12 | C-26 | 18.98 |
| C-12 | 39.83 | C-27 | 20.12 |
| C-13 | 42.32 | C-28 | 23.81 |
| C-14 | 56.89 | C-29 | 12.18 |
| C-15 | 25.17 |
Table 4. 1H-NMR data of β-sitosterol.
| Position H | δH |
|---|---|
| H-3 | 3.50 (m) |
| H-6 | 5.348 (d) |
| H-14 | 2.279 (s) |
| H-18,18’,18” | 1.021 (s) |
| H-19,19’,19” | 0.677 (s) |
| H-21,21’,21” | 0.916 (m) |
| H-26,26’,26” | 0.842 (m) |
| H-27,27’,27” | 0.861 (m) |
| H-29,29’,29” | 0.771 (m) |
Anti-TMV activity in the greenhouse
As shown in Table 6 and Fig. 1, the acetone extract showed the highest anti-TMV activity during all the compounds tested, regardless of its concentration (100 or 500 μg mL-1). Antiviral activity of the acetone extract was much better than 8% Ningnanmycin, with inactivation effect at 71.5%, protection effect at 67.6% and curative effect at 71.2% for the extract mixture (Table 6), but with inactivation effect at 63.5%, protection effect at 61.7% and curative effect at 58.1% for Ningnanmycin (Table 6). When compared with its effective components gossypol and β-sitosterol, the acetone mixture also showed much better antiviral effect, even though higher concentrations (500 μg mL-1) of gossypol and β-sitosterol were used. Antiviral effect of gossypol was higher (inactivation effect at 57.5%, protection effect at 62.9% and curative effect at 54.4%) than that of β-sitosterol (inactivation effect at 40.2%, protection effect at 52.5% and curative effect at 45.6%) at the concentration of 500 μg mL-1.
Table 6. Anti-TMV activity of acetone extract, β-sitosterol, β-sitosterol gossypol mixture, gossypol, and Ningnanmycin.
| Compounds | concentration (μg mL-1) | Inactivation effect (%) | Protection effect (%) | Curative effect (%) |
|---|---|---|---|---|
| acetone extract | 100 | 64.1 | 61.4 | 56.3 |
| 500 | 71.5 | 67.6 | 71.2 | |
| β-sitosterol | 100 | 35.4 | 43.5 | 37.8 |
| 500 | 40.2 | 52.5 | 45.6 | |
| a β-sitosterol and gossypol | 100 | 53.3 | 55.6 | 49.7 |
| 500 | 68.2 | 72.5 | 67.1 | |
| gossypol | 100 | 47.2 | 41.6 | 43.5 |
| 500 | 57.5 | 62.9 | 54.4 | |
| Ningnanmycin | 500 | 63.5 | 61.7 | 58.1 |
a Both of β-sitosterol and gossypol were at the concentration of 100 and 500 μg mL-1.
Dot-ELISA assay (Fig. 2) shown that, signal of TMV in tobacco leaves gradually became weaker with the increased concentration of acetone extract from 100 μg mL-1 to 800 μg mL-1, indicating that the acetone extract may inhibit replication of TMV in plants.
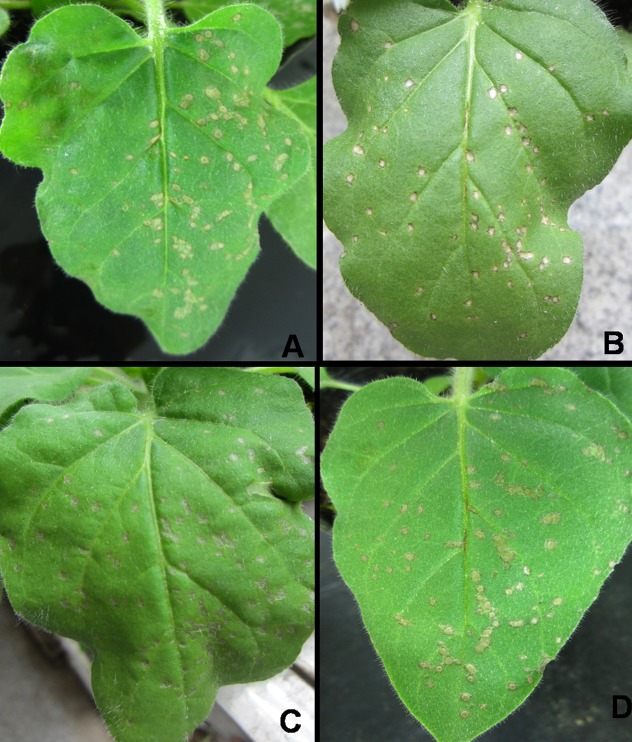
Fig 1. Comparison of curative effects of the acetone extract, β-sitosterol, gossypol and Ningnanmycin against TMV in Nicotiana glutinosa leaves.
A: acetone extract at 500 μg mL-1; B: β-sitosterol at 500 μg mL-1; C: gossypol at 500 μg mL-1; D: Ningnanmycin at 500 μg mL-1. All the left side of the leaves were treated with the tested solutions and the right side with the solvent acetone as control.
Fig 2. Inhibitory effect of the acetone extract and Ningnanmycin on TMV in tobacco tested by Dot-ELISA.
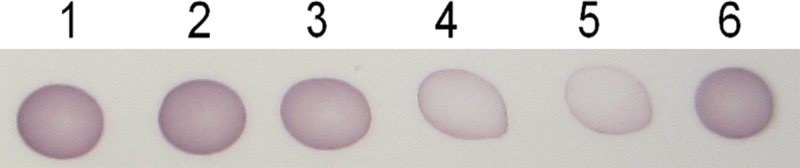
1: positive control, 2: acetone extract at 100 μg mL-1, 3: acetone extract at 200 μg mL-1, 4: acetone extract at 400 μg mL-1, 5: acetone extract at 800 μg mL-1, 6: Ningnanmycin at 200 μg mL-1.
Antiviral activity in the field trials
Antiviral activity of the acetone mixture against RSV was tested in the field in Fujian (Zhangzhou, Longyan), Jiangsu (Guanyun, Yangzhou), Jiangxi and Hunan Provinces, including six host varieties (Table 1). For the control treatment (only sprayed with water), disease indexes were all very high from 2.77 to 11.5. After sprayed with the acetone extract and 8% Ningnanmycin, disease indexes were much lower than those of control treatment. As the dose of the acetone extract increases from 300 to 600 g ha-1, disease index gradually decreased and control effect increased. The best antiviral activity was reached at the dose of 600 g ha-1, which results in better antiviral effect than 8% Ningnanmycin at 750 g ha-1. These results were all similar during all the fields tested, regardless of the host variety used, indicating that the acetone extract is antiviral effective against RSV and widely applicable in different fields and different host varieties.
The acetone extract also show similar and effective antiviral activity against SRBSDV, which was tested in Fujian (Zhangzhou, Longyan), Jiangsu (Guanyun), Jiangxi and Hunan provinces, including 7 host varieties (Table 2). At the dose of 600 g ha-1, rice plants in all the fields tested resulted lowest disease index and highest control effect, compared with 8% Ningnanmycin at 750 g ha-1, and the acetone extract at 300 and 450 g ha-1. These results indicated that the acetone extract also show effective antiviral activity against SRBSDV and is widely applicable in different fields and different host varieties in the south of China.
Phytotoxic activity and environmental toxicology
The acetone extract was tested for their phytotoxic activity, and showed no phytotoxic activity either in tobacco in the greenhouse or in rice in the field trial.
In Japanese quail, 7-d LD50 value of this acetone extract was greater than 66.0 a.i.mg kg-1. In Daphnia magna 48-h EC50 value was 43.1 a.i.mg kg-1. In Scenedesmus obliquus 72-h EC50 value was 9.07 a.i.mg kg-1. EC50 (96 h) for Bombyx mori was 245 a.i.mg kg-1. EC50 (96 h) for fish was 2.18 a.i.mg kg-1. EC50 (48 h) for bees was 119 a.i.mg kg-1. According to "environmental safety evaluation test guidelines of chemical pesticides" of China, this acetone extract was slightly toxic to Japanese quail, Daphnia magna, Scenedesmus obliquus and Bombyx mori, and moderately toxic to fish and bees. In conclusion, the environmental toxicology testing revealed that this acetone extract is safe enough to be applied in the field and is environmentally friendly.
Discussion
Gossypol and β-sitosterol were separated from acetone mixture extracted from cottonseed oil sludge and tested for its antiviral effect against TMV in greenhouse, and RSV and SRBSDV in the field trials. As a result, its antiviral effect is better than the commercial agent Ningnanmycin in all three types of viruses including TMV, RSV and SRBSDV, and all infected plant species, indicating that this acetone extract is effectively against a broad spectrum of plant viruses in various plant species including both dicots (tobacco) and monocots (rice). Gossypol and β-sitosterol were seperated from the acetone extract and tested active against TMV. Environmental toxicology showed that this new acetone extract is environmentally friendly and no phytotoxic activity was found in the treated plants.
Gossypol (GOS), a polyphenolic compound isolated from cotton seeds, has been applied as a male contraceptive drug for many years, and also has several new clinical applications including antiviral, antimalarial, and antitumor effects [12–14]. Gossypol, and its derivatives and analogs have been reported as effective agents against human immunodeficiency virus tipe 1 (HIV-1) [15–17], which is proved to be an HIV-1 reverse transcriptase inhibitor by targeting the non-nucleoside inhibitor binding pocket of reverse transcroptase [15]. An et al. [16] also found that the alanine-(-) gossypol derivative, an effective HIV-1 entry inhibitor, can bind to the gp41 hydrophobic pocket and block the formation of the cell fusion-activated gp41 core. In this study, gossypol was found to exhibit effective anti-TMV activity with 54.4% curative effect at 500 μg ml-1.
β-sitosterol, the dominant phytosterol with chemical structures similar to that of cholesterol, has been reported to have several medical uses such as hypocholesterolemic activity [18, 19], anti-inflammatory activity [20], induction of apoptosis [21, 22], immunomodulatory activity [23] and anti-oxidant effect [24, 25]. In addition, β-sitosterol isolated from aerial parts of Cissus sicyoides showed antibacterial activity against Bacillus subtilis with minimal inhibitory concentration (MIC) of 50 μg mL-1 [26]. Peres et al. [27] analyzed chemical compositions and antimicrobial activity of Croton urucurana, and found. Hex/DCM fraction containing β-sitosterol exhibited the highest inhibitory effect against Staphylococcus aureus (0.8 mg mL-1). Gebre-Mariam et al. [28] revealed that extracts containing β-sitosterol from Euclea schimperi showed antiviral activity against coxsackievirus B3 (CVB3), influenza A virus and herpes simplex virus type 1 Kupka (HSV-1). Alphaamyrin, uvaol, ursolic acid and its lactone, and β-sitosterol were detected in the leaves of Euclea schimperi [29]. But there are no relevant reports on antiviral activity of ouabain, amyrin, uvaol, and β-sitosterol. Lin et al. [30] found that β-sitosterol, one of the five major compounds of the Isatis indigotica root, inhibited cleavage activities inhibited cleavage activities of the SARS coronavirus 3C-like protease in cell-free and cell-based assays. In this study, we confirmed that β-sitosterol has antiviral properties in a certain degree, although not better than that of gossypol, with 45.6% curative effect at 500 μg mL-1 against TMV.
When compared with its individual components (gossypol and β-sitosterol), the extract mixture resulted in the best curative effect (71.2%). In the form of complex mixture, the bioactive compounds may simultaneously function as antiviral activity, dependently or independently with each other, thus reinforcing the inhibiton effect against viruses. Another advantage of mixture of antiviral agents has been discussed by previous researches. One important challenge for any antiviral drugs is the development of resistance by various viruses. In this case, mixture of antiviral compounds is more efficient and practical than the single ones. Because a virus that has developed resistance to a particular drug may not be resistant to other antiviral compounds, which have the potential to possess similar, if not identical, antiviral activities [31].
Natural-product-based antiviral agents have the ability to decompose rapidly, thereby reducing their risk to the environment [32, 33]. In this study, no toxicity was found in tobacco or rice plants. And this acetone extract was slightly toxic to Japanese quail, Daphnia magna, Scenedesmus obliquus and Bombyx mori, and moderately toxic to fish and bees. This environment friendly acetone extract holds potential promise for commecial application in the future. Firstly, from economic considerations, the starting material cottonseed is widely available in China and the cost may be very low. Secondly, cottoseed oil sludge rather than cottonseed oil was served as the raw material, which is the precipitation and enrichment of antiviral effective compounds, so that maximum efforts have been made to improve the concentration of active compounds. Most importantly, the in vivo and field trials demonstrated that this acetone extract was broad-spectrum antiviral, not only against different plant virus (TMV, RSV, SRBSDV), but also more effective in different host varieties from different geographic distributions in China, compared with the commecial agent Ningnanmycin.
In summary, in this study, we firstly discovered acetone extract from cottonseed oil sludge as novel antiviral agent against plant viruses such as TMV, RSV and SRBSDV. Also to our knowledge this is the first report on the application of gossypol and β-sitosterol to plant virus control. Further study will be focused on the antiviral mechanism of these compounds against plant viruses. Hopefully, our results will provide some useful insights for the future virus control strategies.
Acknowledgments
The study was supported by the Special Fund for Agro-scientific Research in the Public Interest (No. 200903052) and the 111 Project from the Education Ministry of China (No.B07049).
Data Availability
All relevant data are within the paper.
Funding Statement
The study was supported by the Special Fund for Agro-scientific Research in the Public Interest (No. 200903052) and the 111 Project from the Education Ministry of China (No.B07049). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hari V, Das P (1998) Ultra microscopic detection of plant viruses and their gene products. In Plant Disease Virus Control; Hadidi A.; Khetarpal R. K.; Koganezawa H. Eds.; APS Press: St. Paul, MN: pp 417−427. [Google Scholar]
- 2. Karen-Beth G, Scholthof S A, Henryk C, Peter P, Emmanuel J, et al. (2011) Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 12: 938–954. 10.1111/j.1364-3703.2011.00752.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang ZW, Feng AZ, Cui MB, Liu YX, Wang LZ, et al. (2012) First Discovery and Stucture-Activity Relationship Study of Phenanthroquinolizidines as Novel Antiviral Agents against Tobacco Mosaic Virus (TMV). PLOS ONE 7(12), e52933 10.1371/journal.pone.0052933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang JQ, Song BA, Bhadury PS, Chen Z, Yang S, et al. (2010) Synthesis and antiviral bioactivities of 2-cyano-3-substitutedamino(phenyl) methylphosphonylacrylates (acrylamides) containing alkoxyethyl moieties. J Agric Food Chem 58: 2730–2735. 10.1021/jf902861m [DOI] [PubMed] [Google Scholar]
- 5. Ouyang MA, Wein YS, Zhang ZK, Kuo YH (2007) Inhibitory activity against tobacco mosaic virus (TMV) replication of pinoresinol and syringaresinol lignans and their glycosides from the root of rhus javanica var. roxburghiana. J Agric Food Chem 55: 6460–6465. [DOI] [PubMed] [Google Scholar]
- 6. Chou CH (1999) Roles of allelopathy in plant biodiversity and sustainable agriculture. Crit. Rev. PlantSci. 18, 609–636. [Google Scholar]
- 7. Huang HC, Chou CH (2005) Impact of plant disease biocontroland allelopathy on biodiversity and agricultural sustainability. Plant Pathol. Bull. 14:1–12. [Google Scholar]
- 8. Scholthof KBG (2004) Tobacco mosaic virus: a model system for plant biology. Annu. Rev. Phytopathol. 42:13–34. [DOI] [PubMed] [Google Scholar]
- 9. Cheng ZB, Li S, Gao RZ, Sun F, Liu WC, et al. (2013) Distribution and genetic diversity of Southern rice black-streaked dwarf virus in China. Virol. J. 10:307–313. 10.1186/1743-422X-10-307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wei TY, Yang JG, Liao FL, Gao FL, Lu LM, et al. (2009) Genetic diversity and population structure of rice stripe virus in China. J. Gen. Virol. 90, 1025–1034. 10.1099/vir.0.006858-0 [DOI] [PubMed] [Google Scholar]
- 11. Gooding GV, Hebert TTJ (1967) A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 57: 1285 [PubMed] [Google Scholar]
- 12. Janero DR, Burghardt B (1988) Protection of rat myocardial phospholipid against peroxidativeinjury through superoxide- (xanthine oxidase)-dependent, iron-promoted fentonchemistry by the male contraceptive gossypol. Biochem. Pharmacol. 37: 3335–3342. [DOI] [PubMed] [Google Scholar]
- 13. Lin TS, Schinazi RF, Zhu J, Birks E, Carbone R, et al. (1993) Anti-HIV-1 activity and cellular pharmacology of various analogs of gossypol. Biochem. Pharmacol. 46: 251–255. [DOI] [PubMed] [Google Scholar]
- 14. Bauer JA, Trask DK, Kumar B, Los G, Castro J, et al. (2005) Reversal of cisplatin resistance with a BH3 mimetic, (-)-gossypol, in head and neck cancer cells: Role of wild-type p53 and Bcl-xL. Mol. Cancer Ther. 4: 1096–1104. [DOI] [PubMed] [Google Scholar]
- 15. Keller PA, Chris B, Scott PL, David T, Renate G (2003) Novel pharmacophore-based methods reveal gossypol as a reverse transcriptase inhibitor. J. Mol. Graph. Model. 21: 365–373. [DOI] [PubMed] [Google Scholar]
- 16. An T, Ouyang WJ, Pan W, Guo DY, Li JR, et al. (2012) Amino acid derivatives of the (-) enantiomer of gossypol are effective fusion inhibitors of human immunodeficiency virus type 1. Antivir. Res. 91: 276–287. [DOI] [PubMed] [Google Scholar]
- 17. Yang J, Zhang F, Li J, Chen G, Wu SW, et al. (2012) Synthesis and antiviral activities of novel gossypol derivatives. Bioorg. Med. Chem. Lett. 22:1415–1420. 10.1016/j.bmcl.2011.12.076 [DOI] [PubMed] [Google Scholar]
- 18. Sugano M, Morioka H, Ikeda I (1977) A comparison of hypocholesterolemic activity of betasitosterol and beta-sitostanol in rats. J. Nutr. 107:2011–2019. [DOI] [PubMed] [Google Scholar]
- 19. Zak A, Zeman M, Vitkova D, Hrabak P, Tvrzicka E (1990) Beta-sitosterol in the treatment of hypercholesterolemia. Cas. Lek. Cesk. 129:1320–1323. [PubMed] [Google Scholar]
- 20. Prieto JM, Recio MC, Giner RM (2006) Anti-inflammatory activity of β-sitosterol in a model of oxazolone induced contact-delayed-type hypersensitivity. Bol. Latinoam Caribe Plant Med. Aromat. 5: 57–62. [Google Scholar]
- 21. Awad AB, Downie AC, Fink CS (2000) Inhibition of growth and stimulation of apoptosis by beta-sitosterol treatment of MDA-MB-231 human breast cancer cells in culture. Int J Mol Med. 5: 541–546. [DOI] [PubMed] [Google Scholar]
- 22. Park C, Moon DO, Rhu CH, Choi BT, Lee WH, et al. (2007) β-sitosterol induces antiproliferation and apoptosis in human leukemic U937 cells through activation of caspase-3 and induction of Bax/Bcl-2 ratio. Biol. Pharm. Bull. 30: 1317–1323. [DOI] [PubMed] [Google Scholar]
- 23. Bouic PJ, Etsebeth S, Liebenberg RW, Albrecht CF, Pegel K, et al. (1996) beta-Sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 18: 693–700. [DOI] [PubMed] [Google Scholar]
- 24. Vivancos M, Moreno JJ (2005) β-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radical Biol. Med. 39: 91–97. [DOI] [PubMed] [Google Scholar]
- 25. Baskar AA, Al NKS, Paulraj MG, Alsaif MA, Al MM, et al. (2012) β-Sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer. J Med Food. 15: 335–43. 10.1089/jmf.2011.1780 [DOI] [PubMed] [Google Scholar]
- 26. Beltrame FL, Greisiele LP, Dani LD, Benedito PDF, Roberto BB, et al. (2002) Evaluation of the antidiabetic and antibacterial activity of Cissus sicyoides. Braz. Arch. Boil Techn. 45: 21–25. [Google Scholar]
- 27. Peres MT, Delle MF, Cruz AB, Pizzolatti MG, Yunes RA (1997) Chemical composition and antimicrobial activity of Croton urucurana Baillon (Euphorbiaceae). J. Ethnopharmacol. 56: 223–226. [DOI] [PubMed] [Google Scholar]
- 28. Gebre-Mariam T, Neubert R, Schmidt PC, Wutzler P, Schmidtke M (2006) Antiviral activities of some Ethiopian medicinal plants used for the treatment of dermatological disorders. J. Ethnopharmacol. 104: 182–187. [DOI] [PubMed] [Google Scholar]
- 29. Lall N, Meyer JJ (2000) Antibacterial activity of water and acetone extracts of the roots of Euclea natalensis. J. Ethnopharmacol. 72: 313–316. [DOI] [PubMed] [Google Scholar]
- 30. Lin CW, Tsai FJ, Tsai CH, Lai CC, Wan L, et al. (2005) Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir. Res. 68: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yasuhara-Bell J, Lu Y (2010) Marine compounds and their antiviral activities. Antivir. Res. 86: 231–240. 10.1016/j.antiviral.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qian XH, Lee PW, Cao S (2010) China: Forward to the green pesticides via a basic research program. J. Agric. Food Chem. 58: 2613–2623. 10.1021/jf904098w [DOI] [PubMed] [Google Scholar]
- 33. Seiber JN (2011) Sustainability and agricultural and food chemistry. J. Agric. Food Chem. 59: 1–21. 10.1021/jf1046078 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.