Abstract
Background and Purpose
Dysphagia after intracerebral hemorrhage (ICH) contributes significantly to morbidity, often necessitating placement of a percutaneous endoscopic gastrostomy (PEG) tube. This study describes a novel risk prediction score for PEG placement after ICH.
Methods
We retrospectively analyzed data from 234 ICH patients presenting during a 4-year period. One hundred and eighty nine patients met inclusion criteria. The sample was randomly divided into a development and a validation cohort. Logistic regression was used to develop a risk score by weighting predictors of PEG placement based on strength of association.
Results
Age (OR 1.64 per 10 years increase in age, 95% CI 1.02–2.65), African American race (OR 3.26, 95% CI 0.96–11.05), Glasgow Coma Scale (GCS; OR 0.80, 95% CI 0.62–1.03), and ICH volume (OR 1.38 per 10 cc increase in ICH volume) were independent predictors of PEG placement. The final model for score development achieved an AUC of 0.7911 (95% CI 0.6931–0.8892) in the validation group. The score was named the GRAVo score: GCS ≤12 (2 points), Race (1 point for African-American), Age >50 years (2 points), and ICH Volume >30 cc (1 point). A score >4 was associated with nearly 12 times higher odds of PEG placement compared to a score ≤4 (OR 11.81, 95% CI 5.04–27.66), predicting PEG placement with 46.55% sensitivity and 93.13% specificity.
Conclusion
The GRAVo score, combining information about GCS, race, age, and ICH volume, may be a useful predictor of PEG placement in ICH patients.
Keywords: intracerebral hemorrhage, gastrostomy tube, PEG, feeding tube
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a devastating form of stroke, accounting for 15–20% of all strokes worldwide1. ICH carries a high risk of poor long-term outcome, and treatment is largely supportive, aimed at promoting recovery2,3. Oropharyngeal dysphagia is a common sequela after ICH, contributing significantly to overall morbidity4,5.
While most patients recover adequate swallowing function within a week, dysphagia may persist in some patients, often necessitating long-term parenteral feeding via a percutaneous endoscopic gastrostomy (PEG) in order to prevent malnutrition and to reduce aspiration6,7.
Previously identified predictors of PEG placement in stroke patients include variables largely associated with stroke severity, such as lesion volume and mental status impairment8–10. Among the different stroke subtypes, patients with ICH have generally been identified as having higher risk for PEG tube placement than ischemic stroke patients10. ICH patients undergoing PEG placement are more likely to be African American10, have low Glasgow Coma Scale (GCS) scores, intraventricular blood, and hydrocephalus8. However, to date, no established scoring system uses individual-level variables to comprehensively and reliably predict risk of PEG placement in ICH patients. A scoring tool aiding in early identification of high risk patients for PEG may aid physicians in clinical decision-making and may help guide counseling of patients. Furthermore, reliably predicting risk for PEG placement may result in shorter hospital stays and allow for expedited transition to rehab, thus potentially reducing costs and improving long-term outcomes.
In this study, we hypothesized that factors associated with ICH severity would be important predictors of subsequent need for a PEG tube. The present study aims to develop a clinically feasible risk prediction score to assist physicians in predicting PEG placement in ICH patients.
Methods
Patients and study design
This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board. We retrospectively analyzed medical records of patients in our prospective stroke database. Consecutive patients presenting with primary ICH to our academic centers (Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center) between January 2010 and December 2013 were included. Patients with in-hospital ICH and inter-hospital transfers were excluded, as were patients with known intracerebral metastatic disease, known arterio-venous malformation or cavernoma in the location of the hemorrhage. In addition, patients with preexisting dysphagia and patients who died, were made comfort care, or transferred to hospice within the first 3 days of admission were excluded from analysis. Early deaths (≤3 days) were excluded since long-term feeding plans are typically not addressed by the neurological and neurocritical care team within the first 3 days of hospitalization. Patients alive on day 4 were included since a recovery trajectory can be established in some patients by this time, and most patients will have undergone at least one formal swallow evaluation. A few patients who were alive on day 4 and were possible candidates for PEG tube placement did not receive a PEG because they died before a PEG could be placed. In addition, a few patients for whom PEG placement was planned died before a PEG tube could be placed. These patients were included in the “no PEG” group since most practitioners have discussions about long-term feeding plans with other team members and family during this time period.
Dysphagia evaluation
The primary outcome was placement of a PEG. At our institutions, all ICH patients are administered a validated bedside swallow screen by a trained nurse prior to any oral intake in order to evaluate for risk of dysphagia and aspiration. This swallow screen incorporates a clinical symptom checklist along with the 3-ounce water swallow test, a standardized and validated test for dysphagia11. Patients who fail bedside swallow screening are referred for a swallow evaluation by a speech and language pathologist (SLP) within 24 hours of admission, unless unable to participate, and are started on enteral feeding via a nasogastric tube according to national guidelines12,13. SLP assesses clinical indicators of swallowing dysfunction, including vocal quality, cough or throat clearing after swallow, and weakness of cough. SLP also evaluates cranial nerve function pertaining to swallowing, such as strength and mobility of the tongue, jaw, and face. In addition, SLP evaluates for the presence of changes in respiratory rate, oxygen desaturation, shortness of breath, sneezing, and watery eyes after swallow as subtle signs of aspiration. A subset of patients (45/189; 23.8%) underwent videofluoroscopic swallowing (VFS) assessments at the discretion of SLP, if necessary. Patients who were deemed unsafe for oral intake of any kind underwent subsequent follow-up evaluations for early recovery of swallowing function by SLP, often in conjunction with VFS assessments. PEG was considered in patients diagnosed with dysphagia over serial SLP evaluations if no adequate trajectory for timely recovery of swallowing function could be demonstrated. The final decision for PEG placement was individualized to each patient via multidisciplinary evaluation by SLPs and stroke neurologists after patients had failed serial attempts to demonstrate adequate swallowing function.
Clinical data collection
Demographic data including age, sex, and race were collected for all patients. In order to study the potential relationship of PEG placement and stroke risk factors, the presence of the following variables was recorded: hypertension, hyperlipidemia, diabetes mellitus, smoking status, history of atrial fibrillation, chronic kidney disease, and prior history of ICH. In order to assess the potential association of PEG placement and medications commonly prescribed in patients with cerebrovascular disease, the pre-hospital use of antiplatelet agents, anticoagulation, and statins was also recorded. The following physiologic parameters at presentation thought to be potentially related to ICH severity and outcome were recorded: GCS at presentation, blood pressure, international normalized ratio (INR), serum glucose, and estimated glomerular filtration rate (eGFR) by Modification of Diet in Renal Disease (MDRD) equation. In addition, data on length of ICU stay, total length of hospitalization, and discharge location were collected.
Neuroimaging analysis
ICH location on admission CT scan was categorized as deep, lobar, cerebellar, or brainstem, and ICH volume was calculated by the ABC/2 method as described previously14. All images were reviewed by a vascular neurologist (RF). A second investigator (VCU) reviewed randomly selected images for just over 10% of the sample, and an intraclass correlation coefficient for a two-way random effects model was used to assess inter-rater agreement of ICH volume (ICC 0.87, 95% CI 0.70–0.95). The presence of intraventricular hemorrhage (IVH), casting of the 4th ventricle, obstructive hydrocephalus, cortical involvement, and subarachnoid component were each recorded. All patients underwent follow-up neuroimaging within the first 24 hours of admission as per standard clinical practice, and repeat imaging was compared to the admission CT scan to assess for hematoma expansion. Hematoma expansion was defined as a proportional increase of more than 33% or an absolute increase greater than 6 cc (if baseline ICH volume ≤15 cc) from the initial ICH volume15. The ICH score as a score predicting mortality in ICH was determined for each patient as described previously16.
Statistical Analysis
Statistical analysis was performed using STATA version 13 (Stata Statistical Software: Release 13. College Station, TX). A p-value<0.05 was considered statistically significant. 95% confidence intervals are reported. For univariate analyses, continuous variables were analyzed using Student’s t-tests for normally distributed variables, and Wilcoxon rank-sum tests (Mann-Whitney U test) for non-normally distributed variables. Categorical variables were analyzed using Pearson’s Chi2 analysis, and Fisher’s exact tests, when appropriate.
The prediction model was developed by using a random sample of 50% of the data set (development group), and subsequently tested upon the remaining 50% (validation group). In addition, the score was tested on the entire population after score development.
Simple logistic regression analysis was performed using basic demographic or physiologic variables as well as other variables previously published to be associated with PEG placement or felt to be potentially clinically relevant for the placement of a PEG tube. A multivariable statistical model of predictors of PEG placement was developed using basic demographic variables including age, gender, and race, as well as statistically significant variables from the simple logistic regression analyses. Independent predictors with p<0.1 were included as score variables in the final score. Continuous variables significantly associated with outcome were transformed into categorical variables based on clinically and statistically meaningful subdivisions in order to facilitate their application in a practical score. Akaike information criterion (AIC) and area under the receiver operating characteristics (ROC) curve were used for final model selection. Calibration was assessed with the HosmerLemeshow test to determine goodness of fit. To generate a risk score, we assigned points to each variable proportional to its regression coefficients rounded to the nearest integer.
Results
Patient characteristics
A total of 234 patients presented to the emergency departments (EDs) at The Johns Hopkins Hospital or The Johns Hopkins Bayview Medical Center for primary ICH between January 2010 and December 2013. Patients who died, were made comfort care, or transferred to hospice within the first 3 days were excluded, leaving 189 patients for further analysis.
The average age was 62.7 years (range 21 – 94 years); 60.3% were male; and 54.0% were African American (table 1). The median GCS at presentation was 14 (IQR 11–15). One hundred fifty-five patients (82.0%) had a history of hypertension, 69 (36.5%) had hyperlipidemia, 39 (20.6%) had diabetes mellitus, and 12 (6.4%) had a prior hemorrhagic stroke. The median ICH volume was 11 cc (IQR 4–25), 56.0% were deep hemorrhages, and 24.5% were lobar hemorrhages. Thirty-five (19.0%) of all ICHs had infratentorial origin.
Table 1.
Baseline characteristics of ICH patients with and without PEG.
| Characteristics | All patients (n=189) | PEG (n=58) | No PEG (n=131) | p-value |
|---|---|---|---|---|
| Age –years: mean (SD) | 62.7 (15.3) | 65.1 (14.9) | 61.6 (15.4) | 0.157 |
| range | 21–94 | 42–94 | 21–91 | |
| Race – African American | 102 (54.0) | 33 (56.9) | 69 (52.7) | 0.591 |
| Gender – male | 114 (60.3) | 36 (62.1) | 78 (59.5) | 0.743 |
| GCS – median (IQR) | 14 (11–15) | 11 (7–14) | 15 (13–15) | <0.001 |
| Seizure at onset | 17 (9.0) | 8 (13.8) | 9 (6.9) | 0.125 |
| BP – mm Hg: median (IQR) | ||||
| SBP | 191 (158–220) | 201 (171–223) | 187 (150–220) | 0.026 |
| DBP | 101 (87–120) | 106 (96–120) | 100 (80–122) | 0.241 |
| Glucose – mg/dl: median (IQR) | 131 (105–157) | 143 (120–174) | 125 (102–155) | 0.044 |
| eGFR < 60 ml/min | 53 (28.0) | 15 (25.9) | 38 (29.0) | 0.657 |
| INR – median (IQR) | 1.1 (1.0–1.1) | 1.1 (1.0–1.1) | 1.1 (1.1–1.2) | 0.545 |
| Comorbidities | ||||
| Hypertension | 155 (82.0) | 49 (84.5) | 106 (80.9) | 0.556 |
| Hyperlipidemia | 69 (36.5) | 19 (32.8) | 50 (38.2) | 0.476 |
| Diabetes mellitus | 39 (20.6) | 10 (17.2) | 29 (22.1) | 0.443 |
| Atrial fibrillation | 18 (9.5) | 4 (6.9) | 14 (10.7) | 0.413 |
| Prior hemorrhagic stroke | 12 (6.4) | 2 (3.5) | 10 (7.6) | 0.350 |
| Chronic kidney disease | 23 (12.2) | 6 (10.3) | 17 (13.0) | 0.610 |
| Current smoking | 53 (29.3) | 12 (22.2) | 41 (32.3) | 0.174 |
| Medications | ||||
| Antiplatelet agent | 68 (36.0) | 20 (34.5) | 48 (36.6) | 0.776 |
| Anticoagulation | 16 (8.5) | 4 (6.9) | 12 (9.2) | 0.606 |
| Statin | 44 (23.3) | 14 (24.1) | 30 (22.9) | 0.853 |
| Imaging | ||||
| ICH volume – cc: median (IQR) | 11 (4–25) | 20.5 (8–47) | 8 (2–19) | <0.001 |
| Hematoma expansion | 18 (9.5) | 5 (8.6) | 13 (9.9) | 0.778 |
| Infratentorial origin | 35 (19.0) | 14 (24.1) | 21 (16.7) | 0.230 |
| Location | 0.216 | |||
| Lobar | 45 (24.5) | 9 (15.5) | 36 (28.6) | |
| Deep | 103 (56.0) | 35 (60.3) | 68 (54.0) | |
| Brainstem | 13 (7.0) | 6 (10.3) | 7 (5.6) | |
| Cerebellum | 23 (12.5) | 8 (13.8) | 15 (11.9) | |
| Isolated IVH | 5 (2.7) | 0 (0) | 5 (3.8) | 0.326 |
| Intraventricular blood | 75 (39.7) | 33 (56.9) | 42 (32.1) | 0.001 |
| Cortical Involvement | 61 (40.9) | 13 (29.6) | 48 (45.7) | 0.067 |
| SAH Component | 28 (15.1) | 11 (19.0) | 17 (13.3) | 0.315 |
| Hydrocephalus | 41 (21.7) | 20 (34.5) | 21 (16.0) | 0.005 |
| 4th ventricle obliteration | 48 (25.4) | 24 (41.4) | 24 (18.3) | 0.001 |
| ICH score: median (IQR) | 1 (0–2) | 2 (2–3) | 1 (0–2) | <0.001 |
| ICU stay – days: median (IQR) | 4 (2–10) | 13.5 (7–23) | 3 (2–6) | <0.001 |
| LOS – days: median (IQR) | 10 (6–21) | 24 (18–32) | 8 (5–12) | <0.001 |
| Discharge | <0.001 | |||
| Home | 37 (19.6) | 0 (0) | 37 (28.3) | |
| ACIR | 69 (36.5) | 21 (36.2) | 48 (36.6) | |
| SA | 51 (27.0) | 32 (55.2) | 19 (14.5) | |
| In hospital death | 32 (17.0) | 5 (8.6) | 27 (20.6) |
P-values are comparing persons with and without PEG placement.
GCS: Glascow Coma Scale; BP: blood pressure; SBP: systolic BP; DBP: diastolic BP; eGFR: estimated glomerular filtration rate; INR: international normalized ratio; ICH: intracerebral hemorrhage; IVH: intraventricular hemorrhage, SAH: subarachnoid hemorrhage, ACIR: acute inpatient rehabilitation, SA: subacute rehabilitation.
Numbers (%) are provided unless otherwise specified.
Fifty-eight patients (30.7%) underwent PEG placement during their hospitalization. The median time to PEG placement was 14.5 days (IQR 12–18). Generally, patients undergoing PEG placement were more likely to present with lower GCS (median 11 vs.15), higher systolic blood pressure (SBP), and higher glucose levels than did patients without PEG placement (table 1). Patients who received a PEG were more likely to present with higher ICH volume (median 20.5 cc vs. 8 cc), were more likely to have IVH (56.9% vs. 32.1%), hydrocephalus (34.5% vs. 16.0%), and obliteration of the 4th ventricle (41.4% vs. 18.3%). The median ICH score was 2 (IQR 2–3) in the PEG group, and 1 (IQR 0–2) in the group without PEG. Patients undergoing PEG placement had a longer total length of stay (median 24 vs. 8 days), longer ICU stay (median 13.5 vs. 3 days), and were more likely to be discharged to a subacute rehabilitation facility/nursing home (55.2% vs. 14.5%).
Development of the PEG prediction model
Simple logistic regression in the development group identified the following clinical and imaging characteristics associated with PEG placement: GCS (OR 0.79 per 1 point increase in GCS, p<0.001), SBP (OR 1.14 per 10 mm Hg increase in SBP, p=0.02), ICH volume (OR 1.37 per 10 cc increase in ICH volume, p=0.002), IVH (OR 3.17, p=0.011), subarachnoid component (OR 3.25, p=0.047), 4th ventricle obliteration (OR 2.88, p=0.025), and ICH score (OR 2.83 per 1 point increase in ICH score, p<0.001). In multivariable logistic regression only age (OR 1.05, p=0.042), African-American race (OR 3.26, p=0.058), GCS on presentation (OR 0.80 per 1 point increase in GCS, p=0.078), and initial ICH volume (OR 1.38 per 10 cc increase in ICH volume, p=0.047) were independent predictors of PEG placement with p<0.1 (Table 2). For score development, the best model included race in addition to age with a cut-point at 50 years, GCS with a cut-point at 12, ICH volume with a cut-point at 30 cc. This model achieved an AUC of 0.8410 (95% CI 0.7504–0.9316) and the HosmerLemeshow test confirmed goodness of fit (p=0.4602).
Table 2.
Multivariable analysis for predictors of PEG placement in ICH patients.
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Age, per 10 years | 1.64 | 1.02–2.65 | 0.042 |
| Race - African American | 3.26 | 0.96–11.05 | 0.058 |
| Gender - male | 1.53 | 0.46–5.13 | 0.491 |
| GCS | 0.80 | 0.62–1.03 | 0.078 |
| SBP, per 10 mm Hg | 1.14 | 0.97–1.32 | 0.105 |
| ICH volume, per 10 cc | 1.38 | 1.01–1.89 | 0.047 |
| Intraventricular blood | 1.59 | 0.41–6.14 | 0.500 |
| SAH | 0.58 | 0.11–3.14 | 0.528 |
| 4th ventricle obliteration | 0.67 | 0.12–3.61 | 0.641 |
| ICH score | 1.18 | 0.44–3.22 | 0.740 |
GCS: Glascow Coma Scale; SBP: systolic blood pressure; ICH: intracerebral hemorrhage; SAH: subarachnoid hemorrhage.
Model validation and risk score
In the validation group, the AUC for the complete model was 0.7911(95% CI 0.6931–0.8892), and the model fit the data well (HosmerLemeshow p= 0.3897). A 4-item risk score was developed based on the following model: Log (odds) PEG = −5.47 + 1.94x1 + 0.99x2 + 2.41x3 + 1.34x4; where x1=GCS≤12, x2=African American race, x3=age>50, x4=ICH volume>30cc. The score was termed GRAVo, representing the 4 components in the score, namely GCS, Race, Age, and Volume. Points for the GRAVo score were assigned as follows: 2 points for GCS ≤12, 1 point for African American race, 2 points for age >50 years, and 1 point for ICH volume >30 cc, with a maximum of 6 points (Table 3). The model for the final score achieved an AUC of 0.7511 (95% CI 0.6722–0.8300) in the entire sample (Figure 1). Each 1-point increase in the score was associated with a 2.27-fold increased odds for PEG placement (95% CI 1.71–3.03, p<0.001). The PEG placement rates for patients with a score of 0–1, 2–3, and 4–6 were 8.7%, 19.6%, and 63.0%, respectively. No patient with a score of 0 underwent PEG placement, while all patients with a score of 6 had a PEG tube placed. The odds of a patients with a score ≥4 undergoing PEG placement was nearly 8 times higher than a patient with a score of 3 or lower (OR 7.86, 95% CI 3.88–15.94). This cut-point predicted PEG placement with 58.62% sensitivity, 84.73% specificity, 62.96% positive predictive value, and 82.22% negative predictive value. A score≥5 predicted PEG placement with 46.55% sensitivity, 93.13% specificity, 75% positive predictive value, and 79.74% negative predictive value (OR 11.81, 95% CI 5.04–27.66).
Table 3.
Determination of the GRAVo score.
| Score component | Score points |
|---|---|
| Age, years | |
| ≤ 50 | 0 |
| >50 | 2 |
| African American | |
| no | 0 |
| yes | 1 |
| GCS | |
| >12 | 0 |
| ≤12 | 2 |
| ICH volume, cc | |
| ≤30 | 0 |
| >30 | 1 |
| Total Score | 0–6 |
GCS: Glascow Coma Scale; ICH: intracerebral hemorrhage.
Figure 1.
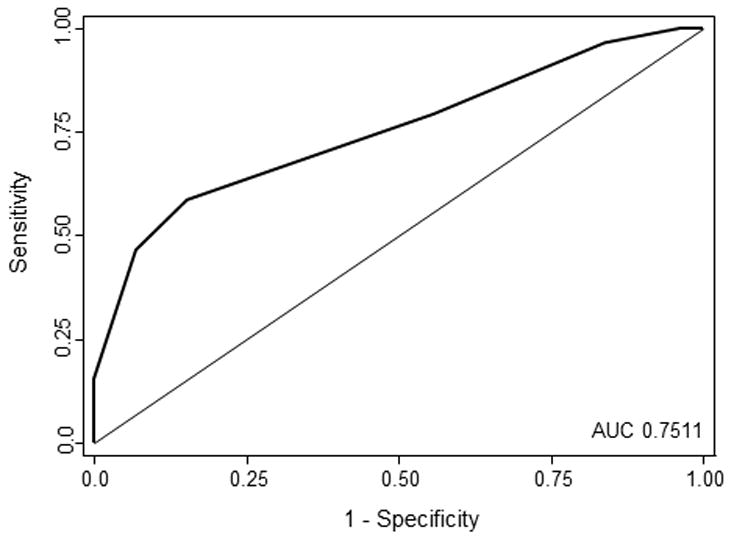
The ROC curve for the score model predicting PEG placement in the entire sample is shown. An area under the curve (AUC) of 0.7511 shows that the model is predictive of PEG placement.
Discussion
In the present study we identified risk factors for PEG placement in patients with primary ICH and derived and validated a simple risk score. This “GRAVo” clinical risk score has 4 predictor variables: GCS on presentation (≤12; equivalent to 2 points), race (African American; 1 point), age (>50 years; 2 points), and ICH volume (>30 cc; 1 point), for a maximum of 6 possible points. All components of the score are easy to obtain, and readily available at the time of presentation.
To be widely applicable in clinical practice, any clinical grading scale should be easy to use without need for complex mathematical calculations, while allowing for a reliable prediction using readily available predictor variables. A recent prognostic model included NIH stroke scale and presence of edema on follow-up imaging10. However, the former is not routinely obtained in patients presenting with ICH, and the latter is difficult to quantify, thus limiting its utility. Our risk score utilizes race and age, both of which are apparent at presentation. GCS is routinely obtained as part of the initial evaluation by emergency personnel and ED staff. In addition, ICH volume on CT by the ABC/2 method is a fast and simple tool routinely employed to estimate ICH size. Our score thus employs readily available variables to allow for calculation of a relatively simple score.
Several features of the individual components of our score are noteworthy. GCS as a quantitative measure of level of consciousness has been shown to be a robust predictor of outcome in previous ICH models16,17. Since post-ICH dysphagia is not uncommonly related to decreased arousal and level of consciousness, it is not surprising that GCS was a reliable predictor in our model. Similarly, ICH volume and age have consistently been associated with outcome in various other prediction models16–18. Interestingly, African American race was a predictor of PEG placement in our model. The reasons for this finding are not entirely clear, however, it is consistent with a recent study suggesting that African American race is associated with PEG placement10. African Americans tend to present with more severe strokes compared to whites19, but since our prediction model adjusted for ICH volume and GCS, stroke severity is unlikely to explain the observed race difference in our model. ICH etiology might possibly explain the observed difference; hypertension is more prevalent in African Americans and is often poorly controlled20–22. ICH in typical hypertensive locations such as the basal ganglia, thalamus, and brainstem may be more likely to be associated with dysphagia compared to non-hypertensive locations, such as lobar hemorrhages of similar size. Thus, a higher rate of ICH in hypertensive locations in African Americans may result in higher rates of dysphagia, thereby contributing to higher rates of PEG placement. Further studies are needed to validate African American race as a predictor of PEG placement, and to determine whether the underlying cause is related to physiological differences, socio-economic differences, or potential disparities in health resource allocation.
The rate of PEG placement of about 30% in our sample is consistent with the rate of long-term dysphagia after stroke, and comparable to other published reports on the frequency of PEG placement8,23. This increases the generalizability of the positive and negative predictive values reported in this study. Every patient who did not receive a PEG tube was able to take adequate nutrition by mouth at the time of discharge. For patients receiving a PEG tube, the median time to PEG was 14.5 days (IQR 12–18). Chart review revealed that in 12 of 58 patients (20.7%) PEG placement was either the sole or a major contributing factor delaying discharge to rehab. Early initiation of rehabilitation is important for optimal chance of recovery24,25. A cut-point of 5 predicted PEG placement with 46.55% sensitivity and 93.13% specificity. Choosing lower cut-points will result in increased sensitivity at the expense of specificity. However, in the context of medical decision making for PEG placement, a score of 5 may be the most clinically useful cut-point since high specificity with concomitant low false positive rate is desired before committing patients to a PEG. Our score may allow for timely initiation and planning for PEG in certain patients (i.e. with a score ≥5), thus potentially reducing hospital length of stay and ensuring smooth and early transition to rehab. The GRAVo score may provide clinicians with a risk-prediction tool for PEG placement based on clinical and demographic variables, however, we acknowledge that our score is no substitution for SLP evaluation, and should rather be utilized in conjuncture with clinical and instrumental evaluation of dysphagia by SLP.
Our study has several limitations. By virtue of limiting the number of score variables any clinical risk scoreen tails simplification at the expense of accuracy of outcome prediction. Decision making about PEG placement is complex and multifactorial involving hospital course and changes on follow-up neuroimaging, as well as family and patient wishes and preferences. Many of such factors were not included in our score because they are not readily assessable on initial presentation or difficult to quantify. We do acknowledge, however, that an individual provider’s a priori expectations about the likelihood of a given patient needing a PEG may influence the decision about placing that PEG. This could lead to bias in this study, particularly if a provider assumes that a patient with a low GCS and large ICH volume is likely to require a PEG, potentially resulting in PEG placement at an earlier time compared to a patient not meeting those criteria. Although we validated our score in a separate cohort, the patients in the validation cohort were cared for by the same physicians and over the same time period as the patients in the development cohort. Thus, patients in both cohorts likely received similar counseling on prognosis and need for PEG, and it is possible that results may vary in an entirely different patient population cared for by different physicians with different viewpoints and a potentially different ethical framework. In addition, although care for ICH patients at our certified stroke centers is consistent with the current standard of care and AHA guidelines, we acknowledge that our model is mainly reflective of the practice pattern at our institutions. In addition, this is a retrospective analysis of a small number of patients from 2 single stroke centers over the course of 4 years, limiting generalizability to larger populations. Further validation of our score in an external dataset is required.
In summary, the GRAVo score includes GCS on admission, African American race, age and ICH volume, and is a reliable clinical score determining the risk of PEG placement after ICH in patients who survive the first 3 days. The score is easy to compute, and a score of >4 predicts need for PEG with 93% specificity. We hope that our score may provide a framework aiding clinicians together with patients and families in informed decision making with regards to PEG placement.
Acknowledgments
We thank Kate Holden and Genevieve McKeon for providing valuable input on the dysphagia and SLP evaluations.
Sources of Funding
RF is supported by an R25 Grant-NIH/NINDS Research Education Program for Residents and Fellows in Neurology and Neurosurgery. EBM was supported by a Johns Hopkins School of Medicine Clinician Scientist Award.
Footnotes
Disclosures
VCU is PI for the investigator-initiated trial SAIL ON, funded by Genentech Inc., and is site PI for the DIAS 4 trial (sponsor: Lundbeck). No other disclosures.
References
- 1.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 2.Rincon F, Mayer SA. Intracerebral hemorrhage: getting ready for effective treatments. Curr Opin Neurol. 2010;23:59–64. doi: 10.1097/WCO.0b013e3283352c01. [DOI] [PubMed] [Google Scholar]
- 3.Morgenstern LB, Hemphill JC, 3rd, Anderson C, Becker K, Broderick JP, Connolly ES, Jr, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng NH, Wang TG, Lien IN. Dysphagia in patients with brainstem stroke: incidence and outcome. Am J Phys Med Rehabil. 2000;79:170–175. doi: 10.1097/00002060-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Sharma JC, Fletcher S, Vassallo M, Ross I. What influences outcome of stroke--pyrexia or dysphagia? Int J Clin Pract. 2001;55:17–20. [PubMed] [Google Scholar]
- 6.Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke. 1999;30:744–748. doi: 10.1161/01.str.30.4.744. [DOI] [PubMed] [Google Scholar]
- 7.Gencosmanoglu R. Percutaneous endoscopic gastrostomy: a safe and effective bridge for enteral nutrition in neurological or non-neurological conditions. Neurocrit Care. 2004;1:309–317. doi: 10.1385/ncc:1:3:309. [DOI] [PubMed] [Google Scholar]
- 8.Kiphuth IC, Kuramatsu JB, Lucking H, Kloska S, Schwab S, Huttner HB. Predictive factors for percutaneous endoscopic gastrostomy in patients with spontaneous intracranial hemorrhage. Eur Neurol. 2011;65:32–38. doi: 10.1159/000322735. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Langmore S, Goddeau RP, Jr, Alhazzani A, Selim M, Caplan LR, et al. Predictors of percutaneous endoscopic gastrostomy tube placement in patients with severe dysphagia from an acute-subacute hemispheric infarction. J Stroke Cerebrovasc Dis. 2012;21:114–120. doi: 10.1016/j.jstrokecerebrovasdis.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubin PH, Boehme AK, Siegler JE, Shaban A, Juengling J, Albright KC, et al. New model for predicting surgical feeding tube placement in patients with an acute stroke event. Stroke. 2013;44:3232–3234. doi: 10.1161/STROKEAHA.113.002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suiter DM, Leder SB. Clinical utility of the 3-ounce water swallow test. Dysphagia. 2008;23:244–250. doi: 10.1007/s00455-007-9127-y. [DOI] [PubMed] [Google Scholar]
- 12.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 13.Martindale RG, McClave SA, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med. 2009;37:1757–1761. doi: 10.1097/CCM.0b013e3181a40116. [DOI] [PubMed] [Google Scholar]
- 14.Kwak R, Kadoya S, Suzuki T. Factors affecting the prognosis in thalamic hemorrhage. Stroke. 1983;14:493–500. doi: 10.1161/01.str.14.4.493. [DOI] [PubMed] [Google Scholar]
- 15.Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 17.Tuhrim S, Dambrosia JM, Price TR, Mohr JP, Wolf PA, Hier DB, et al. Intracerebral hemorrhage: external validation and extension of a model for prediction of 30-day survival. Ann Neurol. 1991;29:658–663. doi: 10.1002/ana.410290614. [DOI] [PubMed] [Google Scholar]
- 18.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 19.Jones MR, Horner RD, Edwards LJ, Hoff J, Armstrong SB, Smith-Hammond CA, et al. Racial variation in initial stroke severity. Stroke. 2000;31:563–567. doi: 10.1161/01.str.31.3.563. [DOI] [PubMed] [Google Scholar]
- 20.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 21.Cooper R, Rotimi C, Ataman S, McGee D, Osotimehin B, Kadiri S, et al. The prevalence of hypertension in seven populations of west African origin. Am J Public Health. 1997;87:160–168. doi: 10.2105/ajph.87.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 23.Geeganage C, Beavan J, Ellender S, Bath PM. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database Syst Rev. 2012;10:CD000323. doi: 10.1002/14651858.CD000323. [DOI] [PubMed] [Google Scholar]
- 24.Indredavik B, Bakke F, Slordahl SA, Rokseth R, Haheim LL. Treatment in a combined acute and rehabilitation stroke unit: which aspects are most important? Stroke. 1999;30:917–923. doi: 10.1161/01.str.30.5.917. [DOI] [PubMed] [Google Scholar]
- 25.Cumming TB, Thrift AG, Collier JM, Churilov L, Dewey HM, Donnan GA, et al. Very early mobilization after stroke fast-tracks return to walking: further results from the phase II AVERT randomized controlled trial. Stroke. 2011;42:153–158. doi: 10.1161/STROKEAHA.110.594598. [DOI] [PubMed] [Google Scholar]


