Abstract
Wingless acts as a morphogen in Drosophila wing discs, where it specifies cell fates and controls growth several cell diameters away from its site of expression. Thus, despite being acylated and membrane-associated, Wingless spreads in the extracellular space. Recent studies have focussed on identifying the route that Wingless follows in the secretory pathway and determining how it is packaged for release. We have found that, in medium conditioned by Wingless-expressing Drosophila S2 cells, Wingless is present on exosome-like vesicles and that this fraction activates signal transduction. Proteomic analysis shows that Wingless-containing exosome-like structures contain many Drosophila proteins that are homologous to mammalian exosome proteins. In addition, Evi, a multipass transmembrane protein, is also present on exosome-like vesicles. Using these exosome markers and a cell-based RNAi assay, we found that the small GTPase Rab11 contributes significantly to exosome production. This finding allows us to conclude from in vivo Rab11 knockdown experiments, that exosomes are unlikely to contribute to Wingless secretion and gradient formation in wing discs. Consistent with this conclusion, extracellularly tagged Evi expressed from a BAC is not released from imaginal disc Wingless-expressing cells. Nevertheless, Evi is present in larval haemolymph, suggesting that exosomes are normally produced in vivo.
Keywords: Wingless, Wnt, Evi, exosomes, Drosophila
INTRODUCTION
Intercellular communication is essential for embryonic development and adult homeostasis. Signalling proteins, such as Wnts, spread within tissues to coordinate cell growth, differentiation and survival (1). Wnt proteins are lipid-modified causing them to be tightly associated with cellular membranes (2, 3). Yet Wnts can be released from producing cells and act at a distance of up to 20 cell diameters from their site of expression (4). It is likely that, as Wnts progress through the secretory pathway they could be packaged in such a way that allows subsequent spread within the extracellular space. Therefore, substantial effort has been devoted to determining the secretory route taken by Wnts. This has led to the identification of several proteins that are specifically required for Wnt secretion (5). One example is the multipass transmembrane protein Evi/Wntless (6-8). However, despite major advances in our understanding of Wnt secretion, an outstanding question remains: in what form are Wnt proteins packaged for release from producing cells?
Wingless (Wg), the main Drosophila Wnt is expressed in a stripe of cells along the dorsal-ventral boundary of wing imaginal discs and spreads throughout most of the prospective wing pouch where it controls patterning and growth (4, 9-13). Thus wing imaginal discs of Drosophila have become a system of choice to study long-range transport of Wnt proteins. Several possible mechanisms of packaging and release that are compatible with long-range transport of Wnts have been suggested (5). These include the formation of micelles, association with lipid-masking chaperone proteins, such as the lipocalin Swim, loading onto long filopodia (cytonemes), or packaging onto membrane vesicles or lipoprotein particles (14-17). The possibility that Wg could be packaged onto small membranous vesicles was suggested in 2001 by Greco et al. These authors showed that membrane tethered GFP could spread within imaginal discs. Since membrane-tethered GFP expressed in Wg secreting cells was found to colocalise with Wg in receiving cells, these authors argued that membranous vesicles, which they named argosomes, could act as transport vehicles for Wg. Although no detail was given on the biogenesis of such vesicles, it was suggested that they must be surrounded by a bilayer because they were marked by GFP targeted to either leaflet of the plasma membrane (15). Subsequent work by the same group suggested that Wg could instead be transported on lipoprotein particles. They reported that Wg is present on lipoprotein particles (LPPs) purified from larvae or imaginal discs. Moreover, knocking down lipophorin, a key component of lipoprotein particles, led to a reduction of the Wg range suggesting a role in transport (16). However, this interpretation is complicated by the pleiotropic effects of lipophorin knockdown. Moreover, the activity of LPP-associated Wg was not tested and only a fraction of total Wg is found on these structures. Argosomes and lipoprotein particles represent distinct structures. The former are surrounded by a bilayer while a single layer of phospholipids encloses the latter. In addition, while argosomes are generated within imaginal discs cells, LPPs are produced in the fat body and transported systemically to imaginal discs via the haemolymph (18). The relative contributions of these two classes of structures to Wg transport remains to be determined. In this study, we assess the possible contribution of exosomes in Wg secretion from cultured Drosophila cells and in the wing imaginal disc.
Exosomes comprise one of several classes of membranous vesicles that are released by cells (19-21). They are 40-100 nm microvesicles that are produced in multivesicular bodies (MVBs), an endocytic compartment, and released into the extracellular space following fusion of the MVB’s outer membrane with the plasma membrane (22-24). They have a characteristic density (1.13-1.19 g/ml), a cup-shape morphology and sediment at 100,000 g (25). They are enriched in cholesterol, sphingomyelin, and ceramide and components of membrane microdomains such as Flotillins (26, 27). Tetraspanins such as CD63 are often used as exosome markers in mammalian cells, although exosomes do not necessarily contain CD63. Exosomes also contain proteins involved in signalling, trafficking, and membrane fusion (28, 29). Although they are produced by a variety of cell types and are found in body fluids such as blood and urine, the functional relevance of exosomes is poorly understood. Exosomes were first demonstrated to be released from reticulocytes (22, 24) and subsequently the majority of exosome studies have been in the fields of blood cells and immunology as they play an important role in antigen presentation and the spread of infectious agents (30, 31). They are also thought to be relevant to cancer because they can carry signalling components (32).
In this paper, we test whether Wg is present on exosomes. Several observations are consistent with this possibility. First, immuno-EM revealed the presence of Wg on 100 nm vesicles located in the extracellular space in Drosophila embryos (33, 34). Second, Wg was found to associate with lipid microdomains, which are commonly found in exosomes (27, 35). Third, Wg cofractionates with Flotillin-2 (Flo2), a common exosome protein, and loss of Flo2 decreases the range of the Wg gradient (36, 37). Finally, recent studies have shown that Wg and Evi cross the neuromuscular junction in an exosome-dependent mechanism (38, 39). We found that Wg and Evi are present in an exosome fraction obtained from culture medium. We then used mass spectrometry (MS) to identify Drosophila exosome markers and developed a cell-based RNAi assay to test the potential role of candidate proteins in exosome production and found Rab11 to be a regulator of this process. However, subsequent analysis showed that Rab11 is not required for Wg secretion and that Evi is not released from Wg expressing cells in wing imaginal discs. It appears therefore that exosomes do not play a role in Wg packaging and release in this tissue.
RESULTS
Wingless and Wnt3A are secreted on exosome-like vesicles
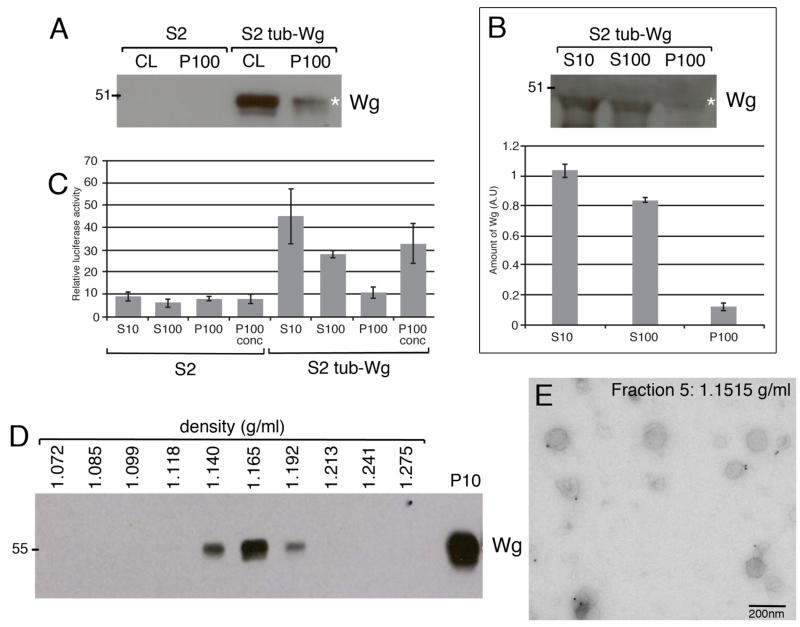
To test if Wg is secreted on exosomes, we obtained conditioned medium (CM) from S2 cells stably expressing Wg (S2 tub-Wg cells) as well as untransfected S2 cells (as controls) and performed differential centrifugation. The CM was first spun at low speed to remove cell debris and any large vesicles (the pellet will be referred to as the P10). The resulting supernatant (the S10) was then spun at 100,000 g to pellet exosome-like vesicles (25). This pellet will be referred to as the P100 and the supernatant as the S100. Western blot analysis revealed the presence of Wg in the P100 obtained from S2 tub-Wg cells, but not from S2 cells (Fig. 1A). The amount of secreted Wg that sediments at 100,000 g (the P100) was compared to the total amount present in the S10 and S100 supernatants. This analysis suggested that approximately 12% of Wg from the S10 (i.e. secreted) is present in the P100 (Fig. 1B). To determine which fractions are capable of activating signal transduction, the S10, the S100, and the resuspended P100 pellets from S2 and S2 tub-Wg cells were added to S2R+ cells transfected with a luciferase reporter of Wg signalling activity (see methods). Luciferase activity was measured 24 hrs after treatment. Several conclusions can be drawn from this experiment. First, approximately 23% of signalling activity present in the S10 is found in the P100 pellet. Second, the majority of signalling activity originally detected in the S10 sample is still present in the S100 sample indicating that non-exosomal associated pools of Wg that can activate signalling are present in this sample. Third, the concentrated P100 pellet (x45) from S2 tub-Wg cells could robustly activate Wg signalling (Fig. 1C). These data indicate that Wg is present in the P100 pellet purified from S2 tub-Wg CM and that this Wg-containing fraction is capable of activating signal transduction.
Figure 1. Wingless is secreted on exosome-like vesicles in S2 cells.
A. Equal amounts of total protein from cell lysates (CL) and P100 pellets were analysed by western blot. Wg is present in cell lysates and the P100 pellet from S2 tub-Wg cells. B. Quantification of Wg levels in S10 and S100 supernatants versus the P100 pellet from S2 tub-Wg cells shows that approximately 12% of secreted Wg sediments at 100,000 g. C. The S10 and S100 supernatants and P100 pellet from S2 tub-Wg cells can activate expression of a Wg signalling reporter in S2R+ cells. D. P100 pellet from S2 tub-Wg cells was fractionated by continuous sucrose density gradient and equal amounts of the resulting fractions analysed by western blot. Wg is present in fractions with densities 1.14 – 1.192 g/ml, corresponding to exosomes. E. Immuno-EM analysis of fraction 5 (1.1515 g/ml) from S2 HA-Wg cells shows cup-shaped vesicles with a mean diameter of 114 nm labelled with anti-HA in addition to smaller structures. Scale bar = 200 nm
The P100 pellet likely contains, in addition to exosomes, a variety of vesicles and cell debris that sediment at 100,000 g. We therefore further fractionated the P100 pellet by continuous sucrose density gradient centrifugation and analysed the various fractions by Western blot. Wg was found to be present in fractions of densities between 1.14 and 1.192 g/ml (Fig.1D), where exosomes are normally found (25). The Wg antibody that is available is not compatible with immuno-EM so we used S2 HA-Wg cells for this analysis. The P100 pellet obtained from these cells was fractionated by continuous density gradient centrifugation and the HA-Wg-containing fractions identified by Western blot analysis (data not shown). These fractions were analysed by immuno-EM and found to contain many cup-shaped vesicles with a mean diameter of 114 nm. 35% of these vesicles were labelled with anti-HA with 1-2 gold particles per vesicle (Fig. 1E). Taken together, these data show that Wg can be secreted on vesicles that have the same size, morphology and density as exosomes.
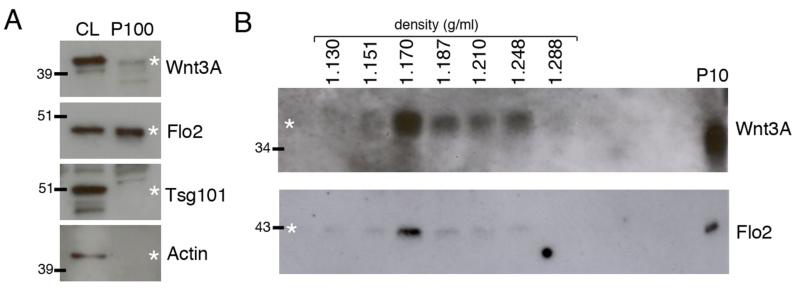
Next, we tested whether a Wnt produced by mammalian cells is present in fractions that sediment like exosomes. We obtained CM from L cells stably transfected with Wnt3A (L-Wnt3A cells) and performed differential centrifugation to sediment exosome-like vesicles at 100,000 g. A small, but reproducible amount of Wnt3A was found in the P100 pellet, as detected by Western blot (Fig. 2A). This is in contrast with an earlier report that Wnt3A is not present in such a pellet (40). The reason for the discrepancy is unknown as similar differential centrifugation methods and cell lines were used. However, our result was further confirmed by subsequent fractionation of the P100 pellet on a continuous sucrose density gradient and Western blot analysis. While Wnt3A is present over a broader range of densities than Wg (1.151 – 1.228 g/ml for Wnt3A compared to 1.14 – 1.192 g/ml for Wg), the highest levels of Wnt3A were found in the 1.170 g/ml fraction (Fig. 2B), which is consistent with exosome association. The P100 pellet was then analysed by Western blot to assess the presence of known mammalian exosome proteins. The ESCRT protein Tsg101 was not found in the pellet even though it was detected in L-Wnt3A cell lysates (41). However, Flo2, a protein required for the formation of lipid microdomains and commonly found in exosomes, was detected in the P100 pellet (Fig. 2A) (27). Furthermore Flo2 was detected in fractions of density 1.151 – 1.228 g/ml with the highest levels observed in the 1.170 g/ml fraction where Wnt3A is present (Fig. 2B). We therefore conclude that both Drosophila Wg and mammalian Wnt3A can be secreted on exosome-like vesicles in cell culture.
Figure 2. Wnt3A is secreted on exosome-like vesicles in L cells.
A. Cell lysates (CL) and P100 pellets from L-Wnt3A cells were analysed by Western blot. Twice as much P100 protein was loaded compared to CL. Wnt3A and Flo2 are both detected in the P100 pellet, while Tsg101 and Actin were only detected in the CL. B. The P100 pellet from L-Wnt3A CM was further fractionated by continuous sucrose density gradient and the fractions analysed by Western blot. Wnt3A was found at low levels in fractions with densities between 1.151 – 1.288 g/ml, but the majority was detected in the exosome fraction with a density of 1.170 g/ml. Flo2 was detected in the same fractions and levels peaked in the exosome fraction with a density of 1.170 g/ml.
Mass spectrometry to identify Drosophila exosome proteins
To begin identifying Drosophila exosome proteins, we performed MS on fractions 2-9 (corresponding to the following densities: fraction 2 = 1.098 g/ml, fraction 3 = 1.113 g/ml, fraction 4 = 1.132 g/ml, fraction 5 = 1.152 g/ml, fraction 6 = 1.172 g/ml for S2 cells, 1.170 g/ml for S2 tub-Wg cells, fraction 7 = 1.193 g/ml, fraction 8 = 1.214 g/ml and fraction 9 = 1.227 g/ml for S2 cells, 1.230 g/ml for S2 tub-Wg cells) obtained from the P100 pellets from S2 and S2 tub-Wg CM. Approximately 200-600 proteins were identified in each fraction. This analysis confirmed that Wg is present in fractions 4-8 obtained from the S2 tub-Wg cells (data not shown).
For ease of subsequent analysis we focused on three fractions: Fraction 5 (1.152 g/ml; referred to as the ‘exosome fraction’), fraction 2 (1.098 g/ml; ‘light non-exosome control’), and fraction 9 (1.227 or 1.230 g/ml; ‘heavy non-exosome control’). These were obtained from media conditioned by either S2 or S2 tub-Wg cells. To ask whether fraction 5 is likely to be enriched in ‘exosome proteins’, we assessed whether it contains Drosophila homologs of known mammalian exosome proteins. A list of 143 human exosome proteins was obtained from ExoCarta (29), and BLAST was used to identify the closest Drosophila homologs. In some cases, no clear Drosophila homolog could be identified. Moreover, in large protein families such as the tetraspanins it was difficult to assign the direct Drosophila homolog for individual proteins, further reducing the number of candidate Drosophila exosome proteins. Ultimately, we identified 80 Drosophila proteins that are likely homologous to a human exosome protein. We then asked which of these proteins were found in fractions 2, 5, and 9. This was first done for fractions derived from untransfected S2 cells. 70% of the putative exosome proteins were detected in fraction 5 while 36.5% and 38.75% were found in fractions 2 and 9 respectively (Fig. 3B, p<0.001 using chi-squared test). 23.75% were specific to fraction 5. Among the fractions obtained from S2 tub-Wg cells, fraction 5 contained 72.5% of putative exosome proteins while fractions 2 and 9 contained 50% and 32.5% respectively (Fig. 3C, p<0.001 using chi-squared test). 21.25% were specific to fraction 5. From this analysis we conclude that exosome-like vesicles purified from S2 are enriched in proteins that are homologous to proteins previously identified in mammalian exosomes.
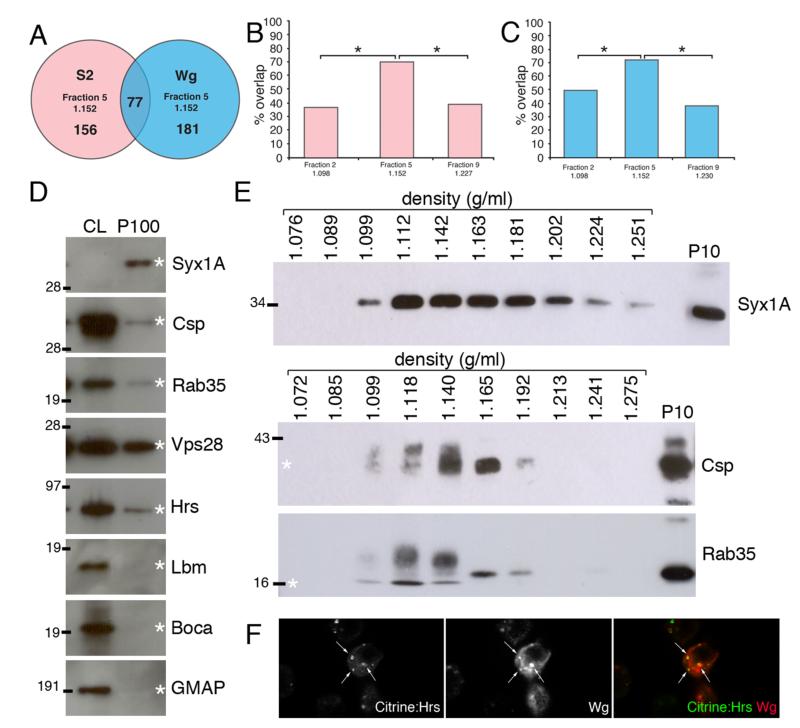
Figure 3. Analysis and validation of mass spectrometry results.
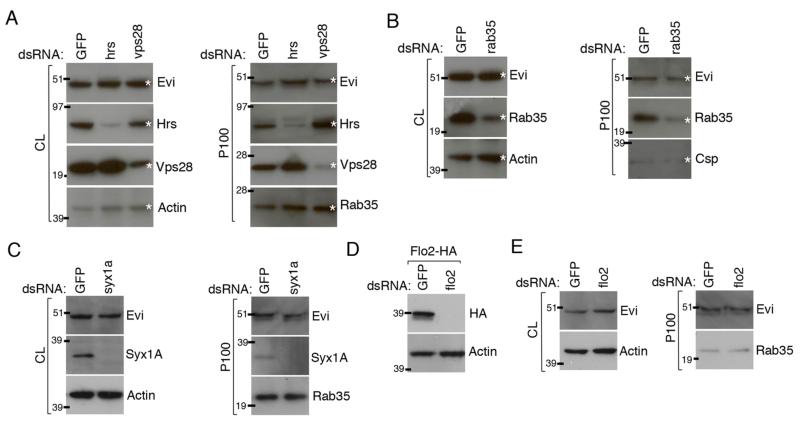
A. Venn diagram showing number of exosome proteins detected (with 2 peptides or more) in exosome fraction 5 (1.151 g/ml) from S2 and S2 tub-Wg cells. 77 proteins were found in exosome fractions from both cell types. B, C. Graph showing percentage overlap between fractions 2, 5 and 9 from S2 (B) and S2 tub-Wg (C) cells and the most common mammalian exosome proteins (* p<0.001). D. Equal amount of protein from cell lysates (CL) and P100 pellets from S2 tub-Wg cells were analysed by western blots. Syx1A, Csp, Rab35, Hrs and Vps28 were all present in the P100 pellet, but the tetraspanin Lbm, the ER protein Boca and the Golgi marker GMAP were only found in the CL. E. Western blots of continuous sucrose density gradients. Syx1A is found in fractions between 1.099 – 1.251 g/ml. Csp and Rab35 are found in fractions between 1.099 – 1.192 g/ml, showing a narrower distribution than Syx1A. The molecular weight of Csp and Rab35 was altered in lighter non-exosome fractions with a density between 1.099 – 1.140 g/ml, but the reason for this change is not understood. F. S2R+ cells were contransfected with plasmids expressing Wg and Citrine:Hrs. Significant colocalisation was observed within Wg secreting cells (white arrows).
We next attempted to identify proteins that are specifically located in the ‘exosome fraction’ without relying on existing knowledge from mammalian systems. To focus on the most significant hits, we decided to exclude all the proteins identified only by a single peptide during the mass spec. Among the remaining proteins, those that were found in all fractions were removed. This left 156 proteins that were specifically present in fraction 5 from S2 cells and 181 proteins in fraction 5 from S2 tub-Wg cells (Fig. 3A). 77 of these proteins are common to both groups and therefore represent core exosome proteins produced by S2 cells (Supplemental Table 1). Among these, many are homologs of mammalian exosome proteins, including proteins involved in trafficking, MVB formation, signalling, the cytoskeleton and metabolism. We also identified a large number of ribosomal proteins in our analysis, but these are commonly found in proteomic analysis of exosomes (42). The above analysis also led us to identify 104 proteins, including Wg itself, that were specific to fraction 5 from S2 tub-Wg cells (not present in fraction 5 from untransfected S2 cells) (Supplemental Table 2).
As suggested above, MS analysis allowed us to draw up a list of potential Drosophila exosome proteins. These proteins for which antibodies are available were validated by Western blot analysis of P100 pellets obtained from S2 tub-Wg cells. For example, mass spec analysis showed that the membrane trafficking proteins Syntaxin 1A (Syx1A), Cysteine string protein (Csp) and Rab35 were present in fraction 5 from S2 tub-Wg cells. The same proteins were also detected by Western blot of the P100 pellet. In particular, Syx1A appeared to be enriched in the P100 pellet relative to its amount in cell lysates (Fig. 3D). Using the same antibodies, we also performed Western blot analysis of the various fractions following density gradient centrifugation of the P100 pellet. All three proteins were found in fractions that overlap with those where Wg was present (Fig. 3E). A similar analysis was performed for ESCRT proteins, which are known to be involved in MVB biogenesis and are commonly found in mammalian exosomes (29, 43). Several ESCRT proteins, including Hrs (Hepatocyte growth factor regulated tyrosine kinase substrate) and Vps28, were found by MS to be present in fraction 5 from S2 tub-Wg cells. Both proteins were found by Western blot to be present in the P100 pellet from S2 tub-Wg cells (Fig. 3D). We next asked whether Wg colocalises with ESCRT proteins in MVBs of secreting cells, as predicted from our data. To test this we cotransfected S2R+ cells with plasmids expressing Wg and a Citrine:Hrs fusion protein and analysed these cells using confocal microscopy. Colocalisation of the two proteins was often seen (Fig. 3F) suggesting that some Wg protein traffics through Hrs-positive MVBs within secreting cells consistent with subsequent export in exosome-like vesicles.
Tetraspanins, which constitute a major class of proteins commonly found in mammalian exosomes, were conspicuously absent from the list of proteins detected in exosome-like vesicles from S2 cells. Tetraspanin family members, specifically CD63, are commonly used as markers of mammalian exosomes (44). There are 43 predicted tetraspanins encoded by the Drosophila genome, but only two were detected by MS in fraction 5 from S2 tub-Wg cells (Tsp42Ee and Tsp42Ef). The only tetraspanin against which we could obtain an antibody was Late bloomer (Lbm, also known as Tsp42Em). Western blot analysis with this antibody showed that this protein is not present in the P100 pellet from S2 cells (Fig. 3D). Overall, MS allowed us to identify many exosome proteins from Drosophila. Many but not all, are homologous to previously identified mammalian exosome proteins. Some of them could be used as markers of exosomes for future functional analysis of exosomes.
Evi is secreted on exosome-like vesicles
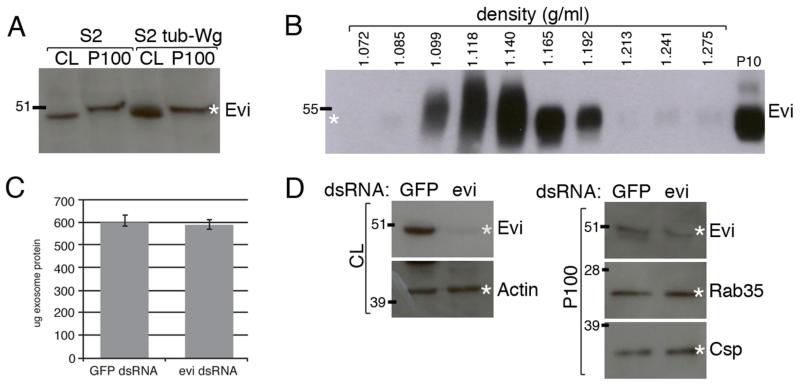
Evi is a multipass transmembrane protein required for Wg secretion (6-8). It has been suggested to chaperone Wg from the Golgi to the plasma membrane (45). Surprisingly for a multipass transmembrane protein, Evi has been shown to move across the larval neuromuscular junction in Drosophila and to be secreted into the medium by S2 cells (38, 39). These observations led to the suggestion that Evi may be secreted on exosomes. No Evi peptide was detected upon MS analyses of ‘exosome fractions’ from S2 or S2 tub-Wg cells. However, this is likely due to technical difficulties associated with detecting peptides from this particular protein since Evi was readily detected by Western blot in the P100 pellets produced from both cell types (Fig. 4A). The P100 pellet contained similar amounts of Evi, irrespective of whether Wg was expressed, indicating that the loading of Evi on exosomes is independent of Wg. Although S2 cells do not express Wg endogenously, they do express one other Wnt (Wnt5) (www.flyrnai.org). We cannot exclude the possibility that Wnt5 could contribute to Evi secretion. However, since exogenous Wg expression (as in S2 tub-Wg cells) has no impact on the amount of Evi found in the P100 pellet, we suggest that Evi secretion is most likely Wnt-independent. To test if Evi secreted by S2 cells is loaded on exosomes, the P100 pellet from S2 tub-Wg cells was fractionated by continuous sucrose gradient centrifugation. Evi was found in fractions overlapping with those containing Wg, as well as in lighter fractions (1.099 – 1.192 g/ml) (Fig. 4B). These data suggest that Evi is indeed secreted on exosome-like vesicles by cultured Drosophila cells.
Figure 4. Evi is secreted on exosome-like vesicles in S2 cells, but is not required for their production.
A. Equal amounts of proteins from cell lysates (CL) and P100 pellets from S2 and S2 tub-Wg cells were analysed by western blot. Similar levels of Evi are detected in the P100 pellet from each cell type, although the apparent molecular weight of Evi is slightly higher in the P100 samples. B. Western blot analysis of a continuous sucrose density gradient from S2 tub-Wg cells shows that Evi is present in fractions between 1.099 – 1.192 g/ml. However, in lighter non-exosome fractions with densities between 1.099 – 1.410 g/ml Evi appears to have a higher molecular weight. We do not understand the reason for this. C, D. S2 cells were treated with dsRNA against GFP or evi. Evi levels were reduced upon RNAi treatment in both the CL and P100 pellet. Exosome production as measured by Rab35, Csp (D) or total protein levels (C) was unaffected.
The above results suggest that Evi could play a role in exosome production and/or in the loading of Wg on exosomes. Unfortunately, a direct test of this suggestion could not be achieved for two reasons. One is that Evi is required for the progression of Wg from the Golgi to the plasma membrane (45), precluding assaying any subsequent role in the production of Wg-containing exosomes. The second reason is that, although Evi could readily be knocked down in S2 cells by RNAi, this was not possible in S2 tub-Wg cells. Nevertheless, we were able to ask whether Evi is generally required for exosome production in S2 cells. S2 cells were treated with dsRNA against Evi or GFP (as a control) and exosome production was assayed by analysing the P100 pellet. Evi RNAi caused robust reduction of Evi levels in cell lysates as well as, although to a lesser extent, in the P100 pellet. The levels (assayed by Western blots) of Rab35 and Csp, two exosome markers, and of total protein (measured by BCA assay) in the P100 pellet were unaffected by Evi RNAi (Fig. 4C-D). We conclude that while Evi is present in exosome-like vesicles it does not seem to be required for their production.
Known Drosophila exosome proteins are not required for exosome production
Several proteins have been shown to participate in exosome production or release in various mammalian cell culture systems and more recently in Drosophila cultured cells (39, 41, 46-50). However, no individual protein or class of proteins has been shown to be universally required for exosome production in all systems (31). We next asked if any of the proteins present in Drosophila exosomes was required for exosome production by S2 cells. For these experiments, Evi was used as an exosome marker.
We first tested the potential role of ESCRT proteins since they play important roles in the formation of MVBs, where exosomes originate (43). As described above, various ESCRT proteins were detected by MS in the Drosophila exosome fractions and this was validated by Western blot for Hrs and Vps28 (Fig. 3D). We therefore tested whether Hrs or Vps28 are required for exosome production in S2 cells. RNAi against Hrs or Vps28 caused a marked reduction in the corresponding protein, both in cell lysates and in the P100 pellets (Fig. 5A). Moreover, the level of ubiquitinated proteins was increased in the lysate of RNAi-treated cells, as expected from reduced trafficking to MVBs and lysosomes (data not shown). Therefore, RNAi against these two proteins was effective. Nevertheless, the level of Evi was unaffected both in cell lysates and P100 pellets following Hrs or Vps28 RNAi. Additionally the levels of Rab35 and total protein were unaltered in the P100 pellets (Fig. 5A and data not shown). Similar experiments were performed in S2 tub-Wg cells, although the levels of Hrs and Vps28 knockdown observed was not as pronounced in this cell line. (We consistently found S2 tub-Wg cells to be partially resistant to RNAi against trafficking proteins but cannot offer an explanation). No effect on Wg or Evi levels in the P100 pellets was observed (data not shown). These data suggest that ESCRT proteins are not required for exosome production in S2 cells, although we cannot exclude the possibility that very small amounts of these proteins could be sufficient.
Figure 5. ESCRT proteins, Rab35, Syx1A and Flo2 are not required for exosome production in S2 cells.
S2 cells were treated with dsRNA against hrs or vps28 (A), rab35 (B), syx1a (C) or flo2 (D). RNAi against GFP was used as a control. The cell lysates (CL) and P100 pellets produced by those cells were analysed by Western blot. A. Hrs and Vps28 RNAi caused reduction in respective protein levels in both the CL and P100 pellets. However, no change in Evi or Rab35 levels in the P100 pellets were observed. B. Rab35 RNAi caused reduction in Rab35 levels in both CL and P100 pellets. No change in Evi or Csp levels in the P100 pellet was observed. C. Syx1A RNAi caused reduction in Syx1A levels in both CL and P100 pellets. No change in Evi or Rab35 levels in the P100 pellet was observed. D. Flo2-HA expressing S2 cells were treated with dsRNA against GFP or flo2. Flo2 RNAi caused reduction of Flo2-HA levels in CL. However, Flo2 RNAi caused no changes in Evi or Rab35 levels in the P100 pellet.
Another class of proteins that could be required for exosome formation are those involved in membrane trafficking such as Rab35, Syx1A and Flo2. We analysed the requirement for these proteins in exosome production in S2 and S2 tub-Wg cells. The results for S2 cells are shown in Figure 5. Similar but milder effects were observed in S2 tub-Wg cells (data not shown), most likely because of the reduced efficacy of RNAi in these cells. We analysed the requirement for Rab35 in S2 cells, since this protein was also present in Drosophila exosomes (Fig. 3D-E) and has been shown by others to be required to regulate exosome secretion in a mammalian oligodendrocyte cell line (50). RNAi against Rab35 in S2 cells caused reduction of Rab35 protein levels in cell lysates and in P100 pellets. However, no effect on Evi levels in the P100 pellet was observed. Csp and total protein levels in the P100 pellet were also unchanged (Fig. 5B and data not shown). These data suggest that wild type levels of Rab35 are not required for exosome production in S2 cells. Similar experiments were performed for Syx1A, which is also present in Drosophila exosomes (Fig. 3D-E), and has recently been suggested to play a role in exosome production in Drosophila S2 cells (39). Despite causing near complete loss of Syx1A protein in both cell lysates and the P100 pellet, RNAi against Syx1a had no impact on Rab35 levels in the P100 pellet from S2 cells (Fig. 5C). Finally we analysed the role of Flo2 in exosome production. Flotillin proteins are known mammalian exosome markers (27) and, as shown above, are present in Wnt3A-containing exosome-like vesicles from L cells (Fig. 2B). Moreover, Flo2 was found in Drosophila exosomes by MS (see above). To overcome the lack of antibody against Flo2, we constructed a vector expressing Flo2 carrying a C-terminal HA tag. Treatment of Flo2-HA expressing S2 cells with dsRNA against Flo2 caused complete loss of Flo2-HA indicating that our dsRNA was functional. However, Flo2 RNAi had no effect on Evi or Rab35 levels in the P100 pellet (Fig. 5D). Overexpression of Flo2-HA also had no effect on Evi levels in the P100 pellet (data not shown). These data suggest that Flo2 levels are not important for exosome production in Drosophila S2 cells.
Rab11 is required for exosome production in S2 cells, but not for Wg secretion in wing imaginal discs
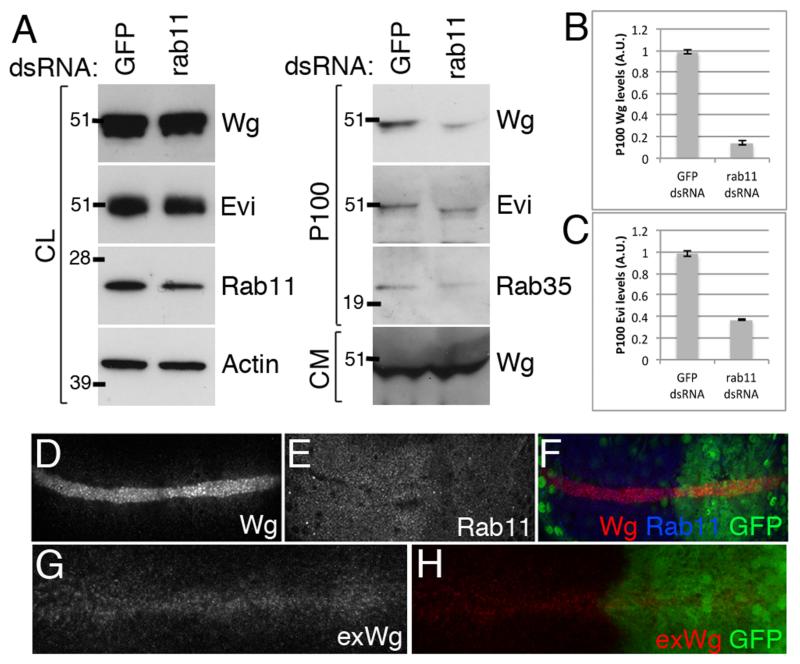
Rab11 is a small GTPase required for exosome production in mammalian reticulocytes that was recently shown to play a similar role in Drosophila S2 cells (49). Knockdown of Rab11 protein levels or expression of a dominant negative form was shown to reduce the levels of Evi secretion in S2 cells and at the larval neuromuscular junction (NMJ) (39). We therefore used our RNAi-based assay to test whether Rab11 plays any role in Wg secretion and/or exosome production by S2 cells. Treatment of S2 tub-Wg cells with dsRNA against Rab11 (shown to be effective by immunoblotting cell lysates with anti-Rab11) led to a reduction of the levels of Wg and Evi in the P100 pellet without significantly impacting on the levels of these proteins in cell lysate. Rab35 was also reduced specifically in the P100. These observations suggest that Rab11 could indeed participate in exosome production by S2 cells. Total secreted Wg present in the CM harvested from Rab11 RNAi-treated cells appeared unaffected (Fig. 6A-C). This is consistent with the small proportion of total Wg present in the P100 pellet and suggests that the other pools of secreted Wg are not affected by Rab11 RNAi. General secretion, assayed with secreted GFP was also unaffected by Rab11 knockdown in S2 cells, (data not shown). We conclude that Rab11 knockdown specifically reduces the exosome-associated pool of secreted Wg while leaving other pools of Wg unaffected.
Figure 6. Rab11 is required for exosome production in S2 tub-Wg cells, but plays no role in Wg gradient formation in the wing imaginal disc.
A-C. S2 tub-Wg cells were treated with dsRNA against GFP or rab11. Rab11 RNAi caused a reduction in Rab11 levels in the CL. Reduced levels of Wg (quantified in B), Evi (quantified in C) and Rab35 levels in the P100 pellet were observed upon Rab11 RNAi. No effect on the levels of secreted Wg in the CM was seen. D-H. Rab11 RNAi was expressed in the posterior compartment of wing imaginal discs and caused strong reduction in Rab11 levels (E), with minimal cell death (data not shown). Total Wg staining showed no changes upon Rab11 knockdown except for aberrant punctae of Wg in the most apical regions of the expressing cells indicating a defect in intracellular trafficking (D, F). However, no changes in extracellular Wg distribution were observed (G, H) indicating that Wg secretion and gradient formation occur normally with reduced Rab11 levels.
Our results showed that Rab11 is required for Wg-containing exosome production in S2 cells. Since Rab11 is also required for Evi and Wg to cross the larval NMJ (39), it is likely that exosomes are the carrier of such transfer. We next asked if exosomes are relevant to another form of Wg transport, from the D-V boundary of wing imaginal discs to surrounding target cells and hence gradient formation in this tissue. We expressed a Rab11 RNAi transgene with en-GAL4, which drives expression in the posterior compartment, leaving the anterior compartment as a control. To overcome the requirement of Rab11 for long-term cell viability, RNAi was expressed for a limited time, using tub-GAL80ts to achieve temporal control of expression. Conditions were chosen so that a strong knockdown was achieved with minimal cell death (see methods). Under such conditions, the distribution of Wg was found to be largely unaffected. The only difference between the posterior (Rab11 knockdown) and the anterior (control) compartment was a slight accumulation of Wg in punctae at the apical region of expressing cells in the former (Fig. 6D-F), which is compatible with altered intracellular trafficking. However, analysis of extracellular Wg showed that both Wg secretion and gradient formation was unaffected by Rab11 RNAi (Fig. 6G-H). If we assume that Rab11 RNAi interferes with exosome production in this tissue (as it does in S2 cells), these results suggest that exosomes do not play a role in Wg secretion and gradient formation in the wing imaginal disc.
Evi, an in vivo exosome marker, is not released by Wg-expressing cells of imaginal discs but is present in the haemolymph
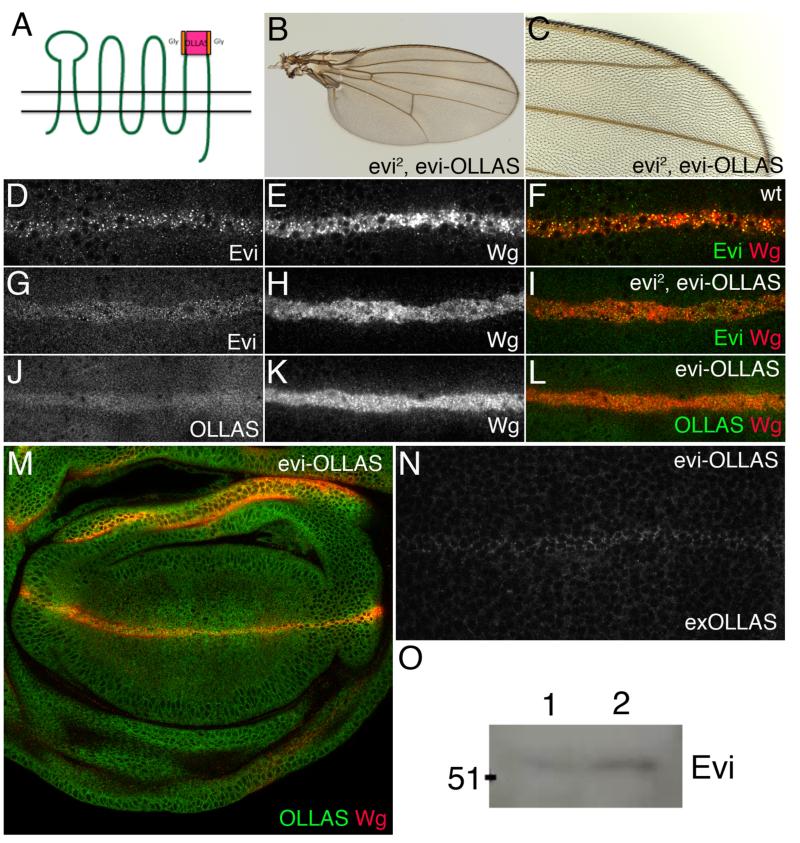
Our results with cultured cells suggest that the multipass membrane protein Evi could be used as an in vivo marker of exosomes. Indeed, Evi is released by motoneurons at the NMJ in structures that could be exosomes (39). We therefore wanted to ask if Evi is secreted by other cell types in vivo. One good place to start is the DV boundary of wing imaginal discs where Wg is produced and from where it spreads to receiving cells. Do these cells release Evi in the extracellular space, as expected from the exosome hypothesis? Since the anti-Evi antibody recognises an intracellular epitope, we created transgenic flies expressing extracellularly tagged Evi at endogenous levels. To achieve physiological levels of expression, which is essential to avoid overexpression artefacts, we started with a BAC comprising 80kb of genomic DNA from the evi locus. This was modified by recombineering to introduce an OLLAS epitope tag in extracellular loop 4 (Fig. 7A). Glycine linkers were used to minimise the impact on protein function. Transgenic flies were obtained and the Evi-OLLAS BAC transgene was introduced onto a chromosome carrying evi2, a null mutation that removes the translational start. As shown in Fig. 7B-C, the Evi-OLLAS BAC rescued homozygous evi2 mutants to viability and the rescued flies had normal wings, showing that this transgene is fully functional. Next we verified that the distribution of Evi-OLLAS is identical to that of the endogenous protein. In wildtype discs, Evi transcripts are produced uniformly but the protein is post-transcriptionaly upregulated at the DV boundary in a Wg-dependent manner (45). This upregulated Evi forms apical punctae that colocalise with Wg (Fig. 7D-F). Upregulation and colocalisation with Wg within Wg-expressing cells was also observed in discs that had the Evi-OLLAS BAC as the only source of Evi either with the same anti-Evi antibody (Fig. 7G-I) or with anti-OLLAS (Fig. 7J-M). These data indicate that Evi-OLLAS reproduces the endogenous expression and subcellular localisation of wildtype Evi. Next, we assessed the distribution of extracellular Evi-OLLAS using a detergent-free protocol that specifically highlights extracellular protein. Very low level uniform staining was seen throughout the disc except at the surface of Wg expressing cells where staining was markedly higher (Fig. 7N). No gradient of OLLAS staining was observed. This indicates that Evi-OLLAS is present at the plasma membrane of Wg expressing cells, but does not appear to be released with Wg and move across the tissue.
Figure 7. Analysis of Evi secretion in vivo.
A. Schematic of Evi protein showing site of OLLAS tag in the fourth extracellular loop. B, C. Wings from evi2 mutant flies, expressing the Evi-OLLAS BAC showing complete rescue of the evi mutant phenotype. D-M. Confocal images of wing imaginal discs from wild type (D-F), evi2, evi-OLLAS (G-I) and evi-OLLAS (J-M) larvae stained with antibodies against Evi (D, F, G, I), Wg (E, F, H, I, K, L, M) and OLLAS (J, L, M). Apical sections showing the Wg expressing cells are shown in panels D-L, while a more basal section of the whole disc is shown in panel M. Evi-OLLAS reproduced the endogenous Evi distribution and colocalises with Wg within Wg expressing cells (compare panel F to panels I and L). N. Extracellular OLLAS staining of wing imaginal disc from Evi-OLLAS larvae shows uniform low levels of staining across the pouch with higher levels on the cell surface of the Wg expressing cells. No gradient of extracellular Evi-OLLAS was observed. O. Western blot of haemolymph from wild type (lane 1) and cg-GAL4 > UAS-evi-V5 expressing (lane 2) larvae shows endogenous Evi is secreted into the larval haemolymph.
Overall, our data do not support a model whereby Evi moves with Wg on exosomes in the wing imaginal disc. However, they do not exclude the possibility that Evi could be secreted and released by other cell types. Exosomes have been found in body fluids of mammals, including blood. Therefore we tested whether Evi was present in larval haemolymph. We obtained haemolymph from wildtype wandering 3rd instar larvae dissected in PBS. The resulting fluid was filtered to remove any cells or debris over 200nm in diameter and the filtered material was analysed by Western blot. Low levels of Evi were reproducibly detected (Fig. 7O lane 1). We have not yet determined the source of Evi found in the haemolymph, but found that tagged Evi expressed from a transgene in the fat body (with cg-GAL4) accumulates in the haemolymph (Fig. 7O lane 2). These data are consistent with the hypothesis that Evi could be secreted on exosomes into the larval haemolymph.
DISCUSSION
In this paper, we have shown that Drosophila S2 cells secrete Wg on vesicles. Analysis of the density, size and morphology of these vesicles leads us to propose that they most likely represent endosome-derived exosomes. Proteomic analysis of the corresponding fractions showed that they contain many homologs of proteins found in mammalian exosomes. These include proteins involved in trafficking, cell adhesion, the cytoskeleton and metabolism, as well as in other cellular processes. Moreover, we have found that a mammalian Wnt, Wnt3A, is also secreted on vesicles of densities similar to those of exosomes. Additionally, we have developed a cell-based RNAi assay that will enable testing the requirements of various proteins in exosome production. Using this assay, we have shown that Evi, Hrs, Vps28, Rab35, Syx1A and Flo2 do not appear to be required for exosome production in S2 cells. However, we have found that Rab11 does participate in the production of exosomes containing Wg and Evi in cell culture. We next tested the potential requirement for exosomes in Wg gradient formation in the wing imaginal disc and found no supporting evidence.
Among the several pieces of evidence that Wg is loaded on exosomes is the presence of Wg in the 1.14 – 1.192 g/ml fraction obtained from conditioned medium. Our results differs from a report that Wg sediments at 1.19 – 1.28 g/ml (51) -- we have no explanation for the discrepancy – but are consistent with those of Budnik (39). Altogether, there is strong support for the notion that a subset of secreted Wg is indeed on exosomes and that this fraction is competent to trigger signalling. Since only 12% of secreted Wg is found in the P100 pellet, it is likely that additional pools of secreted Wg exist. Indeed, in larval extracts, Wg has been shown to associate with LPPs produced in the fat body (16). In culture, it is likely that Wg associates with the LPPs that are present in the serum supplementing culture medium. This is probably functionally significant since Wg secretion is reduced in the absence of serum (K.B. and J.P.V., unpublished observations) and, conversely, serum stabilises purified mammalian Wnt3A (52). However, since LPPs do not sediment at 100,000 g, they are unlikely to contribute to our exosome-like fraction. Most likely, the two fractions coexist in conditioned medium. In addition, Wg could be released in association with the lipocalin encoded by swim, which would shield the lipid moiety on Wg (17). We tested whether manipulating the levels of Swim in S2 tub-Wg cells using RNAi and overexpression would affect the proportion of Wg present in the P100 pellet, but found it to be unaffected (data not shown). The relative abundance and activities of the different pools of secreted Wg remain to be investigated.
What are the trafficking steps that target Wg to exosomes? By definition exosomes are of endocytic origin since they are produced in MVBs (23). Our MS and western blot analyses are consistent with this since they show that proteins involved in endocytosis and MVB formation are present in Drosophila exosomes. We suggest that upon reaching the plasma membrane from the secretory pathway, Wg could be endocytosed and trafficked to specialised MVBs where it would be packaged into exosomes. This hypothesis is supported by our data showing that Wg colocalises with Citrine:Hrs in secreting cells. One possibility is that trafficking of Wg from the plasma membrane to MVBs is mediated by Evi. This is consistent with the documented interaction between these proteins (53) and with our finding that Evi is constitutively secreted on exosomes. Since Evi is known to undergo retrograde transport to the Golgi (45, 54-57), one would have to invoke that only Wg-associated Evi would be targeted to MVBs while Wg-free Evi would return to the Golgi. A second model is that Evi and Wg are independently directed to exosomes. This could occur for example as a result of association with lipid micromains, which could act as a way station for subsequent trafficking to MVBs and packaging into exosomes. Wg is known to associate with lipid microdomains and with microdomain-resident proteins such as Flo2 and the glypicans Dally and Dally-like (35, 36, 58, 59). Moreover, in mammalian systems, microdomain-associated proteins such as GPI-anchored proteins and Flotillins are found in exosomes (27). Further genetic and cell biological analyses will be needed to distinguish between these two models.
Another question raised by our results is whether loading onto exosomes is essential for Wg function. In order to address this question, it is necessary to develop means of inhibiting exosome formation reliably and specifically. While several proteins have been shown to play a role in various cell culture systems, no common regulator of exosome formation has been identified so far (31). ESCRT proteins are required for MVB formation and therefore are likely candidates. We tested the requirement of two ESCRT components, Hrs and Vps28, in our cell-based RNAi assay and found no role in exosome production. One cannot exclude the possibility that this is because these proteins are required in very small amounts. Alternatively exosomes could form in an ESCRT-independent manner, as shown previously in some cell types (27). To overcome the limitations of RNAi, we turned to generating clones of homozygous mutant cells in the larval wing imaginal disc. Unfortunately, long term ESCRT deficiency leads to extensive apoptosis (data not shown), preventing phenotypic analysis. We have therefore sought to identify less pleiotropic factors from our list of MS hits. Rab35 was a good candidate since it is required for exosome production in an oligodendrocyte cell line (50). However our cell-based RNAi assay showed no requirement for Rab35 in exosome production in Drosophila S2 cells. Likewise we found no role for Syx1A or Flo2 in exosome production in our cell culture assay. However, we did confirm that Rab11 plays an important role in exosome production in S2 cells. Rab11 has previously been shown to be required for exosome release in reticulocytes, possibly by regulating the fusion of MVBs with the plasma membrane in a Ca2+-dependent manner (49). Although more components of exosome biosynthesis need to be identified, the role of Rab11 provides a first tool to assess the role that exosomes play in releasing Wg from expressing cells in vivo. This approach has recently been used to show that exosomes could mediate the transfer of Wg and Evi across the NMJ (39). We have similarly assessed the role of exosomes in Wnt transport along epithelia.
The first indication that exosomes could contribute to Wg gradient formation came from a report that Wg spreads on membrane-bound vesicles, which the authors called argosomes (15). Whether these vesicles were exosomes was debatable at the time: they had the right topology but did not colocalise with transgenically expressed human CD63, a potential but unvalidated exosome marker. Wg was subsequently shown to associate with LPPs (16) and interest in exosomes as potential carriers of Wg faded. Our results with cultured cells warrant reviving this hypothesis. Since Rab11 is required for exosome formation in cell culture, Rab11 was knocked down in imaginal discs and the effect on the Wg gradient was assessed. The intracellular distribution of Wg was subtly affected in expressing cells but no effect on secretion levels or the extracellular gradient could be detected. These observations suggest that Rab11 and by inference exosomes (with the caveat that we cannot yet assess exosome formation in the intact epithelium) are not required for Wg gradient formation. As a further test, we analysed the in vivo distribution of Evi, which we showed to be an excellent exosome marker in cell culture. To ask if Evi is released in the extracellular space by Wg-expression cells, as expected from the exosome hypothesis, we devised a transgene expressing extracellularly tagged Evi at endogenous level (Evi-OLLAS BAC). Analysis of transgenic imaginal discs gave no indication of release from Wg-expressing cells. On balance, our results suggest (though do not prove) that exosomes are not required for the spread of Wg at the surface of imaginal discs. However, they leave open the possibility that exosomes could contribute to other physiological functions. In light of the presence of exosomes in many mammalian body fluids, including blood (60, 61), it is intriguing that Evi can be detected in the larval haemolymph. It is conceivable that exosomes carry a Wnt or another lipidated ligand into the haemolymph. These observations highlight the need and potential to uncover the physiological significance of exosomes in a genetically defined, intact organism.
MATERIALS AND METHODS
Cell culture
Drosophila S2, S2R+ (Drosophila Genomics Resource Centre, DGRC), S2 tub-Wg (kindly provided by R. Nusse) and S2 HA-Wg cells (62) were cultured at 25°C in Schneider’s medium + L-Glutamine (Sigma) containing 10% (v/v) Fetal Bovine Serum (FBS; Invitrogen) and 0.1 mg/ml Pen/Strep (Invitrogen). Mouse L and L-Wnt3A cells (ATCC) were cultured at 37°C and 5% CO2 in DMEM + Glutamax (Invitrogen) containing 10% (v/v) FBS and 0.8 mg/ml G418 (Sigma) for the L-Wnt3A cells. Cell lysates were produced using Triton Extraction Buffer (TEB): 50 mM Tris pH7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton-X100. Cells were incubated in TEB for 10 min on ice then spun at 14,000 rpm for 10 min at 4°C to remove remaining cell debris. Total protein levels were measured using a BCA assay (Sigma) against BSA protein standards.
Exosome purification
To collect exosomes cells were incubated in exosome-free medium. This was prepared using Schneider’s or DMEM containing 20% (v/v) FBS spun overnight at 100,000 g at 4°C in a Beckman Coulter L8-70M ultracentrifuge using a SW32Ti rotor to remove any microvesicles from the FBS.
The resulting supernatant was diluted in Schneider’s/DMEM to get a final concentration of 10% (v/v) FBS plus 0.1 mg/ml Pen/Strep was added to the Schneider’s medium prior to filtration using 0.2 μm filters (Corning). For large-scale exosome purification, cells were plated at 2 × 106 cell/ml in exosome-free medium (100-200ml) and incubated for 72 hours. Resuspended cells were spun at 300 g for 5 min to remove the cells and the supernatant spun at 2500 g for 5 min to remove dead cells and large cell debris and produce conditioned medium (CM). The CM was spun at 10,000 g for 30 min at 4°C in an ultracentrifuge to remove cell debris and large microvesicles (P10), then the supernatant (S10) spun at 100,000 g for 90 min at 4°C. The resulting pellet was resuspended in cold PBS and is referred to as the 100,000 g pellet or P100 and contains exosomes.
Exosomes were further purified using a linear sucrose gradient. P100 pellets were resuspended in 1.8 ml 2.5 M sucrose/20 mM Hepes pH 7.4, overlaid with 10 ml of a 0.25 M to 2 M linear sucrose gradient, and spun at least 15 hours at 35,000 rpm (210,000 g) at 4°C. 1.8 ml fractions were collected from top to bottom, refraction index of each measured on 10 μl to indicate the density. They were then diluted in Hepes, and spun at 100,000 g for 1hour. Pellets were then used for MS, for EM, or Western blot analysis
ImmunoEM of exosome fractions
Purified exosome fractions were fixed in 2% paraformaldehyde, deposited on Formwar/carbon-coated electron microscopy grids and immunolabeled with anti-HA antibodies and protein A gold conjugates. Samples were post-fixed in 1% glutaraldehyde, contrasted and embedded in a mixture of methylcellulose and uranyl acetate as described previously (23). Exosomes were observed at 80kV with a CM120 Twin Philipps electron microscope (FEI Company) and digital acquisitions were made with a numeric camera (Keen View; Soft Imaging system). Rat anti-HA was from Roche, mouse anti-rat was from Dako and Protein A gold conjugates from Cell Microscopy Center, Utrecht, The Netherlands.
Mass spectrometry analysis of sucrose density gradient fractions
Proteins were resuspended in 50 mM ammonium bicarbonate, reduced in 0.5 mM tris(2-carboxyethyl)phosphine (Pierce) for 20 min at 37°C and alkylated in 50 mM iodoacetamide for 20 min at 37°C. Excess iodoacetamide was neutralized with 50 mM DTT. Proteins were digested in 50 mM ammonium bicarbonate with immobilized TPCK-treated trypsin overnight at 37°C and high agitation speed. The digest mixture was acidified with TFA and evaporated to dryness prior to MS analyses. LC-MS/MS analyses were performed on an LTQ-Orbitrap XL hybrid mass spectrometer (Thermo Fisher Scientific) coupled to a nano-flow LC system (Eksigent). Peptides were loaded on a C18 trap column prior to separation on a 150 μm ID × 10 cm nano-LC column (Jupiter C18, 3 μm, 300 Å, Phenomex). Peptides were separated on the analytical column using a linear gradient of 5-40% acetonitrile (0.2% formic acid) in 53 min with a flow rate of 600 nL/min. The mass spectrometer operated in data-dependent mode where a survey scan is initiated in full scan at high mass accuracy in the Orbitrap followed by tandem MS in the linear ion trap for the three most abundant precursor ions. The conventional MS spectra (survey scan) were acquired in the Orbitrap at a resolution of 60 000 for m/z 400. Mass calibration used a lock mass from ambient air [protonated (Si(CH3)2O))6; m/z 445.12057], and provided mass accuracy within 15 ppm for precursor ion mass measurements. A dynamic exclusion window was applied to prevent MS/MS analysis of previous selected ions 60 s after its acquisition. Only multiply charged ions with an intensity above 10,000 counts were selected for MS/MS sequencing.
Database Searching with Mass Spectrometry Data
Data were analyzed with Xcalibur (version 2.0.7 SR1) software, and peak lists were generated using Mascot distiller software (version 2.3.2.0, Matrix Science) and LCQ_plus_zoom script. Database searches were performed against an Uniprot Drosophila melanogaster (Dmel) containing 58904 entries (version 3.54, released November 2006) using Mascot (version 2.2.0, Matrix Science). Parent ion and fragment ion mass tolerances were set at 15 ppm and 0.5 Da, respectively. One missed cleavage was allowed for trypsin digestion and phosphorylation (STY), oxidation (Met), deamidation (NQ), and carbamidomethylation (Cys) were selected as variable modifications. All protein identifications and IPI entry numbers were reported for peptide sequences matching more than one protein entry. A Mascot search against a concatenated target/decoy database consisting of a combined forward and reverse version of the Dmel database was performed to establish a cutoff score threshold for a false-positive rate of less than 2% (p < 0.02).
RNAi experiments
dsRNA was produced in vitro using T7-tagged primers to amplify 200-500 bp sequences from the relevant cDNAs. The primers used were:
T7-eGFP-F:
TAATACGACTCACTATAGGGACCCTCGTGACCA
CCCTGAC
T7-eGFP-R:
TAATACGACTCACTATAGGGGGACCATGTGATC
GCGCTTC
T7-evi-F:
TAATACGACTCACTATAGGGGGACACACCGAG
CCAAATGAACC
T7-evi-R:
TAATACGACTCACTATAGGGCGGCGAAGACCA
GCCAGAAA
T7-rab35-F:
TAATACGACTCACTATAGGGGCGCGTAGTTTGT
AGGGTTC
T7-rab35-R:
TAATACGACTCACTATAGGGTTGTTGTCTCTAGT
GGGTGTGAG
T7-hrs-F:
TAATACGACTCACTATAGGGCAGCAGATCATGC
CCCTG
T7-hrs-R:
TAATACGACTCACTATAGGGGGCGCAGCTGCA
TCTC
T7-vps28-F:
TAATACGACTCACTATAGGGGCCGATCTATACG
CAATCA
T7-vps28-R:
TAATACGACTCACTATAGGGAGGAACTGGCGG
ACCTG
T7-syx1a-F:
TAATACGACTCACTATAGGGCGCGGATCCCATC
CTGTCCGCCCCACAAA
T7-syx1a-R:
TAATACGACTCACTATAGGGCGCTCTAGAGCCC
TCCTCCAGCATCTTCTCC
T7-flo2-F:
TAATACGACTCACTATAGGGCGTGAGGCGGAG
TGCCGAAAAG
T7-flo2-R:
TAATACGACTCACTATAGGGCAAGGGTCTGGA
GGCGGAAGG
T7-rab11-F:
TAATACGACTCACTATAGGGGATGGCAAAACAA
TTAAAGCGCAAA
T7-rab11-R:
TAATACGACTCACTATAGGGGTTGAGTCGAGG
GCCGAGGT
PCR products were used to make dsRNA using the T7 Megscript kit (Ambion) and this was purified using the RNeasy mini kit (Qiagen). S2 or S2 tub-Wg cells were plated in 6 well plates at 1×106 cell/ml in 3 ml of exosome-free medium and 15μg of dsRNA was added by bathing or 2 μg of dsRNA was transfected using Effectene (Qiagen). After 48 hours the exosome-free medium was replaced and the cells were incubated for a further 72 hours. The cells were then counted prior to being spun at 300 g for 5 min to pellet the cells. The cells were lysed as described above. The supernatant was spun at 2500 g for 5 min to make CM. The CM was diluted 1:10 in exosome-free medium and spun at 10,000 g for 30 min at 4°C in the ultracentrifuge. The resulting supernatant was spun at 100,000 g for 90 min at 4°C and the pellet resuspended in 0.2 ml cold PBS (P100). The total protein in the cell lysate and P100 pellet was quantified using a BCA assay (Sigma) against BSA protein standards.
Cloning
The Ub-Citrine:Hrs plasmid was made by cloning the citrine cDNA with no stop codon directly upstream of and in frame with the full length hrs cDNA minus a stop codon (kindly provided by H. Bellen). This was subcloned first into an appropriate cloning vector for a stop codon to be introduced using a ds-oligo. This citrine:hrs cDNA containing a stop codon was then subcloned into pCasper containing the ubiquitin promoter (pCaspUb) using EcoRI and KpnI. The pMT-Flo2-HA plasmid was cloned by amplifying the full length Flo2 cDNA (GH22754 from DGRC) by PCR using a 5′ primer containing an EcoRI site upstream of the ATG and a 3′ primer containing the sequence for the HA tag preceded by 4 Glycine residues just prior to a stop codon followed by a NotI site. This Flo2-HA cDNA was subcloned into pMT-V5-His (Invitrogen) using EcoRI and NotI. The pUAS-Evi-V5 plasmid was cloned by adding one V5 tag to the full-length evi cDNA (GH01813 from DGRC Gold collection) by PCR. The Evi-V5 cDNA was then subcloned into pUAST-attB using EcoRI and XbaI.
BAC recombineering
The Evi-OLLAS BAC was designed using an Evi BAC clone (Ch321_67E05) containing 80kb around the evi locus. The BAC ORF was electroporated into SW102 bacteria (NCI). To introduce the GalK selection cassette into the site of choice, the pGalK vector was amplified by PCR using specific primers incorporating sequences from the chosen region for insertion in the BAC. The GalK cassette was inserted between amino acids 474(D) and 475(N) in the fourth extracellular loop of Evi. The GalK fragment containing the evi homology arms was then purified and electroporated into competent SW102 BAC containing cells. Cells were left to recover in LB for 1 hour at 37°C, washed in M9 salts and plated on minimal media + Galactose plates to compete out non-GalK containing bacteria at 30°C for 4 days. Positive colonies were selected by using McConkey plates and screened using GalK containing primers by PCR. To replace the GalK cassette with the OLLAS tag (GAGGG linkers either side of OLLAS – SGFANELGPRLMGK), PCR products were made containing homology arms flanking the OLLAS sequence and electroporated into electrocompetent SW102 BAC-GalK containing cells. Cells were left to recover in LB for 3 hours at 37°C, washed in M9 salts and plated on minimal media DOG plates to compete out GalK containing bacteria at 30°C for 4 days. Positive colonies containing the selected insert instead of the GalK were selected by using McConkey plates and screened using insert specific primers. Positive clones containing the BAC and OLLAS insert were prepared and electroporated into EPI bacteria (NCI). DNA was then prepared for injection from these EPI-BAC containing bacteria using a Pure-Link Maxiprep kit (Invitrogen).
Drosophila genetics
yw was used as a wildtype strain and all flies were kept on standard food at 25°C unless otherwise noted. For the Rab11 RNAi experiments, en-GAL4; tub-GAL80ts flies were crossed to UAS-rab11-RNAi (VDRC 22198) and the resulting cross kept at 18°C. Rab11 RNAi expression was induced by switching crosses to 29°C 24 hours prior to dissection. Evi2 mutant flies were kindly provided by Michael Boutros. Evi-OLLAS and UAS-Evi-V5 transgenic flies were made by Rainbow Transgenics. DNAs were injected into fly strains containing an attP site at 65B2 on the 3rd chromosome.
Cell stainings
S2R+ cells were plated onto glass coverslips and transfected with Ub-Citrine:Hrs and Ub-Wg plasmids using Effectene (Qiagen). After 4 days incubation at 25°C the cells were washed in PBS (Phosphate Buffered Saline) and fixed for 10 minutes in 4% paraformaldehyde in PBS. Cells were washed again, quenched using 50mM NH4CL in PBS and permeabilised using 0.1% Triton-X100 in PBS. Cells were blocked using 2% BSA (Bovine Serum Albumin) in PBS and incubated in anti-Wg (1:1000; DSHB) in 1% BSA in PBS. After incubation with primary antibody the cells were washed in PBS before adding anti-mouse Alexa555 (1:500; Invitrogen) in 1% BSA in PBS. Finally cells were washed again in PBS and distilled water prior to mounting using Prolong Gold (Invitrogen). Cells were imaged using a Leica SP5 confocal microscope.
Disc stainings
For total wing disc stainings, wandering 3rd instar larvae were dissected in PBS and fixed for 20 minutes in 4% formaldehyde in PBS. Discs were washed in PBS, permeabilised in 1% Triton-X100 in PBS and blocked in 4% FCS in PBTx (PBS + 0.5% Triton-X100). Samples were incubated in primary antibodies overnight at 4°C in 4% FCS in PBTx, washed in PBTx, incubated in secondary antibodies (Alexa-488, Alexa-555 or Cy5 conjugated; Invitrogen) in 4% FCS in PBTx for 2 hours at room temperature, washed again and mounted in Vectashield (Vectorlabs). Primary antibodies used were anti-Wg (1:1000; DSHB), anti-Rab11 (1:25; Marcos Gonzalez-Gaitan), anti-Evi (1:1000; Konrad Basler) and anti-OLLAS (1:10; Abnova).
For extracellular stainings, larvae were chilled and dissected in ice-cold Schneiders medium prior to incubation in primary antibody diluted in Schneiders medium on ice for 2 hours. Samples were washed in ice-cold Schneiders medium and PBS and fixed for 40 minutes at room temperature in 4% formaldehyde in PBS. Discs were washed in PBS, permeablised using PBTx, blocked in 4% FCS in PBTx prior to incubation with secondary antibody (Alexa-488 or Alexa-555 conjugated; Invitrogen) diluted in 4% FCS in PBTx. Discs were washed again in PBTx prior to being mounted as above. Primary antibodies used were anti-Wg (1:300) and anti-OLLAS (1:2). Images were acquired using a Leica SP5 confocal microscope.
Haemolymph isolation
For each sample 25 wandering 3rd instar larvae were washed, dried and placed on ice prior. 20μl of ice-cold PBS was added and the larvae torn open. The resulting haemolymph in PBS was filtered using 0.22μm centrifugal filter units (Millipore). 18μl of filtered haemolymph was added to 4 × LDS sample buffer (Invitrogen) and boiled at 95°C for 5 min. 50mM DTT was added and the samples analysed by Western blot immediately.
Western blots
Cell lysates, S10, S100 or P100 samples were mixed with 4 × LDS sample buffer (Invitrogen) and boiled at 95°C for 5 min. 50 mM DTT was added and samples stored at −20°C. For analysis of cell lysates, S10 and S100 supernatants and P100 pellets, samples were run on 4-12% Bis-Tris NuPage gels with MOPS buffer alongside SeeBlue Plus2 Pre-Stained Standard molecular weight marker (Invitrogen). Proteins were transferred onto nitrocellulose membranes using an iBlot dry transfer machine (Invitrogen) for 10 min at 20V. Membranes were washed in dH20 prior to staining with Ponceau to assess protein loading and transfer and then washed again in dH20 to remove stain. Membranes were blocked in 5% dry milk in PBTween (Phosphate Buffered Saline + 0.1% Tween-20) and incubated in primary antibodies diluted in 5% milk overnight at 4°C. Membranes were washed in PBTween prior to incubation in HRP-conjugated secondary antibodies (anti-mouse, rabbit or guinea pig 1:5000; Biorad) for 2 hours at room temperature. Membranes were washed again in PBTween, developed using ECL (Amersham) and exposed to film. Densitometry analysis was performed using Fiji software.
For analysis of fractions from continuous sucrose density gradients, samples were run on 1% acrylamide/bisacrymalide (30%/0.2%) gels, blotted on nitrocellulose membranes using semi-dry transfer. Membranes were stained with Ponceau red to analyse loading and transfer, prior to incubation in 5% dry milk in Western buffer (50 mM TRIS pH7.5; 150 mM NaCl; 0.1% Tween20) for 2 hours at room temperature in the presence of primary antibody, then 1 hour with HRP-coupled secondary antibody. Membranes were developed using Pierce Pico or Dura kit.
Primary antibodies used were: anti-Wingless (1:1000; Developmental Studies Hybridoma Bank, DSHB), anti-Wnt3A (1:500; Cell Signaling Technologies), anti-Flotillin2 (1:1000; BD Transduction Laboratories), anti-Tsg101 (1:500; Abcam), anti-actin (1:1000; DSHB), anti-Evi (1:20,000; kindly provided by K. Basler), anti-GPR177 (1:2500; Lifespan Biosciences), anti-Syntaxin1A (1:500; DSHB), anti-Csp (1:1000; DSHB), anti-Rab35 (1:5000; Arnaud Echard), anti-HrsFL (1:20,000; kindly provided by H. Bellen) and anti-Vps28 (1:5000; Helmut Kramer). Western blots were quantified using ImageJ software.
Topflash signalling assay
S2R+ cells were plated in 6 well plates and transfected with 0.4 μg of a plasmid containing a Wingless-responsive promoter driving Firefly luciferase and a ubiquitous Copia promoter driving Renilla luciferase (made by Cyrille Alexandre). After 24 hours the S2R+ cells were counted and 3×105 cells were plated in each well of a 48 well plate and allowed to attach. After 3-4 hours the medium was removed and replaced by S10, S100 or P100 samples resuspended in exosome-free medium either in the original volume of the S10 sample to be at the same dilution or in a smaller volume to be more concentrated (45×). The P100 pellets were washed in cold PBS and respun at 100,000 g for 1 hour to remove any contaminating S100 sample prior to this. After 24 hours of treatment, the cells were lysed using cold passive lysis buffer (Promega), Firefly and Renilla luciferase levels were measured using the Dual Luciferase Reporter Assay System (Promega) and the Firefly/Renilla ratio calculated to give the Wg signalling activity. Each condition was tested in triplicate and the experiment was repeated four times.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Roel Nusse, Hugo Bellen, Konrad Basler, Michael Boutros, Marcos Gonzalez-Gaitan, Helmut Kramer, Mark Van Doren and Arnaud Echard for providing cell lines, antibodies or fly lines. We also thank the Large Scale Laboratory at the NIMR for help with cell culture and Andrew Bailey and Alex Gould for help with the haemolymph experiments. This work was funded by the Medical Research Council (K.B., L.P., C.A., H.G. and J.P.V.), a Marie Curie International Re-integration Grant (K.B.), a Wellcome Project Grant (K.B. and J.P.V.) an ATIP programme CNRS (R.L.B), Région Bretagne (Programme ACOMB ‘Notasid’ n° 2168) (R.L.B.) and the INCA (R. L. B and G.R.).
REFERENCES
- 1.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136(19):3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 2.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423(6938):448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 3.Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11(6):791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Current biology: CB. 2000;10(6):293–300. doi: 10.1016/s0960-9822(00)00378-x. [DOI] [PubMed] [Google Scholar]
- 5.Port F, Basler K. Wnt trafficking: new insights into Wnt maturation, secretion and spreading. Traffic. 2010;11(10):1265–1271. doi: 10.1111/j.1600-0854.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- 6.Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125(3):509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 7.Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125(3):523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Goodman RM, Thombre S, Firtina Z, Gray D, Betts D, Roebuck J, Spana EP, Selva EM. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133(24):4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- 9.Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87(5):833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]
- 10.Giraldez AJ, Cohen SM. Wingless and Notch signaling provide cell survival cues and control cell proliferation during wing development. Development. 2003;130(26):6533–6543. doi: 10.1242/dev.00904. [DOI] [PubMed] [Google Scholar]
- 11.Johnston LA, Sanders AL. Wingless promotes cell survival but constrains growth during Drosophila wing development. Nat Cell Biol. 2003;5(9):827–833. doi: 10.1038/ncb1041. [DOI] [PubMed] [Google Scholar]
- 12.Herranz H, Perez L, Martin FA, Milan M. A Wingless and Notch double-repression mechanism regulates G1-S transition in the Drosophila wing. The EMBO journal. 2008;27(11):1633–1645. doi: 10.1038/emboj.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baena-Lopez LA, Franch-Marro X, Vincent JP. Wingless promotes proliferative growth in a gradient-independent manner. Sci Signal. 2009;2(91):ra60. doi: 10.1126/scisignal.2000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97(5):599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- 15.Greco V, Hannus M, Eaton S. Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell. 2001;106(5):633–645. doi: 10.1016/s0092-8674(01)00484-6. [DOI] [PubMed] [Google Scholar]
- 16.Panakova D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435(7038):58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- 17.Mulligan KA, Fuerer C, Ching W, Fish M, Willert K, Nusse R. Secreted Wingless-interacting molecule (Swim) promotes long-range signaling by maintaining Wingless solubility. Proc Natl Acad Sci U S A. 2012;109(2):370–377. doi: 10.1073/pnas.1119197109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutty RK, Kutty G, Kambadur R, Duncan T, Koonin EV, Rodriguez IR, Odenwald WF, Wiggert B. Molecular characterization and developmental expression of a retinoid- and fatty acid-binding glycoprotein from Drosophila. A putative lipophorin. J Biol Chem. 1996;271(34):20641–20649. doi: 10.1074/jbc.271.34.20641. [DOI] [PubMed] [Google Scholar]
- 19.Johnstone RM. Exosomes biological significance: A concise review. Blood Cells Mol Dis. 2006;36(2):315–321. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. 2008;18(5):199–209. doi: 10.1016/j.tcb.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21(4):575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 25.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. Chapter 3:Unit 3 22. [DOI] [PubMed] [Google Scholar]
- 26.Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89(2):205–212. doi: 10.1016/j.biochi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 28.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8(19):4083–4099. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 29.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 31.Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 32.Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81(10):1171–1182. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 33.van den Heuvel M, Nusse R, Johnston P, Lawrence PA. Distribution of the wingless gene product in Drosophila embryos: a protein involved in cell-cell communication. Cell. 1989;59(4):739–749. doi: 10.1016/0092-8674(89)90020-2. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez F, Swales L, Bejsovec A, Skaer H, Martinez Arias A. Secretion and movement of wingless protein in the epidermis of the Drosophila embryo. Mech Dev. 1991;35(1):43–54. doi: 10.1016/0925-4773(91)90040-d. [DOI] [PubMed] [Google Scholar]
- 35.Zhai L, Chaturvedi D, Cumberledge S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J Biol Chem. 2004;279(32):33220–33227. doi: 10.1074/jbc.M403407200. [DOI] [PubMed] [Google Scholar]
- 36.Hoehne M, de Couet HG, Stuermer CA, Fischbach KF. Loss- and gain-of-function analysis of the lipid raft proteins Reggie/Flotillin in Drosophila: they are posttranslationally regulated, and misexpression interferes with wing and eye development. Mol Cell Neurosci. 2005;30(3):326–338. doi: 10.1016/j.mcn.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Katanaev VL, Solis GP, Hausmann G, Buestorf S, Katanayeva N, Schrock Y, Stuermer CA, Basler K. Reggie-1/flotillin-2 promotes secretion of the long-range signalling forms of Wingless and Hedgehog in Drosophila. EMBO J. 2008;27(3):509–521. doi: 10.1038/sj.emboj.7601981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139(2):393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, Leszyk J, Zhang B, Budnik V. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287(20):16820–16834. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neumann S, Coudreuse DY, van der Westhuyzen DR, Eckhardt ER, Korswagen HC, Schmitz G, Sprong H. Mammalian Wnt3a is released on lipoprotein particles. Traffic. 2009;10(3):334–343. doi: 10.1111/j.1600-0854.2008.00872.x. [DOI] [PubMed] [Google Scholar]
- 41.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. doi: 10.1038/ncb2000. sup pp 11-13. [DOI] [PubMed] [Google Scholar]
- 42.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40(Database issue):D1241–1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21(1):77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 44.Rana S, Zoller M. Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis. Biochem Soc Trans. 2011;39(2):559–562. doi: 10.1042/BST0390559. [DOI] [PubMed] [Google Scholar]
- 45.Port F, Kuster M, Herr P, Furger E, Banziger C, Hausmann G, Basler K. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol. 2008;10(2):178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- 46.Tamai K, Tanaka N, Nakano T, Kakazu E, Kondo Y, Inoue J, Shiina M, Fukushima K, Hoshino T, Sano K, Ueno Y, Shimosegawa T, Sugamura K. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun. 2010;399(3):384–390. doi: 10.1016/j.bbrc.2010.07.083. [DOI] [PubMed] [Google Scholar]
- 47.van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21(4):708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strauss K, Goebel C, Runz H, Mobius W, Weiss S, Feussner I, Simons M, Schneider A. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J Biol Chem. 2010;285(34):26279–26288. doi: 10.1074/jbc.M110.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6(2):131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 50.Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Gronborg M, Mobius W, Rhee J, Barr FA, Simons M. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189(2):223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koppen T, Weckmann A, Muller S, Staubach S, Bloch W, Dohmen RJ, Schwientek T. Proteomics analyses of microvesicles released by Drosophila Kc167 and S2 cells. Proteomics. 2011;11(22):4397–4410. doi: 10.1002/pmic.201000774. [DOI] [PubMed] [Google Scholar]
- 52.Fuerer C, Habib SJ, Nusse R. A study on the interactions between heparan sulfate proteoglycans and Wnt proteins. Dev Dyn. 2010;239(1):184–190. doi: 10.1002/dvdy.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coombs GS, Yu J, Canning CA, Veltri CA, Covey TM, Cheong JK, Utomo V, Banerjee N, Zhang ZH, Jadulco RC, Concepcion GP, Bugni TS, Harper MK, Mihalek I, Jones CM, et al. WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. J Cell Sci. 2010;123(Pt 19):3357–3367. doi: 10.1242/jcs.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, Maurice MM, Vincent JP. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10(2):170–177. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, Yan D, Selva EM, Lin X. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14(1):120–131. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Pan CL, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell. 2008;14(1):132–139. doi: 10.1016/j.devcel.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell. 2008;14(1):140–147. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Franch-Marro X, Marchand O, Piddini E, Ricardo S, Alexandre C, Vincent JP. Glypicans shunt the Wingless signal between local signalling and further transport. Development. 2005;132(4):659–666. doi: 10.1242/dev.01639. [DOI] [PubMed] [Google Scholar]
- 59.Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128(1):87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- 60.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 61.Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176(3):1534–1542. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 62.Franch-Marro X, Wendler F, Griffith J, Maurice MM, Vincent JP. In vivo role of lipid adducts on Wingless. J Cell Sci. 2008;121(Pt 10):1587–1592. doi: 10.1242/jcs.015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.