Abstract
In this case study, the process of nutritional diagnosis and intervention conducted at a hospital on a malnourished patient who underwent treatment for a chronic illness (chemotherapy for cancer treatment) was recorded. The patient received his first round of chemotherapy for colorectal cancer, and then a second round after the cancer metastasized to the liver. The patient was malnourished and had experienced weight loss (17% loss in the most recent 3 months) due to side effects of chemotherapy including stomatitis, nausea, and vomiting. Nutritional diagnosis and intervention via the nutrition care process were implemented through two screening rounds, and the quantity of oral intake increased from 28% to 62% of the recommended daily intake. The patient required continuous monitoring and outpatient care after hospital discharge. It is speculated that if a more active patient education and dietary regimen with respect to chemotherapy side effects had been offered after the patient's first chemotherapy cycle, it might have been possible to treat ingestion problems due to stomatitis during the second cycle of chemotherapy and prevent the weight loss. Henceforth, patients receiving chemotherapy should be educated about nutrition management methods and monitored continuously to prevent malnutrition.
Keywords: Malnutrition, Nutritional intervention, Nutrition care process
Introduction
Malnutrition from loss of appetite, indigestion, malabsorption, and metabolic problems is a common condition in cancer patients undergoing treatment [1]. Side effects of chemotherapy include anemia, appetite loss, dysgeusia, changes in sensitivity to food temperature, constipation, diarrhea, dysphagia, xerostomia, fatigue, and early satiation [2] that can decrease food intake and lead to extreme weight loss [3].
The goal of nutritional intervention for such patients [4] is to improve their nutritional condition by mitigating treatment side effects, offering individualized patient care, and improving food intake while respecting patients' food habit with regard to administering more progressive interventions. Improved nutritional condition will enhance both treatment and quality of their lives [3].
Additionally, as the main method of nutritional intervention, patients are recommended to eat small and frequent meals 5-6 times a day to increase their overall intake since most patients experience appetite loss resulting from metabolic problems and chemotherapy treatment. If the amount of food does not exceed 60% of the daily recommended intake, tube feeding or intravenous alimentation is performed [5]. If diets are appropriated to each patient's condition, oral food intake is recommended whenever possible. Hot or cold foods and drinks are offered depending on patient's receptivity and variations are made according to patient's condition. When a low bacteria diet is needed, appropriate foods are given and the patient is educated about the dietary practices.
The purpose of the current case study is to record the process of nutritional diagnosis and intervention conducted on malnourished cancer patients with chemotherapy for treatment at a hospital. In this case, after admission to the hospital for general weakness, the patient was identified as being at risk of malnutrition according to an early nutritional assessment program by the nutrition team at hospital. Approval of the institutional review board at (KHNMC IRB 2013-116) the Kyung Hee University Hospital at Gangdong was obtained.
Case
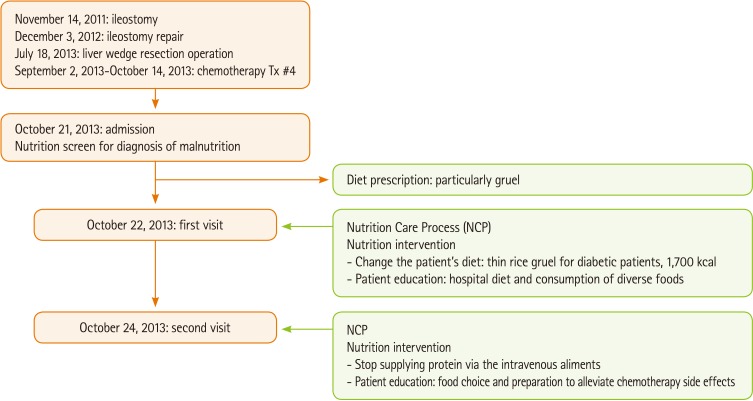
On October 21, 2013, a 61-year-old man was admitted to the gastroenterology ward. To measure the patient's nutritional condition, medical history, physical measurements, biochemical data, medical examinations and treatments, nutritional physical examination data, and food/nutrition-related diet history were examined. Based on the medical history, the principal diagnosis and symptom was general weakness. The patient was diagnosed with diabetes mellitus 10 years ago, hypertension (10 years ago), and rectal cancer 3 years ago, for which the patient underwent an ileostomy on November 14, 2011 and received leucovorin plus fluorouracil (LV5FU2) as preop #1 and postop #8 chemotherapy. Afterward, the patient underwent ileostomy repair on December 3, 2012, and then a liver wedge resection on July 18, 2013 for liver S4 metastasis during the follow-up (f/u). Afterward the patient received chemotherapy #4 from September 2 through October 14, 2013 using oxaliplatin, folinic acid, and 5-fluorouracil (FLOFOX4). The patient's current drug prescriptions include Lantus 10 U/day, Apidra 6 U #3/day, and Nutriflex lipid peri [+ Humulin-R (regular insulin) 15 U] every other day (EOD), and chemotherapy was paused due to general weakness.
The first screening visit was conducted on October 22, 2013. At the time of hospital admission (October 21, 2013), the physical measurements were: height, 167 cm; body weight, 53.2 kg; ideal body weight (IBW), 61.4 kg; %IBW, 86.6%; body mass index (BMI), 19.1 kg/m2; usual weight, 64.8 kg (July 18, 2013); and weight change of -11.6 kg (17.9% loss) over the most recent 3 months. Examinations upon admission showed the following: total protein/albumin, 5.6/3.2 g/dL; hemoglobin/hematocrit (Hgb/Hct), 8.4 g/dL/25.4%; total lymphocyte count (TLC), 741.2 cells/mm3; cholesterol, 162 mg/dL; blood urea nitrogen/creatinine (BUN/Cr), 20/1.0 mg/dL; Ca/P, 8.3/2.3 mg/dL; Na/K/Cl, 132/4.2/98 mEq/L; C-reactive protein, 6.28 mg/dL; hemoglobin A1c, 9.7%; and casual glucose, 470 mg/dL.
The nutritional and physical examination data showed that the patient had stomatitis, nausea, and vomiting as side effects of chemotherapy and the blood pressure was 142/83 mmHg. In terms of food/nutrition-related diet history, the dietary prescription and diet-related experiences were such that the patient experienced a loss of appetite after chemotherapy, did not receive education intended for cancer patients (due to being too tired). The patient's food and beverage consumption over the most recent week (after the fourth round of chemotherapy) includes six glasses of cola (480 kcal/day; 120 g of sugar) due to stomatitis, nausea, and vomiting. At the hospital, the administration of other foods was not attempted since the patient experienced pain and vomiting after consuming soft foods, particularly gruel (not eaten) and intravenous alimentation (Nutriflex lipid peri EOD; energy, 465 kcal; C:P:F ratio, 34.4:17.2:48.4; carbohydrate, 40 g; protein, 20 g; fat, 25 g). No liquid or solid food supplements or biologically active substances were consumed. The patient stayed in the bed whole day.
In the Patient-Generated Subjective Global Assessment [6] conducted for nutritional assessment, the patient scored 15 points, indicating the need for improved symptom and focused nutrition management. Approximately 1600-1860 kcal of energy (baseline weight, 53.2 kg; based on calculation, 30-35 kcal/kg needed) and 64-85 g of protein (baseline weight, 53.2 kg; based on calculation, 1.2-1.6 g/kg needed) were set as the required nutritional intake.
Two nutritional diagnoses were made: frist, malnutrition arising from a decrease in food intake related to the side effects of chemotherapy (stomatitis, nausea, vomiting) as well as a lack of food/nutrition-related knowledge regarding food types and quantities. The evidence for this diagnosis being that the patient experienced a 17.9% weight loss over the most recent 3 months and consumed only 28% of the required nutrients during the most recent week (after the fourth round of chemotherapy); second, the patient's lack of knowledge with regard to foods and nutrients which is possibly attributable to fear for food intake and this was observed through refusal to ingest food and tendency to ingest only cola after chemotherapy.
As a nutritional intervention for the first diagnosis, the medical team was advised to change the patient's diet to diabetic thin rice gruel (Figure 1) (1,700 kcal and 72 g of protein) and supply any intravenous aliments according to the amount of oral intake, which would provide ≥ 30% of the hospital food and the intravenous aliment maintenance to the patients. The hospital-supplied food consisted of soft and non-irradiated foods considering the patient's stomatitis, and in the case of this particular patient, it was decided that close monitoring after dietary changes would be required. As a nutritional intervention for the second diagnosis, the patient was consulted for the need to consume a diverse range of foods and the hospital diet (diabetic thin rice gruel and consider the patient's preferences and lactose intolerance). This intervention would stop for the patient consuming cola, which could worsen the nausea, and try the hospital-supplied foods.
Figure 1.
A 1,700 kcal thin rice gruel diet for diabetic patients.
The second screening visit was conducted on October 24, 2013, and physical measurements, biochemical data, medical examinations and treatments, nutritional physical examination data, and food/nutrition-related diet history were examined. On follow-up, there was no change in physical measurements, and examinations revealed the following: total protein/albumin, 5.1/3.1 g/dL; Hgb/Hct, 8.5 g/dL/25.7%; TLC, 712 cells/mm3; cholesterol, 135 mg/dL; BUN/Cr, 19/1.0 mg/dL; Ca/P, 7.8/1.6 mg/dL; Na/K/Cl, 138/50/107 mEq/L; blood glucose (fasting, postprandial 2 hour glucose #3), 177/208/363/309 mg/dL (10/22), 270/206/206/120 mg/dL (10/23), and 126/133/260/246 mg/dL (10/24). Nutritional and physical examination data, included gastroenterological symptoms such as nausea and vomiting after consuming certain foods. For example, the patient had a vomit after drinking orange juice while apple juice did not cause any gastrointestinal trouble. Vital signs were 135/80 mmHg. In terms of food/nutrition-related diet history, the patient's dietary prescription and diet-related experiences were such that he was informed about diabetic thin rice gruels, non-irritating foods, and nutrient supplement drinks.
The patient's food and beverage consumption consisted of the supplied hospital diet: 20% of rice gruel, 50% of the steamed eggs and nutritious beverages, and 100% of the soup and apple juice (energy, 1,050 kcal; C:P:F ratio, 56.0: 14.4: 29.6; carbohydrate, 147 g; protein, 37.2 g; fat, 34.5 g) along with intravenous aliments (Hepasol inj 500 mL/day; 200 kcal, 50 g protein). After the first screening, the patient tried various foods and started learning to move in a wheelchair. The patient's nutrient requirements were the same as at the first screening. Upon nutrition monitoring and assessment, the goal set after the first screening with regard to nutritional intervention was partially achieved considering that the hospital food consumption was at 62% of the overall consumption and the intravenous aliment had changed (Nutriflex lipid peri EOD to Hepasol/d). After the second nutritional intervention, the patient stopped consuming cola and began to consume a diverse range of hospital foods; thus, the goal of the intervention was reached.
After the second screening, the patient again had two nutritional diagnoses: first, excessive protein supplementation, because extra protein was administered via the intravenous aliments outside oral intake, and the patient was consuming 125% the required protein quantity; second, the patient's lack of knowledge regarding food and nutrition due to absence of education about chemotherapy and food preparation. With regard to nutritional interventions, that for the first nutritional diagnosis involved advising the medical team to stop supplying protein via the intravenous aliments. For the second nutritional diagnosis, advising the medical team educate the patient about food choices and preparation which are appropriate for managing side effects of chemotherapy. The goal of the intervention was consumption of energy and protein > 70% of the requirement and maintenance or increase of body weight. Continuous monitoring was required, and even after discharged, the patient required proper outpatient care.
Discussion
In this case, due to side effects following chemotherapy, the patient experienced significant and unintentional weight loss as a result of stomatitis, nausea, and vomiting and malnourished with poor ingestion. Afterward, through nutritional interventions via the nutrition care process, the patient's quantity of food intake was increased and further increases were expected under continuous care (Figure 2). Similarly, in earlier studies, individualized nutritional education effectively increased caloric and protein intake in cancer patients [7,8]. Thus, it is speculated that, had this patient also received a more active education regarding chemotherapy side effects and food preparation before and after his colon cancer surgery, poor ingestion caused by stomatitis and prevent weight loss after the second round of chemotherapy could have been treated easily.
Figure 2.
Patient's flow chart.
Hereafter, to address this issue, all patients undergoing chemotherapy should be educated about practices that could enhance response to treatment and undergo continuous observation during treatment. Additionally, with regard to this patient, it is regrettable that, despite the fact that repeated malnutrition was diagnosed during several admissions to the hospital during his second round of chemotherapy, the patient was not administered active care for malnutrition.
Footnotes
No conflict interests were declared by any of the authors.
References
- 1.Oh BJ. General oncology nursing. Seoul: Shin-Kwang Press; 2005. [Google Scholar]
- 2.Yang YH, Lee DS. The relationship of anorexia, nausea, vomiting, oral intake and nutritional status in patients receiving chemotherapy. J Korean Acad Nurs. 2000;30:720–730. [Google Scholar]
- 3.Pérez Camargo DA, De Nicola Delfín L, Ñamendys-Silva SA, Copca Mendoza ET, Hernández Méndez M, Herrera Gómez Á, Meneses García A. Nutritional status of patients with cancer of oral cavity. Nutr Hosp. 2013;28:1458–1462. doi: 10.3305/nh.2013.28.5.6591. [DOI] [PubMed] [Google Scholar]
- 4.Escott-Stump S. Nutrition and diagnosis-related care. 6th ed. Philadelphia (PA): Wolters Kluwer/Lippincott Williams & Wilkins; 2008. Section 13. Cancer; pp. 672–688. [Google Scholar]
- 5.Boullata JI, Carney LN, Guenter P American Society for Parenteral and Enteral Nutrition. A.S.P.E.N. enteral nutrition handbook. Silver Spring (MD): American Society for Parenteral and Enteral Nutrition; 2010. [Google Scholar]
- 6.Ottery FD. Patient generated subjective global assessment. In: McCallum PD, Polisena CG, editors. The clinical guide to oncology nutrition. Chicago (IL): American Dietetic Association; 2000. pp. 11–23. [Google Scholar]
- 7.Park KO, Choi-Kwon S. Effects of individualized nutritional education programs on the level of nutrient intake and nutritional status of colorectal cancer patients undergoing palliative chemotherapy. J Korean Acad Nurs. 2012;42:799–809. doi: 10.4040/jkan.2012.42.6.799. [DOI] [PubMed] [Google Scholar]
- 8.Poulsen GM, Pedersen LL, Osterlind K, Bæksgaard L, Andersen JR. Randomized trial of the effects of individual nutritional counseling in cancer patients. Clin Nutr. 2013 doi: 10.1016/j.clnu.2013.10.019. Forthcoming. [DOI] [PubMed] [Google Scholar]