Abstract
Detection of cryptococcal antigen in serum or cerebrospinal fluid allows cryptococcal meningitis diagnosis within few hours with >90% sensitivity. In an HIV-positive patient with Cryptococcus neoformans meningitis, initial antigen detection by immunoagglutination was negative. We thus evaluated a new immunochromatographic detection assay that exhibited a higher sensitivity.
Keywords: Cryptococcus neoformans, HIV, immunochromatography, meningitis, Antigen test
Cryptococcus neoformans has emerged as an important cause of pneumonia and meningoencephalitis among patients with reduced cell mediated immunity. Among patients infected with human immunodeficiency virus (HIV), most of the cases of cryptococcosis occur with a CD4 cell count of <100 cells/mm3[1]. Diagnosis is based on blood or cerebrospinal fluid (CSF) cultures and on immunoassays often used to detect C. neoformans surface capsular polysaccharide glucuronoxylomannan (GXM) shed during infection [2–5]. In HIV-infected patients, sensitivity of cryptococcal antigen detection in CSF approaches 99% [6]. Nevertheless, cryptococcosis with false-negative antigen results has been reported [7–9].
In the present study, we report a case of cryptococcal meningoencephalitis in an HIV-infected patient with initial negative cryptococcal antigen detection. We reviewed cryptococcal antigen assays at our institution over the last 25 years and evaluated a new immunochromatographic detection assay that exhibited a higher sensitivity.
In July 2013, a 29-year-old woman was seen at our outpatient infectious diseases clinic for an HIV infection that was diagnosed 4 months before in Cameroon. Antituberculosis treatment for a likely pulmonary tuberculosis had been started at HIV diagnosis. The patient complained about fever and cough that had lasted for several weeks. At baseline workup, CD4+ cell count was 140/mm3, and HIV virus load was 1.2 × 106 copies/mL. A first serum cryptococcal antigen (July 2013) was negative using the Pastorex immunoagglutination assay (Bio-Rad, Marnes la Coquette, France) (Fig. 1). A right lower lobe infiltrate was seen on chest x-ray. Bronchoalveolar lavage was negative for Mycobacterium tuberculosis (PCR, culture) and fungi (silver stain, culture). Co-trimoxazole for Pneumocystis jirovecii pneumonia prophylaxis was started (while waiting for the result of HIV genotypic analysis to initiate antiretroviral therapy), and antituberculosis treatment was pursued. Two weeks later, fever persisted and the patient developed diffuse intense holocranial headache that led to her admission to the emergency department. At examination, her temperature was 38.2°C, and her vital signs were normal. There was no neck stiffness, and the neurologic examination was normal. Brain magnetic resonance imaging showed a contrast-enhancing nodule about 6 mm of diameter in the left posterior parietal lobe without edema or signs of intracranial hypertension. CSF opening pressure was 7 cm H2O, and CSF examination revealed 192 leucocytes/mm3 (lymphocytes 88%), while protein level was 1775 mg/L, lactate level was 2.8 mmol/L and CSF/blood glucose ratio was 0.4. No microorganisms were observed on CSF staining. Bacterial and mycobacterial cultures were negative. Cryptococcal antigen in serum and CSF was negative (Fig. 1). Two days later, the CSF culture became positive. Microscopic observation revealed the presence of yeastlike organisms that we identified as C. neoformans using MALDI-TOF (matrix-assisted laser desorption-ionization time-of-flight) analysis (Bruker Daltonics, Leipzig, Germany) with a spectral score above 2 [10–12]. The same day, a CSF was collected, which came positive for GXM antigen (titer 1:4) using the Pastorex immunoagglutination assay (Fig. 1). We initiated antifungal therapy with liposomal amphotericin B 5 mg/kg iv once daily and flucytosine 25 mg/kg every 6 hours during the first 2 weeks, followed by fluconazole 400 mg by mouth once daily according to current guidelines [13], with a good outcome. Antiretroviral therapy with tenofovir, emtricitabine, raltegravir, darunavir and ritonavir was started after 2 weeks of antifungal therapy.
Fig. 1.
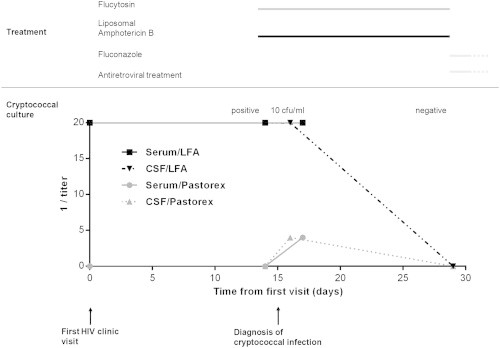
Time course of the case study and treatment. The buttom part of the figure shows results of cryptococcal antigen titers and cryptococcal culture. Cryptococcal antigen titers were determined by immunoagglutination (Pastorex) and lateral flow assay (Immunomycologics Inc., IMMY). Quantitative culture was achieved as follows: four drops of 100 μL, 50 μL, and 10 μL were deposited in duplicate on a brain–heart infusion plate supplemented with blood and incubated at 37°C with CO2. Antifungal and antiretroviral treatment is depicted in the upper part of the figure. Antifungal treatment consisted of liposomal amphotericin B (5 mg/kg) and intravenous flucytosine (25 mg/kg every other day). After 2 weeks of combined therapy, the patient was apyretic and headache disappeared; lumbar puncture revealed normal opening pressure and decreased amount of protein (862 mg/L), and CSF culture was sterile. Cryptococcal antigen detection in the CSF became negative as well. Antifungal treatment was switched to fluconazole (400 mg once daily) and antiretroviral treatment was initiated, without relapse of cryptococcal meningitis.
In our 1027-bed tertiary-care university hospital, we use the immunoagglutination assay Pastorex Crypto Plus 61747 (Bio-Rad), based on latex beads coated with anti-GXM mouth monoclonal antibodies [14,15]. From 1996 to 2013, 1759 samples (sera and CSF) from patients with suspicion of C. neoformans infection were analysed using this assay; among them, 152 samples tested positive (Fig. 2). The present case is the first of meningitis with positive C. neoformans culture and negative cryptococcal antigen agglutination in our hospital. A negative agglutination due to a prozone effect that can occur in high antigen titers has been excluded by serial dilution and by retesting of the sample. We thus hypothesize that the negative result is due to a low fungus load (10 C. neoformans cfu/mL at quantitative culture of the initial sample), possibly explained by partially preserved immunity (CD4 cells above 100/mm3).
Fig. 2.
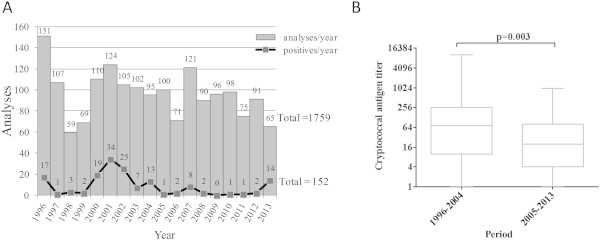
Cryptococcus neoformans infection over 18 years. (A) Total number of cryptococcal antigen detection by immunoagglutination and number of positive results in our hospital from 1996 to 2014. Results were obtained with the Bio-Rad assay Pastorex Crypto Plus 61747. (B) Evolution of cryptococcal antigen titer among positive results: comparison between 1996–2004 and 2005–2013 (comparison by Mann-Whitney test). Antigen titer for positive samples was determined by serial dilution according to the manufacturer's procedure.
Because sensitivity may vary among different commercial immunoassays, we retrospectively tested the negative samples of our patient with the recent lateral flow assay (LFA) detection system from Immunomycologics Inc. (IMMY) [6,16]. This immunochromatographic Point-of-Care test is based on the qualitative and semiquantitative detection of GXM in sera and CSF. The experimental sensitivity of the IMMY-LFA system (C5 to C95 interval = 1.0−1.5 ng/mL) determined with purified GXM is higher than the sensitivity of the Pastorex immunoagglutination system from Bio-Rad (detection limit 50 ng/mL) [17]. To validate the LFA assay in our laboratory, we retrospectively tested 50 CSF and sera from the collection of our hospital (30 positive and 20 negative samples) previously analysed with the Pastorex system. We found 100% agreement between the two systems. Antigen titers measured for positive samples were always higher with the IMMY-LFA system than with the Pastorex assay (Fig. 3; Supplementary Table 1). The samples from our patient that were negative with the Pastorex immunoagglutination assay came positive with the IMMY-LFA system (titer 1:20) (Fig. 1). This suggests a higher sensitivity of the IMMY-LFA compared to immunoagglutination assays. This is consistent with two recent large studies, performed on 421 sera from HIV patients in Colombia and 589 sera and 411 CSF in the United States, respectively [18,19]. The negative case that we report in this article might be due to the low fungus load of C. neoformans (10 cfu/mL), which might be close to the detection limit (Fig. 1).
Fig. 3.
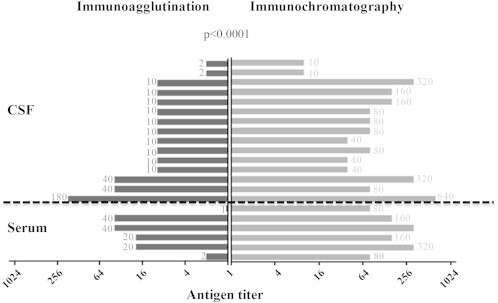
Evaluation of the lateral flow assay (LFA) detection system from Immunomycologics Inc. (IMMY). A total of 50 samples (30 positive and 20 negative) previously analysed with the immunoagglutination assay Pastorex Crypto Plus 61747 (Bio-Rad) were retrospectively tested with the IMMY-LFA (Supplementary Table 1). The 20 samples that were negative with the Pastorex assay also came negative with the LFA assay (data not presented). In 9 cases, immunochromatography was performed but the titration could not be done because the volume of stored CSF was not sufficient. Antigen titers were always higher with the IMMY-LFA system than with the Pastorex assay (comparison by Wilcoxon signed rank test).
The incidence of cryptococcal meningitis has decreased since the advent of effective antiretroviral therapy [20,21]. Similarly, infections with high antigen titer are less likely to occur. In our hospital, we observed decreasing antigen titer for positive samples over the last 18 years (Fig. 2B), with a median titer of 72 during 1996–2004 versus 20 during 2005–2013 (p 0.003, comparison by Mann-Whitney test). Although we did not have comprehensive data on quantitative cultures, this suggests infections with a lower fungus load. In this context, cryptococcosis cases with negative antigen results are more likely to occur. Other causes of false-negative antigen results can be high antigen titers (>1:256) leading to a prozone reaction [22,23] or the presence of immune complexes that prevent the shedding of GXM antigen [24]; alternatively, low antigen release could be associated with poorly encapsulated strains [25–27].
In conclusion, negative antigen results do not permit the exclusion of cryptococcal infection, especially in the setting of low fungus load. Immunochromatography systems should be preferred over immunoagglutination assay, as they are easier to handle and more sensitive.
Conflict of interest
None declared.
Acknowledgements
We are grateful to all of the technicians of the Diagnostic Microbiology Laboratory of the University Hospital of Lausanne for their technical contribution. In particular, we thank Maria Senra-Ortiz and Christian Durussel.
Footnotes
The first two authors contributed equally to this article, and both should be considered first author. The last two authors contributed equally to this article, and both should be considered senior author
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Jarvis J.N., Dromer F., Harrison T.S., Lortholary O. Managing cryptococcosis in the immunocompromised host. Curr Opin Infect Dis. 2008;21:596–603. doi: 10.1097/QCO.0b013e3283177f6c. [DOI] [PubMed] [Google Scholar]
- 2.Kozel T.R., Cazin J., Jr. Immune response to Cryptococcus neoformans soluble polysaccharide. I. Serological assay for antigen and antibody. Infect Immun. 1972;5:35–41. doi: 10.1128/iai.5.1.35-41.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kronstad J., Saikia S., Nielson E.D., Kretschmer M., Jung W., Hu G. Adaptation of Cryptococcus neoformans to mammalian hosts: integrated regulation of metabolism and virulence. Eukaryot Cell. 2012;11:109–118. doi: 10.1128/EC.05273-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon-Chung K.J., Wickes B.L., Stockman L., Roberts G.D., Ellis D., Howard D.H. Virulence, serotype, and molecular characteristics of environmental strains of Cryptococcus neoformans var. gattii. Infect Immun. 1992;60:1869–1874. doi: 10.1128/iai.60.5.1869-1874.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin X., Heitman J. The biology of the Cryptococcus neoformans species complex. Annu Rev Microbiol. 2006;60:69–105. doi: 10.1146/annurev.micro.60.080805.142102. [DOI] [PubMed] [Google Scholar]
- 6.Tanner D.C., Weinstein M.P., Fedorciw B., Joho K.L., Thorpe J.J., Reller L. Comparison of commercial kits for detection of cryptococcal antigen. J Clin Microbiol. 1994;32:1680–1684. doi: 10.1128/jcm.32.7.1680-1684.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton J.R., Noble A., Denning D.W., Stevens D.A. Performance of cryptococcus antigen latex agglutination kits on serum and cerebrospinal fluid specimens of AIDS patients before and after pronase treatment. J Clin Microbiol. 1991;29:333–339. doi: 10.1128/jcm.29.2.333-339.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Currie B.P., Freundlich L.F., Soto M.A., Casadevall A. False-negative cerebrospinal fluid cryptococcal latex agglutination tests for patients with culture-positive cryptococcal meningitis. J Clin Microbiol. 1993;31:2519–2522. doi: 10.1128/jcm.31.9.2519-2522.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessler A.T., Al Kharrat T., Kourtis A.P. Cryptococcus neoformans as a cause of bronchiolitis obliterans organizing pneumonia. J Infect Chemother. 2010;16:206–209. doi: 10.1007/s10156-010-0039-7. [DOI] [PubMed] [Google Scholar]
- 10.Croxatto A., Prod'hom G., Greub G. Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol Rev. 2012;36:380–407. doi: 10.1111/j.1574-6976.2011.00298.x. [DOI] [PubMed] [Google Scholar]
- 11.Prod'hom G., Bizzini A., Durussel C., Bille J., Greub G. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for direct bacterial identification from positive blood culture pellets. J Clin Microbiol. 2010;48:1481–1483. doi: 10.1128/JCM.01780-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prod'hom G., Durussel C., Greub G. A simple blood-culture bacterial pellet preparation for faster accurate direct bacterial identification and antibiotic susceptibility testing with the VITEK 2 system. J Med Microbiol. 2013;62(pt 5):773–777. doi: 10.1099/jmm.0.049361-0. [DOI] [PubMed] [Google Scholar]
- 13.Day J.N., Chau T.T., Wolbers M. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med. 2013;368:1291–1302. doi: 10.1056/NEJMoa1110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dromer F., Charreire J., Contrepois A., Carbon C., Yeni P. Protection of mice against experimental cryptococcosis by anti–Cryptococcus neoformans monoclonal antibody. Infect Immun. 1987;55:749–752. doi: 10.1128/iai.55.3.749-752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dromer F., Salamero J., Contrepois A., Carbon C., Yeni P. Production, characterization, and antibody specificity of a mouse monoclonal antibody reactive with Cryptococcus neoformans capsular polysaccharide. Infect Immun. 1987;55:742–748. doi: 10.1128/iai.55.3.742-748.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarvis J.N., Percival A., Bauman S., Pelfrey J., Meintjes G., Williams G.N. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin Infect Dis. 2011;53:1019–1023. doi: 10.1093/cid/cir613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gates-Hollingsworth M.A., Kozel T.R. Serotype sensitivity of a lateral flow immunoassay for cryptococcal antigen. Clin Vaccine Immunol. 2013;20:634–635. doi: 10.1128/CVI.00732-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escandon P., Lizarazo J., Agudelo C.I., Chiller T., Castaneda E. Evaluation of a rapid lateral flow immunoassay for the detection of cryptococcal antigen for the early diagnosis of cryptococcosis in HIV patients in Colombia. Med Mycol. 2013;51:765–768. doi: 10.3109/13693786.2013.781692. [DOI] [PubMed] [Google Scholar]
- 19.Hansen J., Slechta E.S., Gates-Hollingsworth M.A., Neary B., Barker A.P., Bauman S. Large-scale evaluation of the immuno-mycologics lateral flow and enzyme-linked immunoassays for detection of cryptococcal antigen in serum and cerebrospinal fluid. Clin Vaccine Immunol. 2013;20:52–55. doi: 10.1128/CVI.00536-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bamba S., Lortholary O., Sawadogo A., Millogo A., Guiguemde R.T., Bretagne S. Decreasing incidence of cryptococcal meningitis in West Africa in the era of highly active antiretroviral therapy. AIDS. 2012;26:1039–1041. doi: 10.1097/QAD.0b013e328352d1d8. [DOI] [PubMed] [Google Scholar]
- 21.Mirza S.A., Phelan M., Rimland D., Graviss E., Hamill R., Brandt M.E. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992–2000. Clin Infect Dis. 2003;36:789–794. doi: 10.1086/368091. [DOI] [PubMed] [Google Scholar]
- 22.Hefter L.G., Hix M.A., Stoner M., Cook C.B. False negative hepatitis B surface antigen detection in dialysis patients due to excess surface antigen: postzone phenomenon. J Clin Pathol. 1980;33:993–994. doi: 10.1136/jcp.33.10.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L.L., Lin L.R., Tong M.L., Zhang H.L., Huang S.J., Chen Y.Y. Incidence and risk factors for the prozone phenomenon in serologic testing for syphilis in a large cohort. Clin Infect Dis. 2014;59:384–389. doi: 10.1093/cid/ciu325. [DOI] [PubMed] [Google Scholar]
- 24.Martinez L.R., Moussai D., Casadevall A. Antibody to Cryptococcus neoformans glucuronoxylomannan inhibits the release of capsular antigen. Infect Immun. 2004;72:3674–3679. doi: 10.1128/IAI.72.6.3674-3679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bottone E.J., Toma M., Johansson B.E., Wormser G.P. Poorly encapsulated Cryptococcus neoformans from patients with AIDS. I. Preliminary observations. AIDS Res. 1986;2:211–218. doi: 10.1089/aid.1.1986.2.211. [DOI] [PubMed] [Google Scholar]
- 26.Bottone E.J., Wormser G.P. Poorly encapsulated Cryptococcus neoformans from patients with AIDS. II. Correlation of capsule size observed directly in cerebrospinal fluid with that after animal passage. AIDS Res. 1986;2:219–225. doi: 10.1089/aid.1.1986.2.219. [DOI] [PubMed] [Google Scholar]
- 27.Robertson E.J., Najjuka G., Rolfes M.A., Akampurira A., Jain N., Anantharanjit J. Cryptococcus neoformans ex vivo capsule size is associated with intracranial pressure and host immune response in HIV-associated cryptococcal meningitis. J Infect Dis. 2014;209:74–82. doi: 10.1093/infdis/jit435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


