Abstract
Objective
To investigate the preliminary effectiveness of surface electromyography (sEMG) biofeedback delivered via interaction with a commercial computer game to improve motor control in chronic stroke survivors.
Design
Single-blinded, one-group repeated measures design A1, A2, B, A3 (A=assessment, B=intervention).
Setting
Laboratory and participants’ homes.
Participants
A convenience sample of nine persons between 40–75 years of age with moderate to severe upper extremity motor impairment and at least six months post-stroke completed the study.
Intervention
The electromyography-controlled video game system targeted the wrist muscle activation with the goal of increasing selective muscle activation. Participants received several laboratory training sessions with the system and then were instructed to use the system at home for 45 minutes five times per week for the following four weeks.
Main Outcome Measures
Primary outcome measures included duration of system use, sEMG during home play and pre/post sEMG measures during active wrist motion. Secondary outcomes included kinematic analysis of movement and functional outcomes, including the Wolf Motor Function Test and the Chedoke Arm and Hand Activity Inventory-9.
Results
One-third of participants completed or exceeded the recommended amount of system use. Statistically significant changes were observed on both game play and pre/post sEMG outcomes. Limited carryover, however, was observed on kinematic or functional outcomes.
Conclusion
This preliminary investigation indicates that use of the electromyography-controlled video game impacts muscle activation. Limited changes in kinematic and activity level outcomes, however, suggest that the intervention may benefit from the inclusion of a functional activity component.
Keywords: stroke, technology, video games, rehabilitation, tele-therapy
In the United States, approximately 795,000 persons experience a new stroke each year, and 50% of stroke survivors experience difficulty using their impaired upper extremity six months post-stroke.1 Persons with poor upper extremity motor function after stroke exhibit a variety of impairments, including hemiparesis and spasticity.2–4 Voluntary selective muscle activation is often difficult due to excessive co-contraction of agonists and antagonists, leading to an inability to achieve movement using typical activation patterns.2 Impairments in upper extremity motor function are associated with decreased quality of life and difficulty resuming daily activities.4,5
While impairments can be severe, stroke survivors can partially improve motor function with therapy and repetitive practice of specific tasks.6–8 Rehabilitation therapists use a variety of treatment approaches to address hemiparesis and spasticity. Most current approaches to outpatient therapy, however, provide too little practice to produce recovery in the chronic phase of stroke for those who actually receive therapy services.9,10 While clinical practice guidelines strongly recommend follow-up services for persons with residual impairments following acute rehabilitation, only 30.7% of stroke survivors receive outpatient therapy.11,12 Even for those receiving outpatient therapy the amount is variable, with a median of six outpatient therapy visits (interquartile range 1–21 visits) in the first year after stroke.13
In contrast, the amount of practice needed to induce functional improvements for chronic stroke survivors is extensive. A review paper, reported that a study by Pang and colleagues found that 57 hours of practice was needed to make functional changes that impact performance in self-care and leisure tasks.6,14 With this amount of practice suggested in literature, typical outpatient therapy provides insufficient practice time for motor recovery during clinical sessions. While practice can be extended through home programs, adherence is generally poor with multiple barriers reported.15,16
We sought to address the challenges of providing sufficient and specific practice outside of the clinic. We developed a home-based program using surface electromyographic (sEMG) biofeedback interfacing with a computer game. sEMG biofeedback has been used in motor rehabilitation following stroke since the 1960s.17 While the evidence base for sEMG biofeedback is inconclusive, several small studies have found it to benefit upper extremity motor recovery of stroke survivors.17–19 We utilized this biofeedback method with an engaging, commercially available computer game in order to increase practice and subsequent repetitions using the impaired upper extremity at home. The use of sEMG biofeedback provides the participant with specific feedback of muscle activation as an agonist/antagonist pair over multiple repetitions. Specificity and repetition are two elements found to induce neural plasticity.8 We tested the hypothesis that use of the electromyography-controlled video game system improves voluntary muscle activation and functional performance on outcome measures for adults in the chronic stage of recovery from stroke.
METHODS
Study Design
This preliminary study used a single-blinded, one-group repeated measures design: A1, A2, B, A3 (A=assessment, B=intervention). A1 and A2 were scheduled approximately four weeks apart, prior to system use. A3 occurred immediately after completion of system use in the home. This design was selected because of the heterogeneous nature of stroke survivors and the preliminary nature of this investigation. All procedures were approved by the local Human Subjects Division, and all participants gave written informed consent prior to participation in the study.
Participants
Participants were a convenience sample of volunteers more than six months post-stroke with an average age of 60 (SD = 8). Participants’ level of impairment ranged from no active extension in the digits to full digit extension. Participants had vision and hearing sufficient to play a computer game, and were cognitively able to give informed consent. Participants were excluded if they: (1) had a skin condition that would interfere with the sEMG assessment or intervention, (2) reported significant pain in their affected upper extremity, (3) had a secondary neurological diagnosis such as Parkinson’s disease, (4) had a contracture at the wrist that would prevent the wrist from being passively extended to a neutral position, (5) had received neurolytic injections in the previous four months, or (6) had variations in dosage of oral anti-spasticity medication in the previous three months.
Twelve participants were enrolled and nine completed all three assessments and the intervention. Two withdrew due to lack of time to participate, and one was asked to withdraw secondary to a change in his medical condition unrelated to the study. The characteristics of the nine participants who completed the study are presented in Table 1.
Table 1.
Participant Characteristics
| ID | Age | Gender | Hemiplegic Side | Years Post Stroke | In Therapy | Full Digit Extension | Self-Reported Location | Number of Home Sessions* | Hours of Home Use† |
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| A | 53 | Male | Left | 5 | Yes | No | Unknown | 10 | 5 |
| B | 54 | Female | Left | 9 | No | No | Parietal/Frontal | 10 | 6 |
| C | 67 | Female | Right | 3 | Yes | No | Unknown | 8 | 7 |
| D | 54 | Female | Right | 1 | Yes | No | Brainstem | 12 | 10 |
| E | 47 | Female | Right | 3 | Yes | No | Brainstem | 18 | 11 |
| F | 68 | Male | Right | 9 | No | No | Frontal | 19 | 12 |
| G | 58 | Male | Right | 6 | No | Yes | Unknown | 23 | 15 |
| H | 69 | Male | Left | 27 | No | No | Unknown | 27 | 20 |
| I | 69 | Male | Right | 2 | No | No | Basal Ganglia | 24 | 21 |
Only included sessions that lasted at least five minutes and displayed modulations in recorded signals from both muscles from the day of home setup to the day the system was returned
Includes home play that lasted at least five minutes and displayed modulations in recorded signals from both muscles
Intervention
The electromyography-controlled video game system, called Neurogame Therapy (NGT), consists of a laptop computer, NGT console, sEMG leads, and disposable electrodes. The NGT system console uses a custom Neurochip circuit to amplify and digitize bi-polar analog sEMG signals from two muscle groups and transmit these signals via USB to the computer.20,21 Custom software converts muscle activity into movements used to control the computer game. The system’s sensitivity can be adjusted to detect very low levels of activation, thus allowing persons with minimal muscle activation to participate. The conversion from sEMG activity to game movement was adjusted as needed during the intervention phase to facilitate challenging but successful game play (i.e. the ‘just-right’ challenge). If participants had an Internet connection at home, the investigators could make adjustments to game settings remotely.
Participants used the muscle activity in their affected wrist flexors (i.e., flexor carpi radialus) and extensors (i.e., extensor digitorum) to perform pre-game maximum voluntary contractions (MVCs) and then to play the commercially available computer game, Peggle™a. For collection of MVCs, participants were instructed to maximally flex or extend their wrist during a 10 second window, followed by a 10 second relaxation period. This was repeated three times for flexion, followed by three times for extension. In Peggle™a, participants attempt to clear the board of orange pegs by identifying the correct angle to launch a ball to eliminate pegs. Participants controlled the aim using their affected upper extremity and launched the ball by clicking a button using the less-affected hand. The game could be set up in two modes. Mode 1 trained selective muscle activation (i.e., quieting one muscle group while activating the other). Mode 2 trained activation of a weak muscle group independent of the activity of the antagonist group.
Measures
Home therapy outcome measures
The NGT software captured raw sEMG during each home therapy session. To be included in the analysis, home sessions must have lasted at least five minutes and have displayed modulations in recorded signals from both muscles to assure that the sensors were properly connected to the arm. Outcome variables included number of home game sessions, hours of play, number of usable hours, repetitions per session, independent activity, and maximal voluntary contractions.
Assessment outcome measures
Assessment outcome measures were collected across the impairment and activity levels of the International Classification of Functioning, Disability and Health (ICF)22 in order to determine the level of impact for NGT. These included sEMG, joint kinematics, and activity test described below.
Surface electromyography (sEMG)
sEMG electrodes were placed over the motor points for the wrist flexors and extensors of the participants’ affected extremity (Delsys Bagnoli systemb).23 The electrode placements were measured relative to bony landmarks and were recorded for consistency in future testing. sEMG was collected at 500 Hz during laboratory assessments and low-pass filtered at 200 Hz.
Joint kinematics
During two simple movement tasks, 3-dimensional trajectories were collected using a Qualisys, Oqus 300 camera systemc with eight cameras capturing reflective marker data at 100 Hz (error residuals < 3mm for each camera). Reflective markers were placed on the participant’s trunk, forehead, sternal notch, bilateral acromion processes, and throughout the affected upper extremity (lateral epicondyle, ulnar styloid process, radial styloid process, and head of the third metacarpal). In the first movement task, participants were asked to reach out to pick up a cup of water in midline at arm’s length away.24 If a participant was unable to pick up the cup, they were instructed to reach out as if they were going to pick up the cup and make the cup move. The second task evaluated active range of motion at the wrist. Participants’ affected upper extremity was supported at the forearm and they were instructed to move their wrist as far as possible into extension and then flexion. Each of these tasks had a minimum of five trials. Joint kinematics were measured to identify changes in upper extremity movement efficiency and compensatory movements.
Activity tests
Participants were video recorded during the Wolf Motor Function Test (WMFT)25–27 and the Chedoke Arm and Hand Activity Inventory-9 (CAHAI-9)28–30 as secondary measures. The participants were tested by one of three trained team members. Scoring was completed by an occupational therapist with 20 years of experience, who was not involved with the study, and was blinded to the order of the participants’ videos.
Procedures
In the first assessment (A1) participants completed a health history form. Subsequent assessments began with an update on current therapy routines, followed by the WMFT and CAHAI-9. After placement of the sEMG electrodes, participants performed maximal voluntary contraction against a stabilized dynamometer with their forearm resting on a table, first using wrist extension followed by wrist flexion. Kinematic markers were then placed to permit simultaneous kinematic and sEMG data collection during the reach and wrist active range of motion tasks.
Participants were trained to use the NGT system after the second assessment (A2). Electrode placement for the wrist extensors varied slightly across participants (i.e., proximal versus more distal on the extensor digitorum) with the goal of promoting digit extension in the affected extremity when possible. Extensor digitorum contributes to both wrist and digit extension. It was also targeted for wrist extension as it is able to produce a greater moment about the wrist compared to other wrist extensors such as extensor carpi unlaris.31 Participants received up to five training sessions, during which they learned to attach the sEMG electrodes over ink markings applied to the skin and use the NGT system.
For eight of the participants NGT was set up within a suitable space in their homes. Participants were asked to use NGT for four weeks, playing the game five days a week for up to 45 minutes per day, or a total of 15 hours. One participant determined that her home was unable to accommodate the game system and completed game play at the laboratory one to three times a week over 8 weeks, unsupervised, in a quiet room. Participants had intermittent contact with the research team during the intervention period in order to ensure that the system was working and that the level of challenge was appropriate. Participants were able to contact the team at any time should they encounter difficulties. These difficulties were typically solved over the phone, but at times required a home visit.
Data Analysis
Raw sEMG signals recorded in the home were filtered and wavelet analysis was used to establish a reliable baseline level of sEMG for the detection of bursts in activity. Independent activity was then calculated as the percentage of bursts detected in the agonist muscle when no simultaneous burst was detected in the antagonist. Wavelet analysis allows for the detection of muscle activity against background noise, even when the signal recorded in the home was noisy. The sEMG signal is then iteratively transformed into subsets of coefficients, soft thresholding is applied, and the signal is recovered using the inverse transform.
sEMG data recorded in the laboratory were processed using custom LabViewf software. The sEMG level during MVC was calculated for wrist flexion and wrist extension as the average of the peak amplitudes over 5 trials per assessment. To calculate normalized co-contraction ratio over the period of extensor activation, the integrated signal for the wrist extensors was divided by the extensor MVC and then divided by the integrated signal for the wrist flexors over the flexor MVC (Figure 1). This was done so that a ratio greater than one would indicate greater agonist activation. See Appendix for further details.
Figure 1.
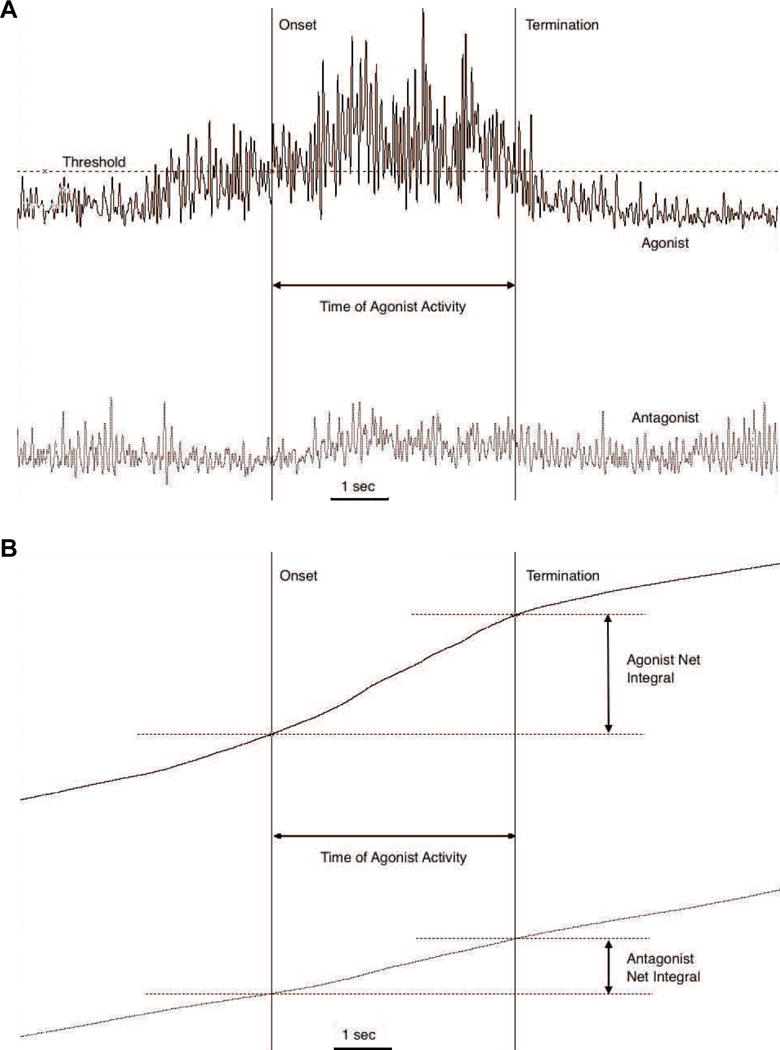
Example EMG from the agonist extensor muscle (EDC, black traces) and antagonist flexor (FCR-grey traces) during the reach task. The co-contraction ratio is calculated by comparing the relative integrated area under the rectified EMG of each muscle, during the time of the agonist activity. During the period of the reach movement determined from video records (not shown), the agonist muscle onset and offset (vertical lines) are detected when activity crosses a threshold of 5SD above baseline activity (shown in A). The ratio of the resulting cumulative integrals of each muscle (shown in B) is the co-contraction value.
Kinematic analysis of the two tasks determined: 1) reach time 2) number of movement segments 3) trunk displacement 4) maximal elbow extension 5) overall amount of wrist extension. Details on the calculations used for each of these variables can be found in the Appendix.
Trends in co-contraction and maximal activation for sEMG recorded during home game play were analyzed using linear regressions (Matlab 2011ae). For laboratory assessment analysis, variables that contained multiple trials were averaged to create a mean for each variable. Secondary to the small sample size and preliminary nature of the data, the Wilcoxon signed-rank test was used to compare performance across no-treatment (A1 to A2) and treatment (A2 to A3) phases (SPSS Version 18d). The alpha level for all tests was 0.05.
RESULTS
Duration of Game Play
Three participants completed or exceeded the number of 20 recommended home training sessions, and two additional participants were close to completing the recommended number of sessions (Table 1). The remaining four participants played the game about two to three times per week. The amount of system use in the home was relatively stable across weeks for most participants (Figure 2). Across both training and home use, participants averaged a total of 11.6 hours of system use.
Figure 2.

The number of hours of game play across the four week intervention for each subject. Subjects are ordered by the amount of time they chose to participate in the NGT intervention in the home. Additional game therapy as part of training was performed in the laboratory prior to using the system in the home for each subject.
sEMG During Game Therapy
Five out of nine participants increased independent activation of wrist extensors and flexors during game therapy. Of the participants who did not increase independence, three performed less than 10 hours of game play (Figure 3). In addition, six of nine participants increased maximal voluntary contraction (MVC) for either the flexor or extensor muscles measured during the daily calibration prior to game therapy (Figure 4). Although there was a trend toward more improvement with greater amounts of game play, linear regressions against the number of hours of game play were not significant for either MVC measures or muscle independence (R2 ≤ 0.12, p ≥ 0.35).
Figure 3.

Independent activity across game play sessions. Subjects are ordered by the number of hours of game play suitable for analysis including training and home play. Of subjects that played 10 or more hours (dashed line), five of six subjects improved muscle independence based on a significant (* p<0.05) positive regression between independence and game play session.
Figure 4.
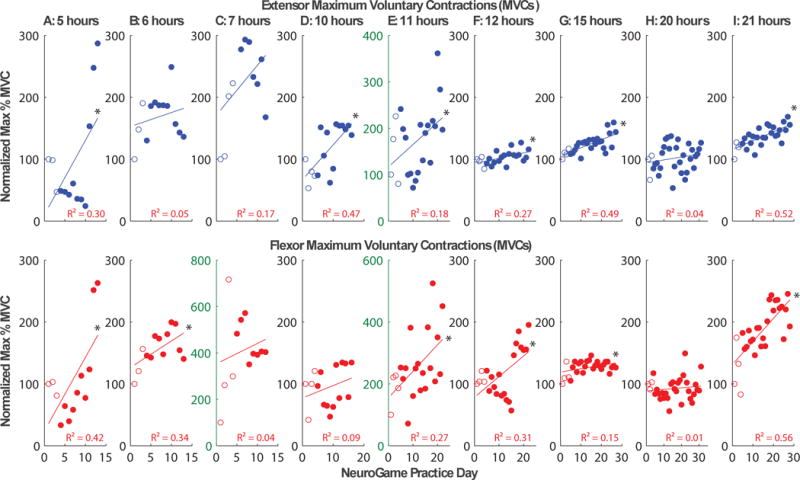
Maximal voluntary contraction (MVC) during pre-game calibration plotted across game play sessions. Subjects are ordered by the number of hours of game play suitable for analysis including training and home play. Six of nine subjects improved muscle activation of at least the extensor (top row) or flexor (bottom row) muscles based on a significant (* p<0.05) positive regression between muscle activity and game play session, while four of these subjects improved activation of both muscles. Note that three subjects improved EMG greater than 300%, requiring greater range on the abscissa, and select axes are thus marked with green text to highlight this difference.
Laboratory Assessment Outcomes
sEMG
During the reach task, five out of the six participants with complete data for analysis demonstrated a change toward increased selective activation of the extensor. This was reflected in a statistically significant difference across the treatment period (A2 to A3) for the normalized co-contraction ratios (Z= −1.992, p = 0.046; Table 2). No other statistically significant differences were found for the extensor and flexor MVCs or for normalized co-contraction ratios for extensor active range of motion (Table 2).
Table 2.
Laboratory Assessment Outcomes
| n | A1 Mean (SD) | A2 Mean (SD) | A3 Mean (SD) | |
|---|---|---|---|---|
|
|
||||
| MVC Extensor (microvolt)† | 8 | 30 (29) | 41 (33) | 37 (26) |
| MVC Flexor (microvolt)† | 7 | 38 (19) | 42 (25) | 37 (14) |
| CC-Extensor AROM† | 6 | 3.14 (1.72) | 4.03 (5.62) | 3.31 (3.63) |
| CC-Reach† | 6 | 2.92 (2.56) | 3.47 (5.84)* | 5.84 (9.78)* |
| Reach Time (seconds) | 8 | 3.97 (2.67)* | 2.52 (1.00)* | 2.54 (1.18) |
| Movement Segments | 8 | 7 (6) | 4 (3) | 4 (3) |
| Elbow extension (degrees)† | 8 | 95.3 (22.7) | 96.8 (24.7) | 95.5 (22.1) |
| Trunk Displacement (mm) | 8 | 121.9 (47.2) | 123.22 (65.1) | 131.7 (49.6) |
| Active Range of Motion (degrees) | 8 | 30.4 (19.1) | 31.6 (17.7) | 25.4 (17.7) |
| WMFT Functional Activity Score† | 9 | 1.79 (0.71) | 1.77 (0.68) | 1.79 (0.66) |
| WMFT MeanTime (s) | 9 | 67.54 (35.09) | 66.07 (33.69) | 67.85 (35.17) |
| WMFT Grip Strength (kg)† | 9 | 5 (6) | 6 (6) | 7 (8) |
| CAHAI Percent Score† | 9 | .27 (.18) | .26 (.17) | .25 (.17) |
Note:
p < 0.05 via Wilcox Signed Rank non-parametric test
Higher number indicates improved performance
Kinematics
Eight participants had usable data for group analysis from the kinematic measures. Of the four variables generated from the reach task (reach time, number of movement segments, elbow extension, and trunk displacement), a statistically significant difference was found across A1 to A2 for reach time, but no change was found across A2 to A3 (Table 2). No other variables demonstrated statistically significant differences.
Activity measures
No differences were found across time on any of the WMFT subscales or the CAHAI-9 (Table 2).
DISCUSSION
Nine adults at least six months post-stroke completed this study to evaluate the preliminary effectiveness of NGT as a home program to improve motor control in chronic stroke survivors. The majority of participants improved maximal activations of at least one wrist muscle and independence of antagonist muscles measured during the game therapy sessions. Co-contraction was also reduced in one of the functional sEMG tasks following the game intervention. No significant changes were seen on standardized functional activity tests.
There was a statistically significant increase in the amount extensor activation in comparison to flexor activation present during the simulated reaching task in the laboratory assessment. A similar change in independent activation during game play was observed in most participants who played the game for more than a total of 10 hours. Daily MVC tests prior to game play showed an increase for some participants. Parallel changes in MVC, however, were not seen in the laboratory assessments. This could be due to the different nature of the test as well as the additional visual feedback provided in the pre-game MVC test.
The changes in independent activity observed in game play and laboratory assessment suggest that the intervention, which was designed to improve selective muscle activation, was functioning as anticipated. These changes observed at the level of muscle activation are encouraging as voluntary muscle activation post stroke is the primary source of muscle weakness.32 A lack of robust findings in voluntary movement activity based outcomes, however, suggest that the outcomes are specific to the training provided – consistent with neuroplasticity research.8 NGT may therefore benefit from combination with active functional movement practice in order to impact functional movement outcomes. Previous research using sEMG biofeedback suggests that a combination of sEMG and more conventional therapy interventions is most successful.19
The participants in the study had chronic movement impairment that substantially limited their success completing activities with their upper extremity. This may have contributed to the limited improvements in the present functional outcomes. Chronic movement impairments of this complexity require more time to create functional changes and are accompanied by additional challenges such as spasticity and learned co-contraction patterns.6 Therefore, it would be beneficial for future studies to investigate the application of NGT during the acute phase of rehabilitation.
Three participants completed or exceeded the number of sessions recommended, and two more participants were one to two sessions away from completing the recommended number of sessions. While this is promising, it does appear that even those participants, who initiated system use at the recommended number of times, did not use the system for the amount of time requested. This suggests that changes to the dosage will need to be considered in future studies. Furthermore, even if the participants achieved perfect adherence, the total intervention duration will likely need to be increased in future studies in order for participants to receive the large amount of practice and repetitions suggested in the literature.6
Adherence to NGT was only fair among some participants, suggesting that five sessions per week may not be a feasible home therapy frequency. Shorter daily sessions were originally selected to minimize the possibility of overuse injuries, however, no soreness or progressive injuries were reported in interviews accompanying this study. In fact, results from our companion study found that participants on the whole found the game enjoyable and even suggested allowing use for a longer period of time. Therefore, requiring fewer sessions of longer duration may also provide a greater level of therapeutic intensity.
Study Limitations
Limitations of this study include the small convenience sample and the variability in participants’ age and time post-stroke. Care should also be taken when interpreting the sEMG results as maximal sEMG signal can be influenced by a number of factors outside of muscle activation, such as the condition of the participant’s skin, electrode placement, electrode contact with the skin, and the nature of the task. Another limitation was the lack of control for confounding factors, such as receiving other motor therapies. It should be noted, however, that of the participants who were receiving additional therapy, most were seen just one or two times a month. Nevertheless, it is interesting to note the participants who were not receiving additional therapy had the greatest adherence. This suggests that stroke survivors who are provided with an engaging home therapy program, in absence of other direct therapy services, may be more apt to follow through with NGT.
CONCLUSIONS
NGT is an engaging combination of biofeedback with a commercial computer game, targeting activation of a particular muscle group or co-contraction within an agonist/antagonist pair. In this preliminary study, we found an effect at the level of sEMG showing a decrease in co-contraction, but no changes at the level of functional movement. NGT may benefit from a longer intervention time and the inclusion of more functional activity training to assist in the transfer of changes at the muscle activation level to changes in function. Further research is needed to determine the value of this intervention in this clinical population.
Acknowledgments
Dianne Rios, ScD, OTR/L; Karli Gutman, DPT and Kathryn E. Forrest Miller, BS made key contributions to data collection and processing of this study.
Presentation of this Material: The contents of this paper were presented October 19, 2012 to the first author’s doctoral committee and the general public as part of a dissertation defense. Material was also presented as part of a “job talk” at the University of North Carolina and Duquesne University, primarily to the faculty and students of these schools.
Funding: The project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1RR025014, the Bayley Family Foundation and, and a Washington Research Foundation gif to the Center for Sensorimotor Neural Engineering (CNSE), a National Science Foundation Engineering Research Center (EEC-1028725). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies including NIH and NSF.
Abbreviations
- sEMG
surface electromyography
- NGT
NeuroGame Therapy
- WMFT
Wolf Motor Function Test
- CAHAI-9
Chedoke Arm and Hand Activity Inventory-9
APPENDIX: Details of sEMG and Kinematic Data Reduction
sEMG
sEMG data from the game play sessions were down-sampled at 500 Hz and low-pass filtered at 200 Hz before rectification and binning into 10 ms windows. Data greater than 3 standard deviations above the mean were considered signal outliers likely due to non-physiological signals (e.g., wire movement artifacts). Wavelet analysis was used to establish a reliable baseline level of EMG, which varied in the home due to changes in environmental noise and variations in electrode placement. Daubechies order 7 (MATLAB Wavelet Toolbox version 2011ae) was used as the mother wavelet function to perform feature extraction from the signal recorded during each game play session.1,2 Daily maximal contraction baseline values were calculated using the mean signal amplitude during all times where features were not detected, and this was subtracted from the rectified signal. The maximum muscle signal for each session was then calculated as the average of the three largest peaks for each muscle. For data collected during game play, a burst of activity in each muscle was subsequently defined as features in the binned data that exceeded 15% of the maximum activity that day for at least 0.5 s. Independent activity was then calculated as the percentage of bursts detected in the agonist muscle when no simultaneous burst was detected in the antagonist. Independence of muscle activity was calculated for the game play sessions rather than a co-contraction ratio due to an inability to determine whether the subject was attempting to move in flexion or extension during game play.
sEMG during assessments were processed using custom software created in LabViewf. Due to technical difficulties during testing some sEMG and kinematic outcome variables have fewer than the nine participants in the group data analysis First, the sEMG recording and the video recording were synchronized using an LED that flashed when the sEMG recording began. The MVC level was determined by creating an envelope of activity with a very low pass (0.5 Hz), 8th order Butterworth filter applied to the rectified, null-offset sEMG signal during the MVC task. The peak amplitude of the envelope was determined to be the MVC level for each trial. The five MVC trials for flexion and for extension were averaged to provide an MVC level for each assessment.
The start and end of each task was identified in video recordings to define the range of data for subsequent analysis (See Figure 1). The start of the task was marked as the initiation of movement and the end of the task was identified when the participant made contact with the cup. Only two participants grasped the cup, and they were excluded from this analysis. The sEMG signal for determining timing and amplitude parameters for the reaching task was rectified and 20 Hz low pass filtered using a forward and reverse pass Butterworth filter with order of 4 per pass. In order to determine the amount of sEMG co-contraction, the signal’s onset and termination were determined using an automated threshold level. This threshold method was applied after filtering without integration of the signal. The automated threshold of the quiescent data level was set using the following equation (Threshold Multiplier) × (mean + n × SD of quiescent level). Events less than the quiescent level were not used in determining onset and termination. Events beginning or ending above the threshold were ignored.
To calculate co-contraction, the integrated sEMG signal for the agonist was divided by the integrated signal for the antagonist (over the period of agonist activity). In order to control for potential variability in the placement of electrodes and participants’ daily variability in performance, the sEMG signals were normalized using the mean MVC level for wrist flexors and extensors calculated from the MVC task for each participant, and no other weighting was applied. The normalized co-contraction ratio is the integrated agonist value divided by the mean agonist MVC level over the integrated antagonist value divided by the mean antagonist MVC level. For the calculation of co-contraction, extensor digitorum commnus was always considered the agonist and flexor capri radialis was always considered the antagonist. MVC values could not be collected during one or more assessments for several participants, and these participants are excluded from the results of this co-contraction analysis.
Kinematics
After data collection, markers were identified and files were exported for analysis using a custom LabVIEW program. The number of repetitions was identified using the 3rd metacarpal marker as the metacarpal of interest. Then each trial’s start and finish were visually identified using the Qualisys Track Manager program, at the first movement of the 3rd metacarpal marker the time was noted.
The variables of interest during the reaching task were reach time and number of movement segments during the reach phase. Reach time was calculated as the time from the start of movement at the 3rd metacarpal that was greater than 2% of the maximal velocity until the cup was moved a minimum of 2 mm from its starting position (the average of the first 100 frames of data). The number of movement segments was calculated by first identifying the local minimum and maximum velocity for the hand marker during the reach phase. Velocity peaks were then identified as the difference between a minimum velocity and the next maximum velocity that was equal to or greater than 20 mm/s that occurred at least 150 ms after the prior peak. The number of velocity peaks that met these criteria was considered the number of movement segments.
Maximal trunk displacement was defined as the resultant displacement of the trunk from the starting position in mm. The maximal elbow extension was calculated from the vector dot product of two line segments formed by the shoulder to the elbow marker and the elbow marker to the average position of the two wrist markers. Kinematics were also used to assess active range of motion, specifically extension at the wrist. This was computed using a vector cross-product method to calculate the angle between the two planes formed from the elbow marker and two wrist markers and the two wrist markers and the hand marker, with respect to the axis between the two wrist markers (i.e., flexion-extension axis). The amount of wrist extension was calculated as the absolute value of the angle of the wrist at the start of the movement minus the maximum angle of wrist extension that was completed during the trial.
- 1.Brechet L, Lucas M-F, Doncarli C, Farina D. Compression of Biomedical Signals With Mother Wavelet Optimization and Best-Basis Wavelet Packet Selection. IEEE Trans Biomed Eng. 2007;54(12):2186–2192. doi: 10.1109/tbme.2007.896596. [DOI] [PubMed] [Google Scholar]
- 2.Rafiee J, Rafiee MA, Yavari F, Schoen MP. Feature extraction of forearm EMG signals for prosthetics. Expert Syst Appl. 2011;38(4):4058–4067. [Google Scholar]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Explanation of Conflicts of Interest: Authors Sarah McCoy and Chet Moritz have a potential intellectual property interest in the reported work, which is managed by the University of Washington.
SUPPLIERS LIST
Peggle, Manufacturer: PopCap Games Address: 2401 4th Ave #300 Seattle, WA 98121
Bagnoli EMG System, Manufacturer: Delsys, Address: P.O. Box 15734 Boston, MA, 02215
Oqus 300 Camera System, Manufacturer: Qualisys Motion Capture Systems, Address: Packhusgatan 6S-411 13 Gothenburg Sweden
SPSS Version 18, Manufacturer: IBM, Address: 1 New Orchard Road Armonk, New York 10504-1722
Matlab 2011a, Manufacturer: The Mathworks, Address: 3 Apple Hill Drive Natick, MA 01760-2098
LabView, Manufacturer: National Instruments, Address: 11500 N Mopac Expwy Austin, TX 78759-3504
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart Disease and Stroke Statistics—2012 Update A Report From the American Heart Association. Circulation. 2012 Jan 3;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kheder A, Nair KPS. Spasticity: pathophysiology, evaluation and management. Pract Neurol. 2012 Oct;12(5):289–98. doi: 10.1136/practneurol-2011-000155. [DOI] [PubMed] [Google Scholar]
- 3.Kong K-H, Chua KS-G, Lee J. Symptomatic upper limb spasticity in patients with chronic stroke attending a rehabilitation clinic: frequency, clinical correlates and predictors. J Rehabil Med. 2010 May;42(5):453–7. doi: 10.2340/16501977-0545. [DOI] [PubMed] [Google Scholar]
- 4.Watkins CL, Leathley MJ, Gregson JM, Moore AP, Smith TL, Sharma AK. Prevalence of spasticity post stroke. Clin Rehabil. 2002 Aug;16(5):515–22. doi: 10.1191/0269215502cr512oa. [DOI] [PubMed] [Google Scholar]
- 5.Franceschini M, La Porta F, Agosti M, Massucci M. Is health-related-quality of life of stroke patients influenced by neurological impairments at one year after stroke? Eur J Phys Rehabil Med. 2010 Sep;46(3):389–99. [PubMed] [Google Scholar]
- 6.Oujamaa L, Relave I, Froger J, Mottet D, Pelissier J-Y. Rehabilitation of arm function after stroke. Literature review. Ann Phys Rehabil Med. 2009 Apr;52(3):269–93. doi: 10.1016/j.rehab.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Urton ML, Kohia M, Davis J, Neill MR. Systematic literature review of treatment interventions for upper extremity hemiparesis following stroke. Occup Ther Int. 2007;14(1):11–27. doi: 10.1002/oti.220. [DOI] [PubMed] [Google Scholar]
- 8.Kleim JA, Jones TA. Principles of Experience-Dependent Neural Plasticity: Implications for Rehabilitation After Brain Damage. J Speech Land Hear R. 2008 Feb 1;51(1):S225–239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 9.Lang CE, MacDonald JR, Gnip C. Counting repetitions: an observational study of outpatient therapy for people with hemiparesis post-stroke. J Neurol Phys Ther. 2007 Mar;31(1):3–10. doi: 10.1097/01.npt.0000260568.31746.34. [DOI] [PubMed] [Google Scholar]
- 10.Lang CE, MacDonald JR, Reisman DS, Boyd L, Jacobson Kimberley T, Schindler-Ivens SM, et al. Observation of Amounts of Movement Practice Provided During Stroke Rehabilitation. Arch Phys Med Rehabil. 2009 Oct;90(10):1692–8. doi: 10.1016/j.apmr.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control. Outpatient rehabilitation among stroke survivors–21 States and the District of Columbia, 2005. MMWR Morb Mortal Wkly Rep. 2007 May 25;56(20):504–7. [PubMed] [Google Scholar]
- 12.National Stroke Foundation. Clinical Guidelines for Stroke Management 2010. Melbourne, Australia: 2010. [Google Scholar]
- 13.Chan L, Wang H, Terdiman J, Hoffman J, Ciol MA, Lattimore BF, et al. Disparities in outpatient and home health service utilization following stroke: results of a 9-year cohort study in Northern California. PM R. 2009 Nov;1(11):997–1003. doi: 10.1016/j.pmrj.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang MY, Harris JE, Eng JJ. A community-based upper-extremity group exercise program improves motor function and performance of functional activities in chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2006 Jan;87(1):1–9. doi: 10.1016/j.apmr.2005.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Touillet A, Guesdon H, Bosser G, Beis J-M, Paysant J. Assessment of compliance with prescribed activity by hemiplegic stroke patients after an exercise programme and physical activity education. Ann Phys Rehabil Med. 2010 May;53(4):250–7. 257–65. doi: 10.1016/j.rehab.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Jurkiewicz MT, Marzolini S, Oh P. Adherence to a home-based exercise program for individuals after stroke. Top Stroke Rehabil. 2011 Jun;18(3):277–84. doi: 10.1310/tsr1803-277. [DOI] [PubMed] [Google Scholar]
- 17.Woodford H, Price C. EMG biofeedback for the recovery of motor function after stroke. Cochrane Database Syst Rev. 2007;(2):CD004585. doi: 10.1002/14651858.CD004585.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreland J, Thomson MA. Efficacy of Electromyographic Biofeedback Compared With Conventional Physical Therapy for Upper-Extremity Function in Patients Following Stroke: A Research Overview and Meta-Analysis. Phys Ther. 1994 Jun 1;74(6):534–43. doi: 10.1093/ptj/74.6.534. [DOI] [PubMed] [Google Scholar]
- 19.Doğan-Aslan M, Nakipoğlu-Yüzer GF, Doğan A, Karabay İ, Özgirgin N. The Effect of Electromyographic Biofeedback Treatment in Improving Upper Extremity Functioning of Patients with Hemiplegic Stroke. J Stroke Cerebrovasc Dis. 2012 Apr;21(3):187–92. doi: 10.1016/j.jstrokecerebrovasdis.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Zanos S, Richardson AG, Shupe L, Miles FP, Fetz EE. The Neurochip-2: an autonomous head-fixed computer for recording and stimulating in freely behaving monkeys. IEEE Trans Neural Syst Rehabil Eng. 2011;19(4):427–35. doi: 10.1109/TNSRE.2011.2158007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson A, Moritz CT, Mavoori J, Lucas TH, Fetz EE. The Neurochip BCI: towards a neural prosthesis for upper limb function. IEEE Trans Neural Syst Rehabil Eng. 2006;14(2):187–90. doi: 10.1109/TNSRE.2006.875547. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Towards a common language for functioning, disability, and health. World Health Organization. 2002 [Google Scholar]
- 23.Cram JR, Ph D, Kasman GS, Holtz J. Introduction to Surface Electromyography. 1. Aspen Pub; 1998. p. 408. [Google Scholar]
- 24.Murphy MA, Willén C, Sunnerhagen KS. Kinematic variables quantifying upper-extremity performance after stroke during reaching and drinking from a glass. Neurorehabil Neural Repair. 2011 Jan;25(1):71–80. doi: 10.1177/1545968310370748. [DOI] [PubMed] [Google Scholar]
- 25.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001 Jul;32(7):1635–9. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 26.Fritz SL, Blanton S, Uswatte G, Taub E, Wolf SL. Minimal detectable change scores for the Wolf Motor Function Test. Neurorehabil Neural Repair. 2009 Sep;23(7):662–7. doi: 10.1177/1545968309335975. [DOI] [PubMed] [Google Scholar]
- 27.Morris DM, Uswatte G, Crago JE, Cook EW, III, Taub E. The reliability of the Wolf Motor Function Test for assessing upper extremity function after stroke. Arch Phys Med Rehabil. 2001 Jun;82(6):750–5. doi: 10.1053/apmr.2001.23183. [DOI] [PubMed] [Google Scholar]
- 28.Barreca S, Gowland CK, Stratford P, Huijbregts M, Griffiths J, Torresin W, et al. Development of the Chedoke Arm and Hand Activity Inventory: theoretical constructs, item generation, and selection. Top Stroke Rehabil. 2004;11(4):31–42. doi: 10.1310/JU8P-UVK6-68VW-CF3W. [DOI] [PubMed] [Google Scholar]
- 29.Barreca SR, Stratford PW, Lambert CL, Masters LM, Streiner DL. Test-Retest Reliability, Validity, and Sensitivity of the Chedoke Arm and Hand Activity Inventory: A New Measure of Upper-Limb Function for Survivors of Stroke. Arch Phys Med Rehabil. 2005 Aug;86(8):1616–22. doi: 10.1016/j.apmr.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Barreca SR, Stratford PW, Masters LM, Lambert CL, Griffiths J, McBay C. Validation of Three Shortened Versions of the Chedoke Arm and Hand Activity Inventory. Physiother Can. 2006 Jan 1;58(2):148–56. [Google Scholar]
- 31.Gonzalez RV, Buchanan TS, Delp SL. How muscle architecture and moment arms affect wrist flexion-extension moments. J Biomech. 1997 Jul;30(7):705–12. doi: 10.1016/s0021-9290(97)00015-8. [DOI] [PubMed] [Google Scholar]
- 32.Klein CS, Power GA, Brooks D, Rice CL. Neural and muscular determinants of dorsiflexor weakness in chronic stroke survivors. Motor Control. 2013 Jul;17(3):283–97. doi: 10.1123/mcj.17.3.283. [DOI] [PubMed] [Google Scholar]


