Abstract
Translational control of gene expression contributes to various aspects of immune function [1]. Recent results by Brubaker et al. [2] show how alternative translation initiation produces distinct isoforms of Mitochondrial Antiviral Signaling (MAVS), an adaptor protein associated with RIG-I and MDA5 that possess unique immunomodulatory properties.
Most eukaryotic genes are transcribed into intron-containing mRNAs that can be alternatively spliced to produce one or more structurally related proteins. The processed transcripts are typically monocistronic meaning that they contain one open reading frame (ORF) that is translated into a functional protein. Upstream ORFs found in the leader sequence of selected transcripts tend to be small and are not likely to produce functional polypeptides. In some cases, translation can be initiated at alternative sites within the main ORF to produce N-terminal extensions of the protein. The first example of alternative translation initiation producing two proteins differing by an N-terminal extension was the MHC class II-associated invariant chain, a protein that is essential for antigen presentation during the immune response [3]. Now Brubaker et al. [1] show that a similar mechanism produces functionally distinct isoforms of MAVS, an adapter protein that is required for immunity to viral infection.
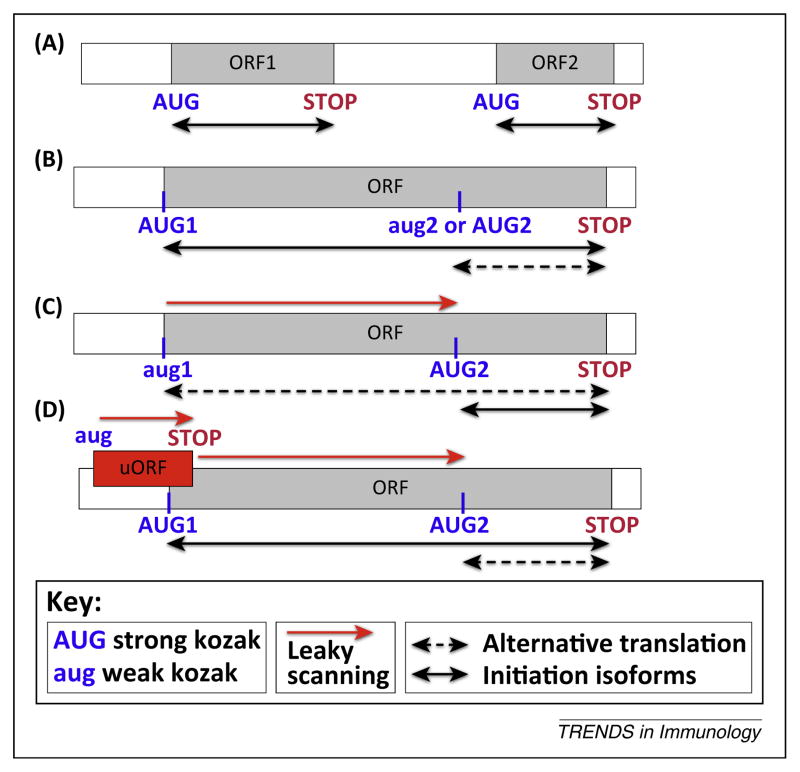
Unlike prokaryotes and some viruses, whose mRNAs are often polycistronic (i.e., more than one independent ORF is translated into functional proteins) (Figure 1A), eukaryotic mRNAs are typically monocistronic (Figure 1B,C). In eukaryotes, an initiation complex including the small ribosomal subunit is assembled on the 5′-cap and scans the untranslated region (UTR) until it finds an AUG (start) codon [4]. Many viral transcripts use leaky scanning, a phenomenon in which the initiation complexes skip upstream AUGs and scan to downstream AUGs to begin synthesis of alternative protein(s)–in the same or different reading frames–to generate protein diversity. The frequency at which individual AUG codons are skipped depends on the sequence of surrounding nucleotides, known as ‘Kozak’ sequences, which determine the efficiency of translation initiation [5]. Some eukaryotic transcripts use a similar mechanism to produce proteins that differ by an N-terminal extension (Figure 1B,C). As these polypeptides often have distinct functions, their relative expression can have important effects on cellular function.
Figure 1.
Alternative translation initiation. (A) Classical bicistronic transcript. Ribosomes initiate at the start (AUG) site of the first ORF (ORF1) followed by termination at the stop codon (STOP). Often ribosomes reinitiate at the following AUG to translate the next ORF (e.g., ORF2). This reinitiation can require ribosome scanning through inter-ORF spacer (non-overlapping ORFs) or ribosome termination/reinitiation at the overlapping stop and start codons of two ORFs. (B) Typical monocistronic eukaryotic transcript. Translation initiation occurs at the first AUG of the ORF, which is typically surrounded by an optimal nucleotide context (‘strong Kozak’). All internal in-frame AUG codons are decoded as methionine. The possibility of 40S leaky scanning through this ‘strong’ start site is minimal. (C) Monocistronic eukaryotic transcript encoding a protein with an N-terminal extension. Translation initiation at a ‘weak Kozak’ AUG (aug1) (or an alternative start codon (e.g., CUG)) is typically a stochastic process, although there are instances of regulation. Ribosomes initiate at the first AUG (aug1) giving rise to a long (N-terminally extended) isoform, and sometimes ribosomes do not recognize the weak start and the transcript undergoes leaky scanning until the ribosomes encounter the following AUG with a ‘strong Kozak’, resulting in the production of the short protein isoform. Two proteins are products of alternative translation initiation from the same cistron. (D) Alternative translation of the MAVS transcript. MAVS mRNA contains an upstream ORF (uORF) overlapping with the MAVS ORF. Because the uORF is surrounded by a ‘weak Kozak’ sequence (aug), ribosomes often skip this start codon (leaky scanning) and initiate translation at the ‘strong Kozak’ (AUG1) of the MAVS ORF synthesizing canonical full length MAVS protein. However, sometimes ribosomes can initiate at the uORF’s AUG (AUG) and after termination (STOP), they scan downstream until they reinitiate at the ‘strong Kozak’ AUG (AUG2) to synthesize N-terminally truncated miniMAVS.
MAVS is an innate immunity protein that functions downstream of RIG-I-like receptors (RLRs) to link RNA virus invasion to the type I interferon (IFN) pathway. Activated RLRs initiate prion-like multimerization of MAVS to trigger a signaling cascade that generates type I IFNs [6]. Brubaker et al. [2] used in vitro assays and transfection studis to show that two related isoforms of MAVS (50 kDa and 72 kDa as observed in Western blots) are produced by alternative translation initiation at inframe AUG codons (Met1 and Met142) found in a single ORF, technically making MAVS mRNA a monocistronic as opposed to a bicistronic transcript. Ribosome profiling [7], a strategy based on deep sequencing of ribosome-protected mRNA fragments, confirmed that MAVS mRNA has two start codons initiating translation of full length (FL)-72 kDa and mini-50 kDa MAVS. How are these alternative initiation sites chosen? Mechanistically, this is achieved by the interplay of translation initiation at the FL-MAVS start site and leaky ribosomal scanning leading to initiation at the miniMAVS start site. Cis-elements in the 5′-UTR of the MAVS mRNA play an important role in the control of miniMAVS expression. Specifically, the 5′-UTR contains an out of frame ORF that encompasses the AUG start codon of FL-MAVS (Figure 1D) [2]. Translation of this ORF would be expected to bypass the FL-MAVS AUG start site. Termination of the upstream ORF (uORF) could then allow re-initiation of 40S scanning to find the miniMAVS AUG start codon to initiate translation of the miniMAVS protein. Consistent with this mechanism, mutating the start codon of the uORF leads to a decrease in miniMAVS levels relative to FL-MAVS [2]. But why would FL-MAVS be expressed at all if initiation at the uORF prevents translation from the FL-MAVS start site? The likely explanation is that uORF AUG is surrounded by a suboptimal nucleotide context (‘weak Kozak’) that promotes leaky scanning [5] to allow translation initiation at the FL-MAVS AUG (‘strong Kozak’) and production of FL MAVS protein.
While the functions of FL-MAVS in immunity are well known, the biological significance of miniMAVS protein and balanced expression of MAVS/miniMAVS by alternative translation remains largely unknown. While MAVS positively regulates the transcription of type I IFNs, miniMAVS interferes with the signaling function of FL-MAVS and attenuates MAVS-mediated immune responses. The molecular details of this inhibition remain to be elucidated, but the manipulation of nucleotide context to promote or inhibit leaky scanning on MAVS mRNA clearly demonstrates that alternative translation regulates the FL-MAVS:miniMAVS ratio to modulate the anti-viral response. Since miniMAVS is a truncated version of FL-MAVS lacking the CARD (Caspase Activation and Recruitment Domain) domain necessary for multimerization, miniMAVS cannot bind FL-MAVS or inhibit MAVS aggregation. Rather, mini-MAVS may compete with FL-MAVS for binding to two other adaptor proteins, TRAF2 and TRAF6, which also contribute to IFN production, antiviral responses and cell survival. Whether such competition takes place is an open question, as is whether FL-MAVS and miniMAVS interact with TRAF2/TRAF6 with different affinities to modulate IFN production and cell death.
It should be noted that in addition to RLRs, viral RNA is also detected by the stress-activated kinase PKR. Upon activation, this kinase phosphorylates Ser51 on the α-subunit of initiation factor 2 (eIF2α), a translation initiation factor that recruits initiator tRNAMet to the 40S ribosomal subunit to recognize the AUG start codon on mRNA. When eIF2α is phosphorylated, translation of most mRNAs is inhibited but a subset of transcripts is selectively translated [1,4]. Within this group of transcripts are mRNAs with uORFs that employ phosphorylated eIF2α to facilitate leaky ribosome scanning to promote alternative translation of stress-responsive proteins (e.g., ATF4). Whether PKR activation/eIF2α phosphorylation similarly facilitates alternative translation on MAVS mRNA is not known. How the FL-MAVS:miniMAVS ratio, and thus signaling through this pathway, is affected by the stress response will be an important area of future investigation.
The use of ribosome profiling analysis to identify translation initiation sites in eukaryotic cells has revealed that uORFs and alternative translation initiation may be more common than previously suspected [8–10]. A similar analysis in human and mouse immune cells identifies multiple examples of transcripts with uORFs and N-terminal extensions [10]. Future investigations will clarify the roles of alternative translation in gene regulation of immune response genes, and will uncover how this mode of regulation is employed in the development and functions of immune system. These findings may in turn pave the way to the development of new therapies for infectious and inflammatory diseases.
References
- 1.Ivanov P, Anderson P. Post-transcriptional regulatory networks in immunity. Immunol Rev. 2013;253:253–272. doi: 10.1111/imr.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brubaker SW, et al. A bicistronic MAVS transcript highlights a class of truncated variants in antiviral immunity. Cell. 2014;156:800–811. doi: 10.1016/j.cell.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Struin M, et al. Two forms of the Ia antigen-associated invariant chain result from alternative initiation at two in-phase AUGs. Cell. 1986;47:619–625. doi: 10.1016/0092-8674(86)90626-4. [DOI] [PubMed] [Google Scholar]
- 4.Jackson RJ, et al. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kozak M. Pushing the limits of the scanning mechanism for initiation of translation. Gene. 2002;299:1–34. doi: 10.1016/S0378-1119(02)01056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs JL, Coyne CB. Mechanisms of MAVS regulation at the mitochondrial membrane. J Mol Biol. 2013;425:5009–5019. doi: 10.1016/j.jmb.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingolia NT, et al. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S, et al. Global mapping of translation initiation sites in mammalian cells at single-nucleotide resolution. Proc Natl Acad Sci USA. 2014:109. doi: 10.1073/pnas.1207846109. http://dx.doi.org/10.1073/pnas.1207846109. [DOI] [PMC free article] [PubMed]
- 9.Menschaert G, et al. Deep proteome coverage based on ribosome profiling aids mass spectrometry-based protein and peptide discovery and provides evidence of alternative translation products and near-cognate translation initiation events. Mol Cell Proteomics. 2013;12:1780–1790. doi: 10.1074/mcp.M113.027540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Damme P, et al. N-terminal proteomics and ribosome profiling provide a comprehensive view of the alternative translation initiation landscape in mice and men. Mol Cell Proteomics. 2014 doi: 10.1074/mcp.M113.036442. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]