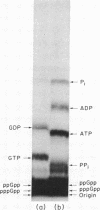
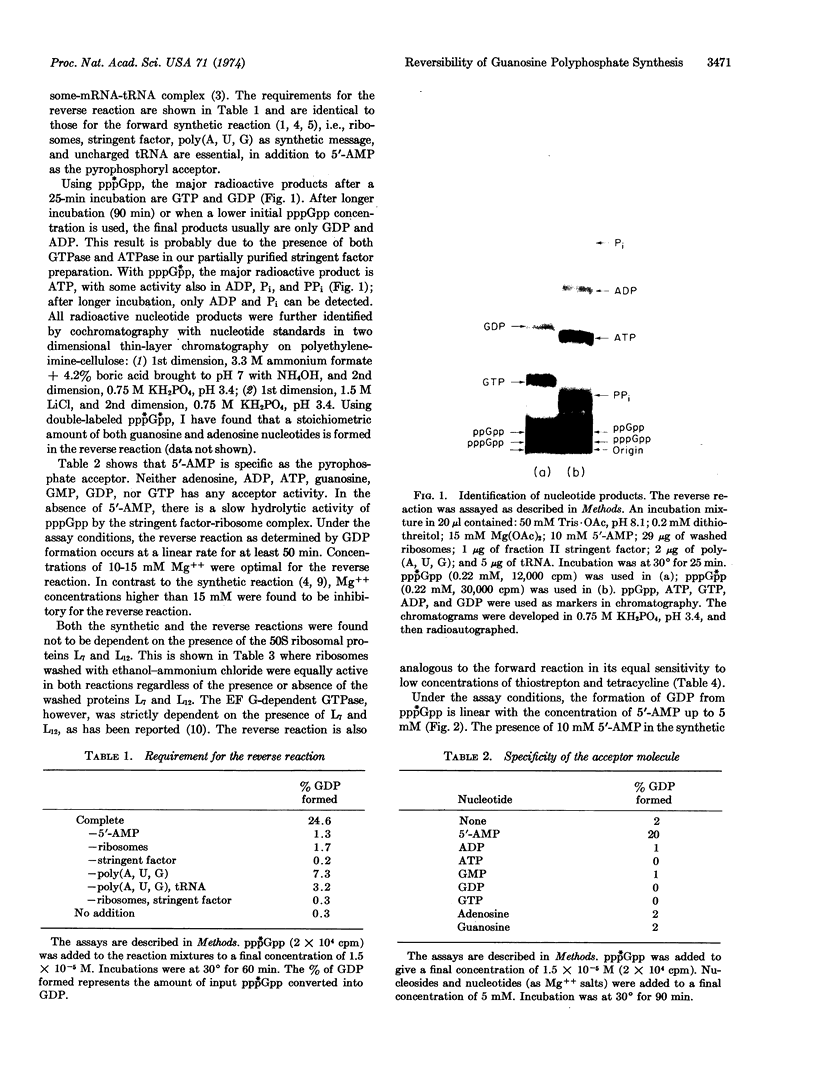
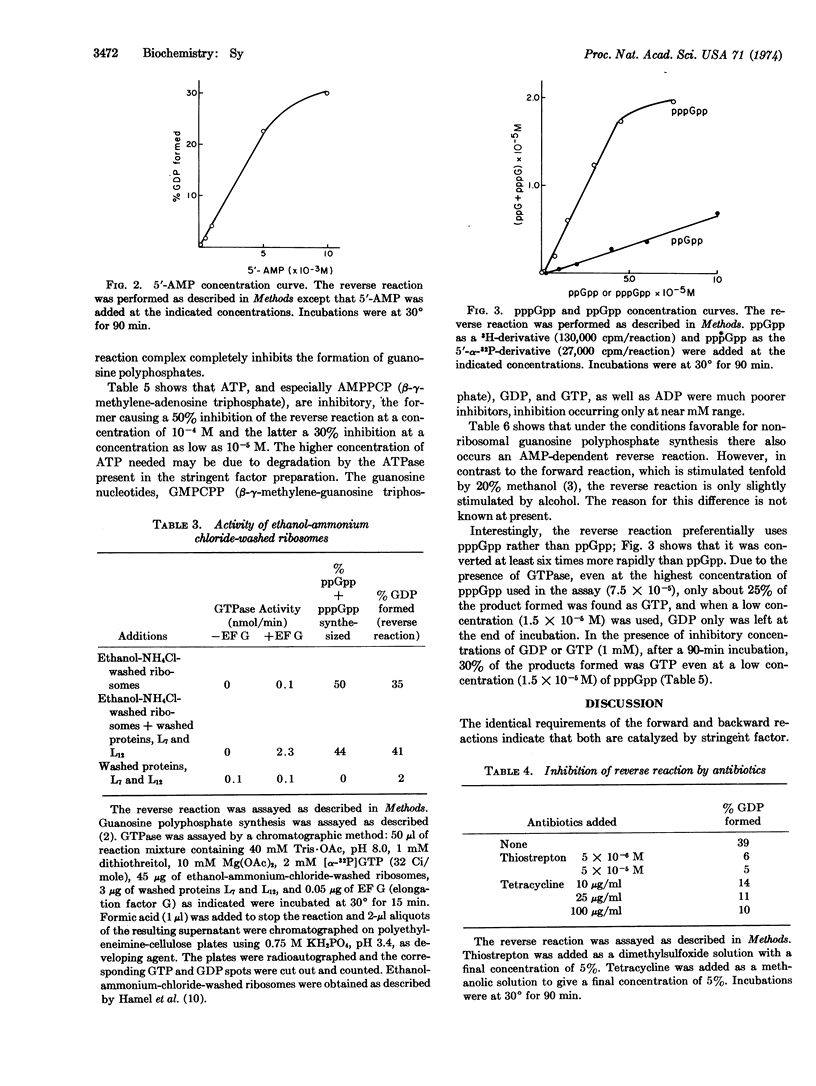
Abstract
The stringent factor-catalyzed, ribosome-dependent synthesis of guanosine polyphosphates is found to be reversible. The reverse reaction specifically requires 5′-AMP as the pyrophosphoryl acceptor, and guanosine 5′-triphosphate-3′-diphosphate is preferentially utilized as the pyrophosphoryl donor. The primary products of the reaction are GTP and ATP. The reverse reaction is strongly inhibited by the antibiotics thiostrepton and tetracycline, and by ATP and β-γ-methylene-adenosine-triphosphate, but not by ADP, GTP, and GDP. The reverse reaction occurs under conditions for nonribosomal synthesis. The overall reaction for stringent factor-catalyzed guanosine polyphosphate formation may thus be formulated: (p)pp5′G + ppp5′A ⇌ (p)pp5′G3′pp + p5′A.
Keywords: guanosine 5′-diphosphate-3′-diphosphate, guanosine 5′-triphosphate-3′-diphosphate, relaxed control, ribosomes
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashel M., Kalbacher B. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem. 1970 May 10;245(9):2309–2318. [PubMed] [Google Scholar]
- Cochran J. W., Byrne R. W. Isolation and properties of a ribosome-bound factor required for ppGpp and ppGpp synthesis in Escherichia coli. J Biol Chem. 1974 Jan 25;249(2):353–360. [PubMed] [Google Scholar]
- Hamel E., Cashel M. Role of guanine nucleotides in protein synthesis. Elongation factor G and guanosine 5'-triphosphate,3'-diphosphate. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3250–3254. doi: 10.1073/pnas.70.11.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel E., Koka M., Nakamoto T. Requirement of an Escherichia coli 50 S ribosomal protein component for effective interaction of the ribosome with T and G factors and with guanosine triphosphate. J Biol Chem. 1972 Feb 10;247(3):805–814. [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipmann F. Effects of cyclic AMP, its mechanism of action, and comments on the energetics of its 3'-phosphate bond. Adv Enzyme Regul. 1970;9:5–16. doi: 10.1016/s0065-2571(71)80035-3. [DOI] [PubMed] [Google Scholar]
- Pedersen F. S., Lund E., Kjeldgaard N. O. Codon specific, tRNA dependent in vitro synthesis of ppGpp and pppGpp. Nat New Biol. 1973 May 2;243(122):13–15. [PubMed] [Google Scholar]
- Sy J., Lipmann F. Identification of the synthesis of guanosine tetraphosphate (MS I) as insertion of a pyrophosphoryl group into the 3'-position in guanosine 5'-diphosphate. Proc Natl Acad Sci U S A. 1973 Feb;70(2):306–309. doi: 10.1073/pnas.70.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy J., Ogawa Y., Lipmann F. Nonribosomal synthesis of guanosine 5',3'-polyphosphates by the ribosomal wash of stringent Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2145–2148. doi: 10.1073/pnas.70.7.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]