Abstract
Epidemiological studies of Rickettsia felis and related bacteria are very important, because the natural cycle of this important infection has not yet been established. The recent emergence of R. felis-associated febrile diseases in West and East Africa demands insightful epidemiological studies of the vectors and reservoirs of this bacterium in Africa. Twenty-nine cat fleas, Ctenocephalides felis, were tested for the presence of rickettsiae, including R. felis, bartonellae, and borreliae, with specific quantitative real-time PCR assays. Supporting our previous studies, R. felis was not detected in the fleas collected. In addition, neither Bartonella nor Borrelia was found. In five (17%) examined fleas, we found another species of rickettsia. We isolated three rickettsial strains, and genetic analysis demonstrated that these strains represent a probable new species, provisionally called Candidatus Rickettsia senegalensis here.
Keywords: Africa, Candidatus Rickettsia senegalensis, flea, Rickettsia, Rickettsia felis, Senegal
Introduction
Recently, several papers have highlighted an emerging problem in tropical medicine in Africa, namely, acute rickettsioses [1], including those caused by Rickettsia felis [2] and related rickettsiae. Rickettsioses appear to be some of the most important and often neglected causes of febrile diseases in tropical countries [3–5]. In Laos, rickettsioses may be responsible for 7% of the cases of patients hospitalized with acute fevers [4]. In Senegal, our previous studies have shown that up to 15% of the cases of fever encountered in rural dispensaries may be caused by R. felis [2]. Since 2010, two teams, one based in Senegal [2] and another in Kenya [6,7], have been working independently on the detection of the causes of febrile illnesses, including R. felis-associated illness, in Africa. However, the natural reservoirs of R. felis have not yet been completely described. Since the first clinical descriptions of R. felis-associated fever, the cat and dog fleas, Ctenocephalides felis and Ctenocephalides canis, respectively, have been implicated as the most probable vectors [8]. However, R. felis has been identified by molecular methods in >20 different species of fleas, soft and hard ticks, mites and booklice [9], and mosquitoes, including Anopheles species [10,11]. Multiple reported cases of R. felis-associated fever in Senegal [2,12] may not be explained by transmission of R. felis by flea bites, because of its actual absence (or rarity) in fleas in Senegal [13]. In this article, we report the results of a study aimed at finding rickettsiae in fleas in Senegal.
Materials and methods
Cat fleas were collected manually from two cats in Dakar, Senegal. Twenty-four fleas were placed directly into 70% ethanol, and five were kept alive. Fleas were identified with a standard taxonomic key [14]. Five live fleas were homogenized and blindly inoculated into a cell culture; DNA was extracted from the supernatant and tested together with the DNA of the fleas initially placed into alcohol. DNA was extracted from fleas and the cell culture suspension with a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions, and stored at 4°C until being used in PCR amplifications. Rickettsial DNA was detected by performing quantitative real-time PCR (qPCR) assays with a BIORAD CFX96 system and software (Bio-Rad Life Science, Marnes-la-Coquette, France). Master mixtures were prepared according to the instructions of the manufacturer, by using Rickettsia-specific primers and probes that target a gltA gene and the bioB gene specific to R. felis [12]. The samples were screened for the presence of Borrelia species and Bartonella species by qPCR with primers and probes that target the 16S rRNA gene of Borrelia [15] and the ITS gene of Bartonella [16]. Amplifications of almost the entire rrs, sca4, ompB, ompA and gltA genes of the new isolates of Rickettsia were performed as previously described [17]. The isolation of rickettsial strains was performed in an XTC-2 cell line with a shell-vial technique [18] at 28°C. Rickettsiae were detected by Gimenez staining followed by Rickettsia-specific qPCR. The gltA sequences and concatenated rrs, gltA, sca4 and ompB sequences were aligned by the use of CLUSTALW, and phylogenetic inferences were obtained with Bayesian phylogenetic analysis [19] with TOPALi 2.5 software (Biomathematics and Statistics Scotland, Edinburgh, UK) by use of the integrated MrBayes application (http://mrbayes.csit.fsu.edu/).
Results
All collected fleas were morphologically identified as C. felis. In total, five of the 29 fleas (17.2%) tested positive by qPCR with the Rickettsia-specific primers and probe, including three fleas inoculated in XTC-2 cells. All five positive fleas were among 12 collected from one cat; 17 fleas collected from the second cat were negative. However, none tested positive for R. felis. Over a period of 2 weeks, we succeeded in isolating three rickettsial strains, namely, PU01-02, PUX1-X2, and PU03-04, from three of five fleas inoculated into the XTC-2 cell cultures. All three strains were visualized by Gimenez staining, and their appearance was typical of cell cultures infected with rickettsiae: Gimenez-positive intracellular bacteria accompanied by a moderate cytopathic effect (Fig. 1).
FIG. 1.
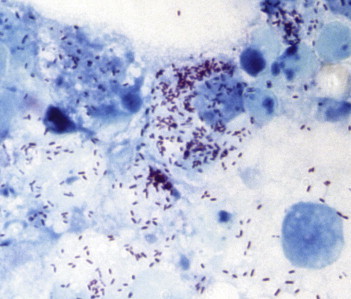
XTC-2 cell line infected with Candidatus Rickettsia senegalensis, strain PU01-02, seventh day post-inoculation, Gimenez staining, 1500×.
We amplified almost the entire lengths of the gltA (for all strains and positive fleas), rrs, ompB and sca4 genes (for the PU01-02 strain only), and sequenced the amplicons. All attempts to amplify the ompA gene typical for the spotted fever group of rickettsiae [20] produced negative results. The gltA gene sequences were identical among all three isolates. BLAST searches of the sequences of all four sequenced genes demonstrated that the isolated PU01-02 strain does not completely share identity with any other known rickettsial strain. The closest officially validated species was R. felis strain California 2 (CP000053); the PU01-02 strain showed 99.65%, 97.68%, 95.24% and 97.2% sequence identity of its rrs, gltA, ompB and sca4 genes, respectively, with R. felis. A phylogenetic tree constructed on the basis of the gltA sequences (Fig. 2) and concatenated rrs, gltA, sca4 and ompB gene sequences (Fig. 3) also indicated a distinct position for the PU01-02 strain. The PU01-02 strain grouped together with R. felis and Rickettsia hoogstraalii and a number of sequences of unisolated rickettsiae that were amplified from fleas, Ornithodoros ticks, and ladybird beetles. A detailed BLAST search found that a very similar rickettsia (‘Rickettsia sp. Rf31’, AF516331, 1149/1150 (99%) for the gltA gene) was identified in a C. canis flea collected from a dog in Thailand [21]. Moreover, other genetically similar rickettsiae were identified in human blood in south-eastern Senegal (D. Raoult, personal communication; GenBank accession number JQ674485) and in the mosquito malaria vectors Anopheles gambiae from Côte d'Ivoire (JN620082) and Anopheles melas from Gabon (JQ354961) [10,11]. Overall, R. felis, R. hoogstraalii, Rickettsia sp. PU01-02 and other genetically related rickettsiae that have been identified in fleas, soft ticks, ladybird beetles and tsetse flies form a well-supported (99/100 bootstrap support) clade, here provisionally called the ‘R. felis group’ (Fig. 2).
FIG. 2.
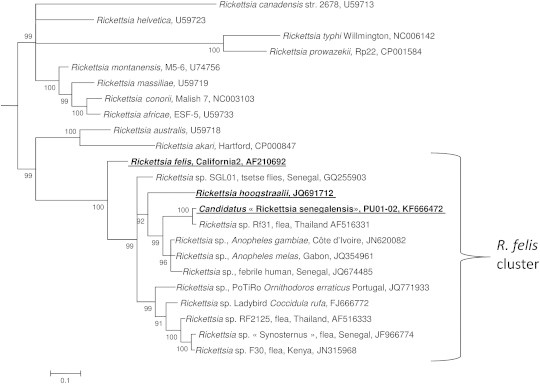
Phylogenetic tree highlighting the position of Candidatus ‘Rickettsia senegalensis’ strain PU01-02 relative to rickettsia type strains and uncultured rickettsiae. The gltA sequences were aligned by the use of CLUSTALW, and phylogenetic inferences were obtained from a Bayesian phylogenetic analysis with the HKY+Г substitution model. The GenBank accession numbers are indicated at the end. The numbers at the nodes are the bootstrap values obtained by repeating the analysis 100 times to generate a majority consensus tree. There were a total of 727 positions in the final dataset. The scale bar indicates a 10% nucleotide sequence divergence.
FIG. 3.

Phylogenetic tree highlighting the position of Candidatus ‘Rickettsia senegalensis’ strain PU01-02 relative to rickettsia type strains. The sequences of the rrs, gltA, sca4 and ompB genes were concatenated and aligned by the use of CLUSTALW, and phylogenetic inferences were obtained from a Bayesian phylogenetic analysis with the GTR+Г substitution model. The GenBank accession numbers of the genomes from which the gene sequences were extracted are indicated at the end. For Rickettsia hoogstraalii, the accession numbers are NR_104877, FJ767737, FJ767736 and EF629536 for the rrs, gltA, sca4 and ompB genes, respectively. The numbers at the nodes are the bootstrap values obtained by repeating the analysis 100 times to generate a majority consensus tree. There were a total of 9392 positions in the final dataset. The scale bar indicates a 5% nucleotide sequence divergence.
The sequences were deposited in GenBank under the following accession numbers: KF666476, KF666472, KF666470 and KF666474 for the rrs, gltA, ompB and sca4 genes of the PU01-02 strain, respectively.
The PU01-02 strain (provisional name, ‘Rickettsia senegalensis’) was deposited in two international collections of bacterial strains under the numbers CSUR R184 and DSM2850.
No fleas tested positive for either Bartonella species or Borrelia species.
Discussion
This article presents the results from a study undertaken to identify the sources and vectors of R. felis in Senegal. The epidemiology of this bacterium is presently an enigma in West Africa. The life cycle of pathogenic rickettsiae necessitates the presence of a blood-sucking vector [1]. However, examinations of the most probable vectors of R. felis in Senegal, including fleas, hard and soft ticks, bed bugs, tsetse flies, and biting midges [2,13], have not uncovered the natural life cycle of this pathogen. No fleas that are considered to be the vectors and reservoirs of R. felis worldwide were found to be positive for R. felis in Senegal. The only arthropods in this country in which R. felis has been identified are mosquitoes [2], and the reservoir of R. felis has still not been identified in Senegal [13].
Our study supported the findings of previous reports indicating the absence of R. felis in Senegalese fleas. This finding indicates that, in Senegal, where R. felis plays an important role as the cause of acute febrile diseases, its epidemiology may differ from that in American or European countries, where it is considered to be transmitted by the bite of an infected flea [8,9]. The presence of R. felis in both haematophagous and non-blood-sucking arthropods [22], its association with human febrile illnesses and its identification in negative control groups have not been explained. However, it is clear that R. felis is not an obligately flea-associated bacterium.
In this study, we announce the isolation of a novel strain of Rickettsia. The application of gene sequence-based criteria for the identification of new rickettsia isolates [23] confirms that the PU01-02 strain may represent a new rickettsial species. We could not, unfortunately, compare the entire ompB and sca4 sequences of the PU01-02 strain with those of other R. felis-like bacteria, because of the almost complete lack of available sequences. However, we found that ompB of the PU01-02 strain was identical to the small portion (15% of the total sequence) of ompB of Rickettsia sp. clone HL15c identified in C. felis fleas in Malaysia [24], and that sca4 was identical to the small portion (25% of the total sequence) of sca4 of Rickettsia sp. cf15 identified in C. felis fleas in the USA (unpublished; GenBank accession number DQ379482). Both of these rickettsia were not isolated in pure culture, and only small portions of genes (ompB, sca4, and gltA) are available. None of the Rickettsia species previously identified in Africa, including Candidatus Rickettsia assemboensis/Rickettsia sp. Synosternus [13,25] and Rickettsia sp. SGL01 [26], showed complete identity with the PU01-02 strain. Hence, we provisionally propose the name Candidatus Rickettsia senegalensis for this strain. The phylogenetic tree based on four concatenated ‘major’ rickettsial genes in Fig. 3 shows an undoubtedly separate position of the PU01-02 strain among officially validated rickettsial species in proximity to R. felis. The phylogenetic tree in Fig. 2 shows the position of the PU01-02 strain, based on gltA gene analysis, among other representatives of the genus, and sequences of rickettsial amplicons from different sources. The gltA gene was chosen for this phylogeny because of the relative richness of the GenBank database: most of the genetically related rickettsia were identified only by sequencing of this gene. Interestingly, the topology of the gltA-based phylogenetic tree contains a well-defined and bootstrap-supported cluster of rickettsiae, mostly comprising uncultured species. Thus, these sequences do not provide evidence for any epidemiological similarity, but they are genetically quite similar. They form a compact isolated clade (with two officially validated species: R. felis and R. hoogstraalii) that is a sister group to the neighbouring Rickettsia australis/Rickettsia akari. The roles of the PU01-02 strain in human and animal diseases and ecology are currently unknown.
Transparency declaration
The authors state that they have no conflicts of interest.
Acknowledgements
We thank N. Duclos, A. Bernard and D. Pyak for technical support. This study was funded by the Agence National de Recherche grant 2010 (MALEMAF) and Foundation Mediterranée Infection, Marseille, France.
References
- 1.Parola P., Paddock C.D., Socolovschi C., Labruna M.B., Mediannikov O., Kernif T. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013;26(4):657–702. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mediannikov O., Socolovschi C., Edouard S., Fenollar F., Mouffok N., Bassene H. Common epidemiology of Rickettsia felis infection and malaria, Africa. Emerg Infect Dis. 2013;19(11):1775–1783. doi: 10.3201/eid1911.130361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman D.O., Weld L.H., Kozarsky P.E., Fisk T., Robins R., von Sonnenburg F. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354(2):119–130. doi: 10.1056/NEJMoa051331. [DOI] [PubMed] [Google Scholar]
- 4.Mayxay M., Castonguay-Vanier J., Chansamouth V., Dubot-Peres A., Paris D., Phetsouvanh R. Causes of non-malarial fever in Laos: a prospective study. Lancet Global Health. 2013;1(1):e46–e54. doi: 10.1016/S2214-109X(13)70008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mediannikov O., Diatta G., Fenollar F., Sokhna C., Trape J.F., Raoult D. Tick-borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Negl Trop Dis. 2010;4(9) doi: 10.1371/journal.pntd.0000821. pii: e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maina A.N., Knobel D.L., Jiang J., Halliday J., Feikin D.R., Cleaveland S. Rickettsia felis infection in febrile patients, western Kenya, 2007–2010. Emerg Infect Dis. 2012;18(2):328–331. doi: 10.3201/eid1802.111372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards A.L., Jiang J., Omulo S., Dare R., Abdirahman K., Ali A. Human infection with Rickettsia felis, Kenya. Emerg Infect Dis. 2010;16(7):1081–1086. doi: 10.3201/eid1607.091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reif K.E., Macaluso K.R. Ecology of Rickettsia felis: a review. J Med Entomol. 2009;46(4):723–736. doi: 10.1603/033.046.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdad M.Y., Stenos J., Graves S. Rickettsia felis, an emerging flea-transmitted human pathogen. Emerg Health Threats J. 2011;4(7168):1–7. doi: 10.3402/ehtj.v4i0.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Socolovschi C., Pages F., Ndiath M.O., Ratmanov P., Raoult D. Rickettsia species in African Anopheles mosquitoes. PLoS One. 2012;7(10):e48254. doi: 10.1371/journal.pone.0048254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Socolovschi C., Pages F., Raoult D. Rickettsia felis in Aedes albopictus mosquitoes, Libreville, Gabon. Emerg Infect Dis. 2012;18(10):1687–1689. doi: 10.3201/eid1810.120178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Socolovschi C., Mediannikov O., Sokhna C., Tall A., Diatta G., Bassene H. Rickettsia felis, a common cause of uneruptive fever in rural Senegal. Emerg Infect Dis. 2010;16(7):1140–1142. doi: 10.3201/eid1607.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roucher C., Mediannikov O., Diatta G., Trape J.F., Raoult D. A new Rickettsia species found in fleas collected from human dwellings and from domestic cats and dogs in Senegal. Vector Borne Zoonotic Dis. 2012;12(5):360–365. doi: 10.1089/vbz.2011.0734. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins G.H., Rothschild M. vol. 1. British Museum (Natural History); London: 1953. (An illustrated catalogue of the Rothschild collection of fleas (Siphonaptera) in the British Museum (Natural History). With keys and short descriptions for the identification of families, genera, species and subspecies of the order). [Google Scholar]
- 15.Parola P., Diatta G., Socolovschi C., Mediannikov O., Tall A., Bassene H. Tick-borne relapsing fever borreliosis, rural Senegal. Emerg Infect Dis. 2011;17(5):883–885. doi: 10.3201/eid1705.100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mediannikov O., Diatta G., Kasongo K., Raoult D. Identification of Bartonellae in the soft sick species Ornithodoros sonrai in Senegal. Vector Borne Zoonotic Dis. 2014;14(1):26–32. doi: 10.1089/vbz.2013.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mediannikov O.Y., Sidelnikov Y., Ivanov L., Mokretsova E., Fournier P.E., Tarasevich I.V. Acute tick-borne rickettsiosis caused by Rickettsia heilongjiangensis in Russian Far East. Emerg Infect Dis. 2004;10(5):810–817. doi: 10.3201/eid1005.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekeyova Z., Mediannikov O., Subramanian G., Kowalczewska M., Quevedo-Diaz M., Kocianova E. Isolation of Rickettsia helvetica from ticks in Slovakia. Acta Virol. 2012;56(3):247–252. doi: 10.4149/av_2012_03_247. [DOI] [PubMed] [Google Scholar]
- 19.Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 20.Fournier P.E., Roux V., Raoult D. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int J Syst Bacteriol. 1998;48:839–849. doi: 10.1099/00207713-48-3-839. [DOI] [PubMed] [Google Scholar]
- 21.Parola P., Cornet J.P., Sanogo Y.O., Miller R.S., Van Thien H., Gonzalez J.P. Detection of Ehrlichia spp., Anaplasma spp., Rickettsia spp., and other eubacteria in ticks from the Thai–Myanmar border and Vietnam. J Clin Microbiol. 2003;41(4):1600–1608. doi: 10.1128/JCM.41.4.1600-1608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thepparit C., Sunyakumthorn P., Guillotte M.L., Popov V.L., Foil L.D., Macaluso K.R. Isolation of a rickettsial pathogen from a non-hematophagous arthropod. PLoS One. 2011;6(1):e16396. doi: 10.1371/journal.pone.0016396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fournier P.E., Dumler J.S., Greub G., Zhang J., Wu Y., Raoult D. Gene sequence-based criteria for identification of new rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol. 2003;41(12):5456–5465. doi: 10.1128/JCM.41.12.5456-5465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tay S.T., Mokhtar A.S., Low K.C., Mohd Zain S.N., Jeffery J., Abdul A.N. Identification of rickettsiae from wild rats and cat fleas in Malaysia. Med Vet Entomol. 2014;28(Suppl. 1):104–108. doi: 10.1111/mve.12075. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J., Maina A.N., Knobel D.L., Cleaveland S., Laudisoit A., Wamburu K. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013 May 15;13(8):555–558. doi: 10.1089/vbz.2012.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mediannikov O., Audoly G., Diatta G., Trape J.F., Raoult D. New Rickettsia sp. in tsetse flies from Senegal. Comp Immunol Microbiol Infect Dis. 2012;35(2):145–150. doi: 10.1016/j.cimid.2011.12.011. [DOI] [PubMed] [Google Scholar]


