Abstract
Neurodegeneration of cholinergic and dopaminergic neurons is a major hallmark in Alzheimer’s or Parkinson’s disease, respectively. A dysregulation in calcium homeostasis may be part of this process and counteracting calcium influx may have neuroprotective properties in both diseases. Therefore, we investigated the putative neuroprotective or neurotoxic activity of L-type calcium channel (LTCC) inhibitors on cholinergic and dopaminergic neurons in a rat organotypic vibrosection model. Sagittal or coronal vibrosections (200 μm thick) of postnatal day 10 rats were cultured on 0.4 μm semipermeable membranes for 2 weeks with 10 ng/ml nerve growth factor (NGF) and/or glial-cell line derived neurotrophic factor (GDNF) to maintain survival of cholinergic or dopaminergic neurons, respectively. Thereafter, sections were incubated with 0.1, 1 or 10 μM isradipine, nicardipine or verapamil for 2 weeks to explore cytotoxicity. Alternatively, in order to explore neuroprotective activity, vibrosections were incubated without growth factors but with isradipine or verapamil or with nicardipine, nimodipine or nifedipine from the beginning for 4 weeks. Our data show that all LTCC inhibitors exhibited no neurotoxic effect on cholinergic and dopaminergic neurons. Further, LTCC inhibitors did not have any neuroprotective activity on cholinergic neurons. However, nimodipine and nifedipine significantly enhanced the survival of dopaminergic substantia nigra (SN) but not ventral tegmental area (VTA) neurons, while nicardipine, isradipine and verapamil had no effect. Nifedipine (and more potently GDNF) reduced inflammatory cytokines (macrophage inflammatory protein-2, tumor necrosis factor-α), but did not influence oxidative stress or caspase-3 activity and did not interfere with iron-mediated overload. Our data show that nifedipine and nimodipine are very potent to enhance the survival of axotomized SN neurons, possibly influencing inflammatory processes.
Keywords: L-type calcium channel inhibitor, Calcium, Neuroprotection, Neurotoxicity, Alzheimer’s disease, Parkinson’s disease
1. Introduction
Neurodegeneration of cholinergic and dopaminergic neurons represents a key pathology in Alzheimer’s (AD) or Parkinson’s (PD) disease pathology, respectively. The most affected areas regarding cholinergic cell loss in AD are the basal forebrain, such as nucleus Basalis of Meynert (nBM), septum and the diagonal band of Broca. In PD, dopaminergic neurodegeneration is predominantly evident in the substantia nigra pars compacta (SNc). The reason for this marked neuronal cell death remains still elusive. Nevertheless, increased evidence shows, that disturbances in calcium homeostasis might be involved in the progression of AD (Berridge, 2010) and PD (Surmeier, 2007; Chan et al., 2009) and that calcium channels may play a neuroprotective role.
Voltage-gated L-type calcium channels (LTCC) are expressed outside the cardiovascular system and in the brain and are formed by the CaV1 family (Striessnig et al., 2006). The CaV1 family (CaV1.1–CaV1.4) can be distinguished from other LTCCs by their high sensitivity to organic calcium channel blockers and activators, such as dihydropyridines (DHP) (Striessnig et al., 2006). LTCC blockers are commonly used to treat hypertension and other cardiovascular disorders. Recently, more and more evidence comes up that LTCC blockers may be effective therapeutic targets in neurodegenerative disorders such as AD (Copenhaver et al., 2011) or PD (Surmeier, 2007; Chan et al., 2009).
It is well known that the LTCC blockers counteract neurodegeneration in AD (Copenhaver et al., 2011) or PD (Surmeier, 2007) animal models in vivo. Usually LTCC blockers, e.g. isradipine, are applied via subcutaneous time-release pellets (Surmeier, 2007). However, the relation between dose and serum levels as well as the entry into the brain remains unclear, especially, that some LTCC inhibitors have a low stability. In fact, isradipine e.g. undergoes extensive first-pass metabolism and nearly 90% of orally administered isradipine is absorbed in the digestive tract (Anekonda and Quinn, 2011). A sharp peak level occurs in plasma about 2 h after administration with a terminal half-life of 8 h (Anekonda and Quinn, 2011). Thus, in order to better understand the direct effects of LTCC inhibitors on brain neurons, in vitro studies are very important. Hence, the aim of the present study was to investigate if LTCC inhibitors may exhibit neuroprotective or neurotoxic activity on cholinergic and dopaminergic neurons. We will use well established organotypic rat brain vibrosections, where we can follow up survival of neurons for up to 4 weeks.
2. Results
2.1. Dopaminergic and cholinergic neurons in vibrosections
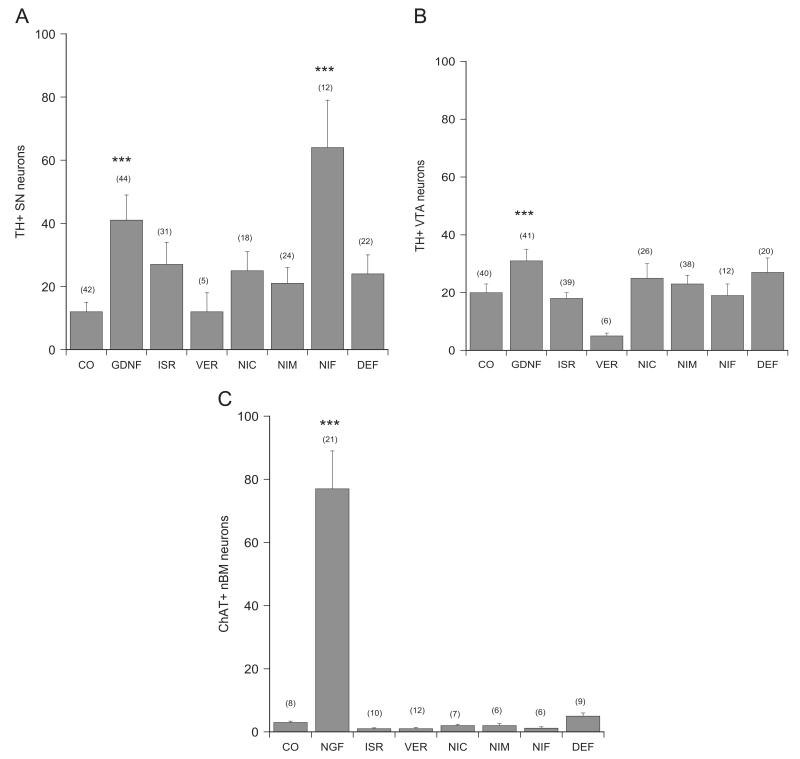
Dopaminergic neurons were stained by immunohistochemistry for tyrosine hydroxylase (Fig. 1A, B, D and F) and 41±8 (n=44) SNc neurons and 31±4 (n=41) VTA neurons were counted when incubated with GDNF (Fig. 1F; Fig. 2A and B). Addition of DMSO equivalent did not change the cell number (48±9 SNc neurons, n=30 and 44±7 VTA neurons, n=32). Incubation of vibrosections without GDNF displayed 12±3 (n=42) SNc neurons and 20±3 (n=40) VTA neurons (Fig. 1D; Fig. 2A and B).
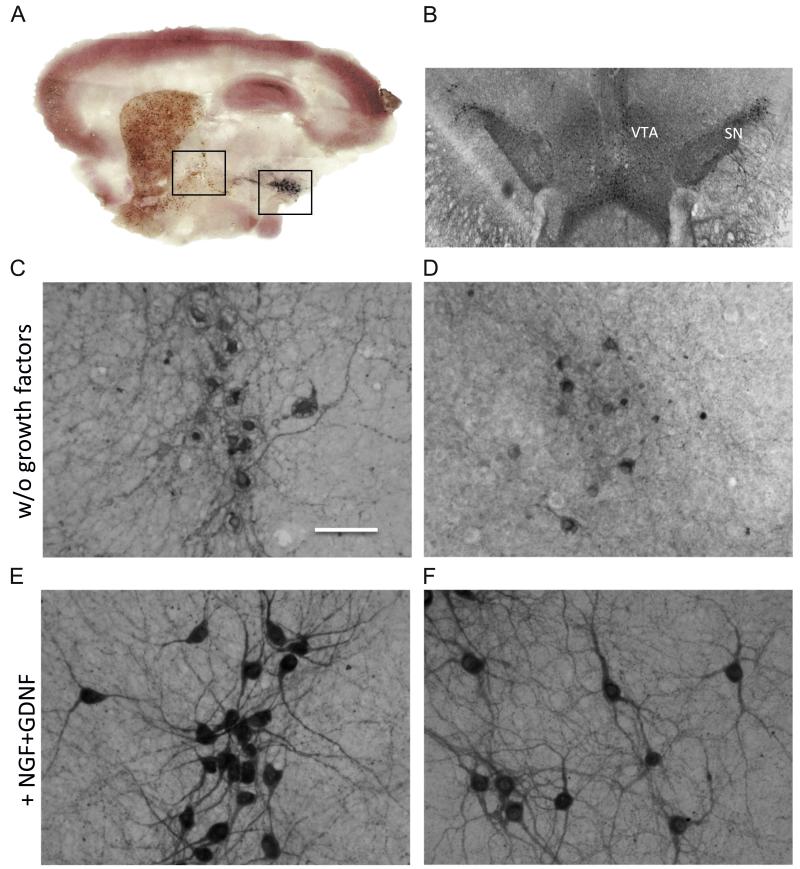
Fig. 1.
A sagittal vibrosection (A) shows cholinergic and dopaminergic neurons stained by immunohistochemistry for choline acetyltransferase (brown) or tyrosine hydroxylase (black), respectively. This vibrosection was counterstained with calbindin (red) to show the brain structures. A coronal vibrosection (B) shows tyrosine-hydroxylase positive dopaminergic neurons of the SN and VTA. When vibrosections were incubated without growth factors, only a few not healthy cholinergic nBM (C) or dopaminergic SNc (D) neurons could be observed. However, when vibrosections were incubated with 10 ng/ml NGF and GDNF the number of healthy cholinergic (E) and dopaminergic (F) neurons markedly increased. Scale bar in C=1800 μm (A), 930 μm (B), 80 μm (C–F).
Fig. 2.
Quantification of neuronal numbers in brain slices after incubation with LTCC inhibitors. Brain slices were incubated without (control, CO) or with 10 ng/ml glial-cell line derived neurotrophic factor (GDNF) or nerve growth factor (NGF) or with 10 μM LTCC inhibitors isradipine (ISR), verapamil (VER), nicardipine (NIC), nimodipine (NIM) or nifedipine (NIF) or 100 μM iron chelator deferoxamine (DEF) for 4 weeks. Slices were then immunohistochemically stained for tyrosine hydroxylase (TH; A and B) or choline acetyltransferase (ChAT; C) and the number of substantia nigra (SN; A), ventral tegmental area (VTA; B) or nucleus basalis of Meynert (nBM; C) neurons were counted. Values are given as mean±SEM; n gives the number of independent slices. Statistical analysis was performed by one-Way ANOVA with a subsequent Bonferroni posthoc test (***p<0.001).
Cholinergic neurons were stained by immunohistochemistry for choline acetyltransferase (Fig. 1A, C, E) and 48±6 (dorsal striatum), 65±9 (ventral striatum) and 77±12 (nBM) neurons (all n=21) were counted when incubated with NGF (Fig. 1E and Fig. 2C). Addition of DMSO equivalent did not change the cell number (71±10 neurons in doStr; 58±9 neurons in vStr; 49±6 neurons in nBM; all n=12). Incubation of vibrosections without NGF displayed only 10±2 doStr neurons (n=17), 22±5 vStr neurons (n=16) and 3±0.4 nBM neurons (n=8) (Figs. 1C and 2C).
2.2. Neurotoxicity assay
Sagittal vibrosections were cultured for 2 weeks with NGF and GDNF and then growth factors were removed for 3 days. After this, the vibrosections were incubated with either nicardipine (0.1–10 μM), verapamil (0.1–10 μM) or isradipine (0.1–10 μM) or without drugs for 2 weeks. No signs of neurodegeneration of cholinergic or dopaminergic neurons after drug treatment were observed compared to controls (56±7 doStr ChAT+ neurons, n=21; 71±11 vStr ChAT+neurons, n=21, 78±12 nBM ChAT+neurons, n=22; 83±22 vMES (SNc+VTA) TH+neurons, n=18).
2.3. Neuroprotection assay
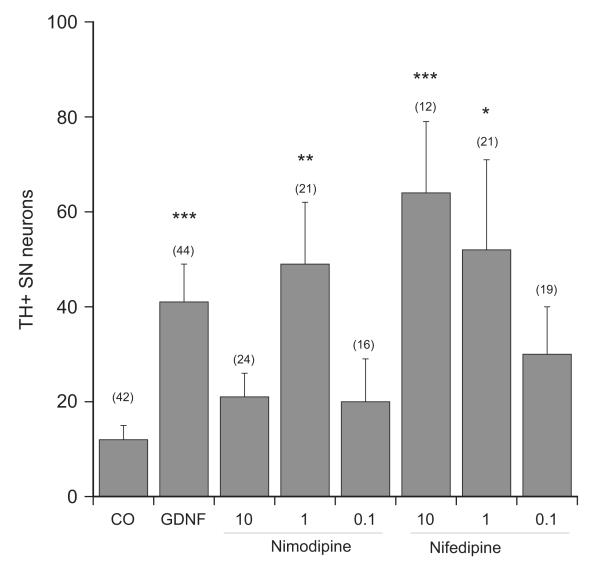
In order to determine any neuroprotective activity of the LTCC inhibitors on SN or VTA neurons coronal vibrosections were cultured. Incubation of coronal sections with 10 ng/ml GDNF significantly improved survival of dopaminergic SNc and VTA neurons (Fig. 2A and B). Incubation of slices with 10 μM nifedipine significantly enhanced the survival of SNc but not VTA neurons (Fig. 2A and B). This effect was dose-dependent (Fig. 3). When slices were incubated with nimodipine only a concentration of 1 μM increased the number of SNc neurons (Figs. 2A and 3). Verapamil, nicardipine or isradipine (10–1–0.1 μM) had no effect on the survival of dopaminergic SNc and VTA neurons (Fig. 2A and B; data for 1 and 0.1 μM are not shown). Incubation of sagittal vibrosections with all LTCC inhibitors had no neuroprotective activity on cholinergic neurons at all concentrations tested (Fig. 2C).
Fig. 3.
Dose response curve of nimodipine and nifedipine. Brain slices were incubated without (control, CO) or with 10 ng/ml glial-cell line derived neurotrophic factor (GDNF) or with 10–1–0.1 μM nimodipine (NIM) or nifedipine (NIF) for 4 weeks. Slices were then immunohistochemically stained for tyrosine hydroxylase (TH) and the number of substantia nigra (SN) neurons was counted. Values are given as mean±SEM; n gives the number of independent slices. Statistical analysis was performed by one-Way ANOVA with a subsequent Bonferroni posthoc test (*p<0.05; **p<0.01; ***p<0.001).
2.4. Effects of the iron-chelator deferoxamine
Incubation of brain slices with the iron-chelator deferoxamine at a dose of 100 μM did neither protect dopaminergic SNc nor VTA nor cholinergic nBM neurons (Fig. 2A–C). Deferoxamine at a dose of 1 mM exhibited severe toxicity to dopaminergic neurons in the slices.
2.5. Effects of nifedipine on LDH release
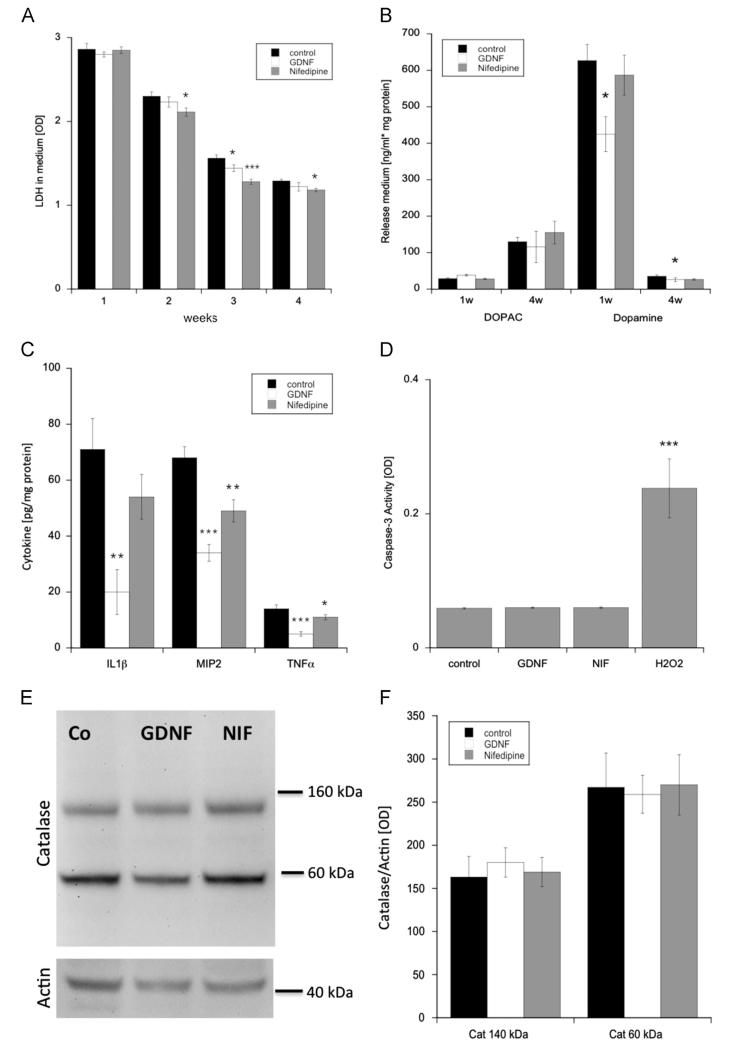
Cell viability was tested by measuring the levels of LDH in medium. After 1 week of incubation, no severe degeneration could be observed with the treatment of nifedipine compared to controls. However, from the second week on, the addition of nifedipine led to a significant decrease of LDH levels compared to controls, with the lowest value in week 4. In week 3, the amount of LDH was found to be significantly reduced by the addition of GDNF (Fig. 4A).
Fig. 4.
Effects of nifedipine (gray bars) on apoptosis, inflammation and oxidative stress during and after 4 weeks of incubation compared to glial-cell line derived neurotrophic factor (open bars) and controls (black bars). Cell viability was tested by investigating slice media for lactate dehydrogenase (LDH) (A, n=10), as well as for DOPAC and dopamine release (B, n=6). Slice extracts were evaluated for proinflammatory cytokines (interleukin-1β, IL-1β; macrophage inflammatory protein-2, MIP-2; tumor necrosis factor-α, TNF-α (C, n=6). Apoptosis was measured by examining caspase-3 activity in slice extracts (D, n=10). Western Blot analysis of catalase (2 isoforms at 60 and 140 kDa) performed on slice extracts revealed the level of oxidative stress (E and F, n=8). Values are given as mean±SEM optical density (A, D, F) or ng/ml correlated to 1 mg protein (B) or pg per 1 mg protein. Western Blots were normalized to actin expression (E and F). Statistical analysis was performed by one-way ANOVA with a subsequent Bonferroni posthoc test (*p<0.05; **p<0.01; ***p<0.001).
2.6. Effects of nifedipine on dopamine/DOPAC release
Cell viability was further tested by investigating DOPAC and dopamine levels in medium after 1 and 4 weeks of incubation. While the amount of DOPAC stayed more or less the same between controls, GDNF and nifedipine treatment, the level of dopamine was statistically decreased by the treatment of GDNF compared to controls in either 1 week or 4 weeks of incubation (Fig. 4B).
2.7. Effects of nifedipine on cytokines
The proinflammatory cytokine markers IL-β, MIP2 and TNFα were used to determine the level of inflammation in homogenates from brain slices. The addition of GDNF to brain slices led to a significant downregulation of all tested proinflammatory markers. Similarly, the treatment with nifedipine showed a significant decrease for the cytokines MIP2 and TNF-α (Fig. 4C).
2.8. Effects of nifedipine on caspase-3
Apoptotic processes were investigated by measuring caspase-3 activity in brain extracts. The treatment with either GDNF or nifedipine showed no effect on caspase-3 activity. However, hydrogen peroxide acutely added to brain slices led to a strong enhancement of caspase-3 level compared to controls (Fig. 4D).
2.9. Effects of nifedipine on catalase
Western blot analysis of catalase expression in brain slice extracts revealed no differences between either the 60 kDa or the 140 kDa band expression of GDNF or nifedipine treated slices compared with controls (Fig. 4E and F).
3. Discussion
The present study demonstrates that LTCC inhibitors did not exert any neurotoxic effects on cholinergic or dopaminergic neurons, and did not have any neuroprotective activity on cholinergic neurons. However, nimodipine and nifedipine had a more pronounced neuroprotection of dopaminergic SNc but not VTA neurons, while nicardipine, isradipine and verapamil did not have any neuroprotective activity. The effect of nimodipine and nifedipine on axotomy-induced cell death may modulate inflammatory processes.
3.1. The vibrosection model
The organotypic brain slice model has been optimized and characterized since several years (Crain et al., 1982; Gähwiler and Hefti, 1984; Stoppini et al., 1991; Buchs et al., 1993; Ostergaard et al., 1990; Gähwiler et al., 1997, 1990). Slices can be cultured as single slices from a respective brain area or as a co-culture where two functional related brain slices are connected. The culturing of organotypic brain slices on membranes is well established in our research group (Humpel et al., 1995; Schatz et al., 1999, 2000; Weis et al., 2001; Humpel and Weis, 2002). We have strong experience in culturing mesencephalic dopaminergic neurons (Schatz et al., 1999), cholinergic nBM neurons (Weis et al., 2001), dorsal raphe serotonergic neurons (Hochstrasser et al., 2011), or cells from cortical or hippocampal slices. Moreover, co-cultures of the nBM together with the cortex (Humpel and Weis, 2002) or the vMes together with the dorsal striatum (dStr) (Schatz et al., 1999) provide an excellent system to study interaction between two brain regions. Recently, we also improved this slice model and provided a large slice containing 4 functionally related brain areas (Ullrich and Humpel, 2009a, 2009b) or whole brain sections (Ullrich et al., 2011). Such slices allow to study developmental aspects, survival of neurons, neurodegeneration or the effect of toxins or trophic molecules.
3.2. Neurotoxicity or neuroprotective assay
The organotypic slice model represents an axotomy-induced model, because dopaminergic or cholinergic neurons are axotomized due to dissection and culturing. Thus, these neurons will undergo rapid cell death in the slices within days. In order to prevent this axotomy-induced neurodegeneration we cultured slices with NGF (Humpel and Weis, 2002) or GDNF (Schatz et al., 1999) to enhance survival of cholinergic and dopaminergic neurons, respectively. This model allows us to use a neurotoxicity and a neuroprotection assay. In order to study neurotoxic effects, we cultured freshly isolated slices for 2 weeks with the growth factors, which results in survival of approximately 80–100 neurons/slice. It is well established that these neurons remain stable for another 3–4 weeks without growth factors. Thus, we withdrew the growth factors and added LTCC blockers to study any neurotoxic effects. Alternatively, this model allows to explore neuroprotection. It is well established that cholinergic and dopaminergic neurons degenerate when incubated from the beginning without any growth factors (axotomy-induced cell death). Thus, in order to study neuroprotection, we added growth factors or LTCC blockers from the beginning to the slices and tested any neuroprotective activity after 4 weeks.
3.3. LTCC blockers do not display neurotoxic properties in vitro
It has been reported that chronic treatment of midbrain slice cultures with the LTCC blockers nicardipine (3–10 μM) or verapamil (10 μM) for 18 days resulted in a drastic decrease of dopaminergic neurons (Katsuki et al., 2001). This seems to be a discrepancy to our data where we do not see any neurotoxicity using all LTCC blockers in our vibrosection model. However, they (Katsuki et al., 2001) report that the neurotoxic effect is only seen when the LTCC blockers were added to the slices from the beginning of the cultures (that means from day 0 up to day 8 or to day 18). However, when they (Katsuki et al., 2001) added the LTCC blocker starting from day 10 to day 18, they did not observe any neurotoxic effects. Thus, these data clearly point to a severe toxicity of the LTCC blockers at the beginning of the cultures, where the neurons are very sensitive. It is important to note that the slice model represents an axotomy-induced model, where neurons are markedly damaged only due to the dissection of the brains. Thus, any treatment strategy at this time point with acute slices seems to be unphysiological. In order to circumvent this problem, we used in our model growth factors (e.g. GDNF in the case of dopaminergic neurons) to enhance the survival of dopaminergic neurons from the beginning. We used LTCC blockers only in cultures with healthy fully stabilized neurons after 2 weeks of GDNF treatment. Thus, our data are in agreement with that of Katsuki et al. (2001) but only when LTCC blockers are added to healthy stabilized neurons, where these LTCC blockers do not exhibit any neurotoxicity.
3.4. LTCC blockers do not protect cholinergic neurons
It has been demonstrated that LTCC activity may contribute substantially to memory loss and neurodegeneration associated with dementia (Copenhaver et al., 2011) and LTCC antagonists can prevent AD or slow its progression. Clinical studies with nimodipine fail to show effects in AD patients, but isradipine may represent a superior alternative (Copenhaver et al., 2011). In fact, chronic treatment of mice with isradipine pellets (3 μg/g/day, 60 days) inhibited the accumulation of beta-amyloid and phospho-tau in a triple transgenic AD model (Copenhaver et al., 2011). To our knowledge the present study is the first to investigate the direct effects of LTCC blockers on cholinergic neurons. We clearly show that none of the tested LTCC blockers have any neuroprotective activity on cholinergic striatal interneurons or on nBM neurons. However, we cannot exclude any effects on septal neurons, since those were not studied in this model. Further, as striatal cholinergic neurons are interneurons, axotomy-induced mechanisms may only partly play a role in this process.
3.5. Neuroprotection of dopaminergic neurons by LTCC blockers
In order to explore the effects of LTCC blockers in vitro, we decided to culture the vMes as coronal sections, where we can differentiate between SNc and VTA. Our data clearly show that verapamil and isradipine and also nicardipine did not exert any significant neuroprotective activity on dopaminergic SNc and VTA neurons. However, our data provide evidence that nimodipine (1 μM) and nifedipine (1 and 10 μM) markedly enhanced the survival of SNc but not VTA neurons. Our data are partly in contrast to studies showing a potent effect of isradipine on 6-OHDA-induced cell death of dopaminergic neurons (Surmeier, 2007). Verapamil has been shown to significantly inhibit LPS-induced dopamine neurotoxicity (Liu et al., 2011), but it did not have any effect on axotomy-induced cell death in our model. On the other hand two further DHP analogons, nimodipine and nifedipine exerted a strong protection on dopaminergic neurons, which was as strong as the effect of GDNF. This effect was dose-dependent and also restricted to the substantia nigra. Finally, previous studies have shown that dopaminergic neurons require a certain level of activity to survive either in primary or slice midbrain cultures and that activation of LTCCs may be required in this process (Katsuki et al., 2001; Michel et al., 2013). This suggests that protection by LTCC blockers may be dependent on the experimental context.
3.6. Neuroprotective activity of nifedipine and nimodipine
The mechanism how nifedipine/nimodipine may counteract axotomy-induced neurodegeneration is unclear. As the other DHP analogons did not exert a similar neuroprotective activity, we suggest a mechanism independent of the calcium channel blocking. Thus, in order to get some insights into neuroprotective mechanisms, we studied iron-overload, oxidative stress, apoptosis, and inflammation. To test cell viability, we examined the release of LDH and dopamine, as well as its metabolite DOPAC into the medium. Axotomy clearly induced an acute degenerative response, which decreased during longer incubation periods. GDNF and nifedipine significantly counteracted the release of LDH into medium, pointing to its neuroprotective effects. The measurement of dopamine showed a marked release during the first week, suggesting the acute axotomy-induced damage of dopaminergic neurons. No further release of dopamine was visible in week 4, but the release of DOPAC was enhanced at week 4, reflecting increased metabolism. The analysis of dopamine/DOPAC, however, was not sensitive enough to see any marked differences between nifedipine/GDNF and controls.
The vMes is a brain area with high iron contents and high sensitivity to iron-induced oxidative stress. Moreover, it is well known that the iron content of the substantia nigra pars compacta increases in the brains of Parkinson’s disease patients. Thus, iron-induced toxicity may account for the neuronal cell death of dopaminergic neurons (Ben-Shachar et al., 1991; Ma et al., 2012). Previous studies have shown that the iron chelators deferiprone and deferoxamine and defer-asirox exert neuroprotection in the 6-OHDA Parkinson model (Ben-Shachar et al., 1991, 2004; Dexter et al., 2011). Recently, it has been reported that nifedipine restores the number of TH-positive neurons reduced by iron dextran overload and prevents increase of iron content in the SN (Ma et al., 2012). Ludwiczek et al. (2007) have shown that the LTCC blocker nifedipine enhanced the divalent metal transporter at concentrations of 1–100 μM. In order to study iron-induced effects, we treated the brain slices from the beginning with the iron-chelator deferoxamine, and show that it did not counteract axotomy-induced cell death of SNc and VTA neurons. This is consistent with others, showing that the iron-chelator deferoxamine failed to provide protection to vulnerable dopaminergic neurons (Rousseau et al., 2013). Thus, we conclude that nifedipine and nicardipine do not exhibit their neuroprotective activity via an iron overload.
It seems possible that the DHP drugs may counteract oxidative stress of SNc neurons after axotomy. In fact, nifedipine has been shown to exhibit antioxidant properties (Yao et al., 2000; Smith and Cass, 2007). It has been demonstrated that the pretreatment of PC12 cells with nifedipine prior to the mitochondrial toxin rotenone decreased the intracellular dopamine levels and ROS formation, increased the cell viability and neurite outgrowth (Sai et al., 2013). In NGF-differentiated PC12 cells, it has been clearly shown that nimodipine (1–100 μM) displayed up to 65% neuroprotection against oxygen–glucose deprivation or NGF withdrawal (Lecht et al., 2012). Nifedipine also displayed neuroprotection but at a lower potency and only at 100 μM (Lecht et al., 2012). Interestingly, nimodipine conferred neuroprotection only in a narrow therapeutic time window within the first 5 h (Lecht et al., 2012). Caspase-3 activity, a hallmark for mitochondrial dysfunction, was tested to analyze apoptosis. It has already been shown, that nifedipine can protect insulin-secreting pancreatic β-cells from caspase-3 activation treated with high doses of either glucose or toxic etoposide (Arora et al., 2013; Syeda et al., 2013). However, neither GDNF nor nifedipine induced caspase-3 activity in our brain slice extracts. Thus, our data provide evidence that nifedipine does neither affect apoptotic nor oxidative stress mechanism to counteract the axotomy-induced neurodegeneration.
Finally, in order to investigate inflammation we tested the effects of nifedipine on three different proinflammatory cytokines: interleukin-1β, macrophage inflammatory protein-2 and tumor necrosis factor-α. Our data show that nifedipine revealed a significant downregulation of MIP2 and TNFα compared to controls. GDNF was even more potent than nifedipine and also counteracted IL1β. Thus, our data support the findings of Li et al. (2008) showing that nifedipine (and possibly nimodipine) protect dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation.
Taken together, our data demonstrate that LTCC inhibitors do not exert any neurotoxicity on dopaminergic and cholinergic neurons, but do also not exhibit neuroprotective activity on cholinergic neurons. However, nimodipine and nifedipine were both very potent to enhance the survival of dopaminergic SNc but not VTA neurons in an axotomy-induced model. The data suggest that these DHP drugs may have an important relevance in counteracting dopaminergic neurodegeneration.
4. Experimental procedures
4.1. Organotypic vibrosection cultures
Vibrosections were performed as described in detail recently (Ullrich et al., 2011). For vibrosection cultures postnatal day 10 Sprague-Dawley rats were used. All experiments were approved by the Austrian Ministry of Science and Research and conformed to the Austrian guidelines on animal welfare and experimentation. The animals were rapidly sacrificed, the brains dissected and sagitally (Fig. 1A) or coronally (Fig. 1B) cut. The brains were glued (Glue Loctite 404) onto the chuck of a water cooled vibratome Leica VT1000A, and triggered close to a commercial shave racer. Under aseptic conditions, 200 μm vibrosections were cut and collected in sterile medium. The organotypic vibrosections were carefully placed onto a sterile 0.4 μm pore membrane (Millipore HTTP02500) which was then placed into a 0.4 μm membrane insert (Millipore PICM03050) within a 6-well plate. Vibrosections were cultured in 6-well plates (Greiner) at 37 °C and 5% CO2 with 1.2 ml/well of the following culture medium: 50% MEM/HEPES (Gibco), 25% heat inactivated horse serum (Gibco/Lifetech, Austria), 25% Hank’s solution (Gibco), 2 mM NaHCO3 (Merck, Austria), 6.5 mg/ml glucose (Merck, Germany), 2 mM glutamine (Merck, Germany), pH 7.2. Medium was fully changed once per week (Monday) or 200 μl were added once per week (Friday). For neurotoxicity assay, vibrosections were cultured for 2 weeks with 10 ng/ml NGF or GDNF to maintain survival of cholinergic or dopaminergic neurons, respectively. Thereafter sections were incubated with 0.1, 1 or 10 μM LTCC inhibitors for 2 weeks. Alternatively for neuroprotection assays, vibrosections were incubated with the LTCC inhibitors from the beginning for 4 weeks. As a control NGF/GDNF was added alone, or slices were incubated without any growth factors of with the highest DMSO equivalent. For some experiments, slices were incubated with the iron-chelator deferoxamine mesylate (Sigma-Aldrich, D9533) at a dose of 1–1000 μM and with hydrogen peroxide (Hochstrasser et al., 2011). Nicardipine (Sigma-Aldrich, N7510), verapamil (Sigma-Aldrich, V4629), isradipine (Sigma-Aldrich, I6658), nifedipine (Sigma N7634) and nimodipine (Sigma N149) were dissolved in Dimethylsulfoxid (DMSO) (Merck) at 1 mg in 100 μl. Chemicals were further diluted in medium to 0.1, 1 or 10 μM. The highest DMSO equivalent was 0.52 μl DMSO per 1 ml medium.
4.2. Immunohistochemistry
Immunohistochemistry was performed as previously described (Ullrich et al., 2011). All incubations were performed free floating for 2 days including 0.1% Triton, such that the antibodies can penetrate from both sides into the vibrosections. Vibrosections were washed 30 min with 0.1% Triton/Phosphate buffered saline (T-PBS) at room temperature and pre-treated 20 min with 20% methanol/1% H2O2/PBS. After thorough rinsing, the vibrosections were blocked with 20% horse serum/0.2% BSA/T-PBS and then incubated for 2 days at 4 °C with primary antibodies against choline acetyltransferase (ChAT, 1:750, Millipore AB144P, raised against human placental enzyme) or tyrosine hydroxylase (TH, 1:500, Millipore AB152, from rat pheochromocytoma). Then, the vibro-sections were washed again with PBS and incubated with secondary biotinylated anti-goat (ChAT) or anti-rabbit (TH) antibodies (1:200, Vector Lab., USA) for 1 h at room temperature. Following further washing steps with PBS, vibrosections were incubated in an avidin-biotin complex solution (ABC-Elite Vectastain reagent Vector Lab.) for 1 h. After being washed with 50 mM Tris-buffered saline (TBS), the signal was detected by using 0.5 mg/ml 3,3′-diaminobenzidine (DAB) including 0.003% H2O2 as a substrate in TBS. The vibrosections were mounted on glass slides, air dried and coverslipped with Entellan (Merck, Germany). Unspecific staining was defined by omitting the primary antibody. Immunolabeling was visualized with a Leica DMIRB fluorescence inverse microscope equipped with an Apple computer and Openlab software.
4.3. Triple immunohistochemical staining
Organotypic vibrosections were first processed for ChAT immunohistochemistry and stained with DAB-nickel giving a black color. Sections were then washed in PBS, blocked with the avidin/biotin blocking kit (Vector SP-2001), and then processed for TH immunohistochemistry. Dopaminergic neurons were stained with Vector SG substrate (SK-470) giving a gray color. Sections were then again washed in PBS and blocked with the avidin/biotin blocking kit and were then processed for mouse-anti-calbindin immunohistochemistry (abcam, 1:250) and stained with the Vector VIP substrate (SK-4600) giving a violet color.
4.4. Measurement of LDH
Cell viability was assessed by lactate dehydrogenase (LDH) cytotoxicity assay (Roche) as previously described (Hochstrasser et al., 2011). Briefly, 100 μl of brain slice media were mixed with 100 μl of reaction mixture and incubated at 20 °C in the dark for 30 min. Light absorbance was measured at 490 nm with an ELISA reader.
4.5. Analysis of dopamine/DOPAC
DOPAC and dopamine in medium were measured by high-performance liquid chromatography (HPLC) and electrochemical detection. Samples were thawed and 10 μl of a 2 M perchloric acid were added to 100 μl extracts, vortexed and centrifuged at 16,000 × g for 5 min at 4 °C. The supernatant (20 μl) was injected onto the HPLC. The samples were separated on a reversed-phase C18 Nucleosil column (Bartelt, Graz, Austria) at a flow rate of 0.8 ml/min using the following mobile phase: 0.05 M trichloric acid (Merck), 0.26 mM EDTA (Merck), 1.36 mM NaCl (Roth), 1.81 mM heptane sulfonic acid (Sigma), and 8% acetonitril (BDH Prolabo, Vienna, Austria) in HPLC water. Detection was performed with an electrochemical detector (Antec II, Leyden, Netherlands) at +0.55 V and 30 °C. All unknown samples were correlated to external standards of DOPAC and dopamine (both Sigma) by measuring peak areas.
4.6. ELISAs for cytokines
Brain slices were dissolved in PBS containing a protease inhibitor cocktail (Sigma), and sonicated on ice (10 s, 125 W/cm2, 140 μm amplitude, 100%), and centrifuged (10 min, 4 °C, 14,000g). These supernatants were analyzed for the inflammatory markers interleukin-1β (IL-1β), macrophage inflammatory protein-2 (MIP-2) and tumor necrosis factor-α (TNF-α) by using the Thermo Scientific SearchLight Protein Array Technology (THP Medical Products, Vienna, Austria) as described recently (Hochstrasser et al., 2011). Briefly, 50 μl standards or brain extracts were added to coated wells and incubated for 3 h. After a washing step, the biotinylated antibodies were added and subsequently incubated for 30 min. Then, wells were washed again and incubated with streptavidin-horseradish peroxidase conjugate. After the final washing step, the SuperSignal Chemiluminescent Substrate was added. All incubation steps were carried out on a shaker at room temperature. The luminescent signal was detected using a CCD imaging and analysis system. The concentration of each sample was quantified by comparing the spot intensities with corresponding standard curves calculated from the standard sample results using the SearchLight Array Analyst software.
4.7. Western blot analysis for catalase
Western blot analysis was performed as described previously (Hochstrasser et al., 2011). The brain extracts (see 4.5) were used and total protein was determined by the Bradford method with Coomassie brilliant blue G250 dye (Bio-Rad, Vienna, Austria). Brain extracts (25 μg) were loaded onto 10% Bis-Tris polyacrylamide gel (Invitrogen) and electrophoresis was performed for 30 min at 200 V. Samples were electrotransferred to nylon PVDF Immobilon-PSQ membranes (Millipore) for 90 min at 30 V with 20% methanol blotting buffer (Invitrogen). For detection, the Western Breeze Chemiluminescent System (Invitrogen) was used. Blots were blocked for 30 min with blocking buffer and then incubated overnight at 4 °C with the primary antibody anti-catalase (1:10,000; Thermo Scientific, Rockford, IL) or anti-actin (1:500; Sigma). After that, blots were washed and incubated with alkaline phosphatase-conjugated anti-rabbit antibodies for 30 min at room temperature. Then, blots were washed again and subsequently incubated in CDP-Star chemiluminescent substrate solution (Invitrogen), and the signal was visualized with a cooled CCD camera (SearchLight; Thermo Scientific).
4.8. Caspase-3 assay
To investigate apoptotic processes , caspase-3 activity was measured with a Caspase-3/CPP32 Colorimetric Assay Kit (BioVision, Mountain View, CA) according to manufacturer’s instructions. Briefly, 50 μl of a 2× reaction buffer containing 10 mM dithiothreitol was added to each sample (20 μg) along with 5 μl of 4 mM DEVD-pNA substrate (200 μm final concentration) and incubated for 4 h at 37 °C. The samples were read at 405 nm with an ELISA microplate reader.
4.9. Analysis, quantification and statistics
The brain areas were identified by the respective immunohistochemial stainings. The immunoreactive neurons were photographed in the striatum, nBM or vMes using a 20× objective. The digital image was then processed by computer-assisted image analysis. The multistatistical analysis was obtained by one-way ANOVA, followed by a Bonferroni posthoc test. Controls were compared against the respective treatments, where p<0.05 represents statistical significance.
Acknowledgments
This study was supported by the Austrian Science Funds (SFB F04405-B19). We thank Ursula Kirzenberger-Winkler and Karin Albrecht for excellent technical assistance. We thank Prof. Jörg Striessnig for carefully reading the MS.
Footnotes
Declarations of the Authors The authors declare that they have no competing interests. The corresponding author declares that the MS has not been submitted elsewhere.
REFERENCES
- Anekonda TS, Quinn JF. Calcium channel blocking as a therapeutic strategy for Alzheimer’s disease: the case for isradipine. Biochim. Biophys. Acta. 2011;1812(12):1584–1590. doi: 10.1016/j.bbadis.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora DK, Mohammed AM, Kowluru A. Nifedipine prevents etoposide-induced caspase-3 activation, prenyl transferase degradation and loss in cell viability in pancreatic β-cells. Apoptosis. 2013;18(1):1–8. doi: 10.1007/s10495-012-0763-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar D, Eshel G, Finberg JP, Youdim MB. The iron chelator desferrioxamine (Desferal) retards 6-hydroxydopamine-induced degeneration of nigrostriatal dopamine neurons. J. Neurochem. 1991;56(4):1441–1444. doi: 10.1111/j.1471-4159.1991.tb11444.x. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar DB, Kahana N, Kampel V, Warshawsky A, Youdim MB. Neuroprotection by a novel brain permeable iron chelator, VK-28, against 6-hydroxydopamine lession in rats. Neuropharmacology. 2004;46(2):254–263. doi: 10.1016/j.neuropharm.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium hypothesis of Alzheimer’s disease. Pflüg. Arch.—Eur. J. Physiol. 2010;459:441–449. doi: 10.1007/s00424-009-0736-1. [DOI] [PubMed] [Google Scholar]
- Buchs PA, Stoppini L, Muller D. Structural modifications associated with synaptic development in area CA1 of rat hippocampal organotypic cultures. Brain Res. Dev. 1993;71:81–91. doi: 10.1016/0165-3806(93)90108-m. [DOI] [PubMed] [Google Scholar]
- Chan CS, Gertler TS, Surmeier DJ. Calcium homeostasis, selective vulnerability and Parkinson’s disease. Trends Neurosci. 2009;32:249–256. doi: 10.1016/j.tins.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhaver PF, Anekonda TS, Musashe D, Robinson KM, Ramaker JM, Swanson TL, Wadsworth TL, Kretzschmar D, Woltjer RL, Quinn JF. A translational continuum of model systems for evaluating treatment strategies in Alzheimer’s disease: isradipine as a candidate drug. Dis. Model Mech. 2011;4(5):634–648. doi: 10.1242/dmm.006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain SM, Crain B, Peterson ER. Development of cross-tolerance to 5-hydroxytryptamine in organotypic cultures of mouse spinal cord-ganglia during chronic exposure to morphine. Life Sci. 1982;31:241–247. doi: 10.1016/0024-3205(82)90584-7. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Statton SA, Whitmore C, Freinbichler W, Weinberger P, Tipton KF, Della Corte L, Ward RJ, Crichton RR. Clinically available iron chelators induce neuroprotection in the 6-OHDA model of Parkinson’s disease after peripheral administration. J. Neural Transm. 2011;118(2):223–231. doi: 10.1007/s00702-010-0531-3. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Hefti F. Guidance of acetylcholinesterase-containing fibers by target tissue in co-cultured brain slices. Neuroscience. 1984;40:235–243. doi: 10.1016/0306-4522(84)90088-5. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Tietschin L, Knöpfel T, Enz A. Continuous presence of nerve growth factor is required for maintenance of cholinergic septal neurons in organotypic slice cultures. Neuroscience. 1990;36:27–31. doi: 10.1016/0306-4522(90)90348-8. [DOI] [PubMed] [Google Scholar]
- Hochstrasser T, Ullrich C, Sperner-Unterweger B, Humpel C. Inflammatory stimuli reduce survival of serotonergic neurons and induce neuronal expression of indoleamine 2,3-dioxygenase in rat dorsal raphe nucleus organotypic brain slices. Neuroscience. 2011;184:128–138. doi: 10.1016/j.neuroscience.2011.03.070. [DOI] [PubMed] [Google Scholar]
- Humpel C, Johansson M, Marksteiner J, Saria A, Strömberg I. Mesencephalic grafts increase preprotachykinin-A mRNA expression in striatal grafts in an in oculo co-graft model. Regul. Pept. 1995;56:9–17. doi: 10.1016/0167-0115(95)00122-r. [DOI] [PubMed] [Google Scholar]
- Humpel C, Weis C. Nerve growth factor and cholinergic CNS neurons studied in organotypic brain slices. J. Neural Transm. 2002;62:253–263. doi: 10.1007/978-3-7091-6139-5_23. [DOI] [PubMed] [Google Scholar]
- Katsuki H, Takenaka C, Kume T, Kaneko S, Akaike A. Requirement of neural activity for the maintenance of dopaminergic neurons in rat midbrain slice cultures. Neurosci. Lett. 2001;300:166–170. doi: 10.1016/s0304-3940(01)01570-1. [DOI] [PubMed] [Google Scholar]
- Lecht S, Rotfeld E, Arien-Zakay H, Tabakman R, Matzner H, Yaka R, Lelkes PI, Lazarovici P. Neuroprotective effects of nimodipine and nifedipine in the NGF-differentiated PC12 cells exposed to oxygen-glucose deprivation or trophic withdrawal. Int. J. Dev. Neurosci. 2012;30(6):465–469. doi: 10.1016/j.ijdevneu.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu X, Liu Y, Bao Y, An L. Nimodipine protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. Neuropharmacology. 2008;56(3):580–589. doi: 10.1016/j.neuropharm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lo YC, Qian L, Crews FT, Wilson B, Chen HL, Wu HM, Chen SH, Wei K, Lu RB, Ali S, Hong JS. Verapamil protects dopaminergic neuron damage through a novel anti-inflammatory mechanism by inhibition of microglial activation. Neuropharmacology. 2011;60(2-3):373–380. doi: 10.1016/j.neuropharm.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwiczek S, Theurl I, Muckenthaler MU, Jakab M, Mair SM, Theurl M, Kiss J, Paulmichl M, Hentze MW, Ritter M, Weiss G. Ca2+ channel blockers reverse iron overload by a new mechanism via divalent metal transporter-1. Nat. Med. 2007;13(4):448–454. doi: 10.1038/nm1542. [DOI] [PubMed] [Google Scholar]
- Ma Z, Zhou Y, Xie J. Nifedipine prevents iron accumulation and reverses iron-overload-induced dopamine neuron degeneration in the substantia nigra of rats. Neurotox. Res. 2012;22(4):274–279. doi: 10.1007/s12640-012-9309-8. [DOI] [PubMed] [Google Scholar]
- Michel PP, Toulorge D, Guerreiro S, Hirsch EC. Specific needs of dopamine neurons for stimulation in order to survive: implication for Parkinson disease. FASEB J. 2013;27(9):3414–3423. doi: 10.1096/fj.12-220418. [DOI] [PubMed] [Google Scholar]
- Ostergaard K, Schou JP, Zimmer J. Rat ventral mesencephalon grown as organotypic slice cultures and co-cultured, hippocampus, and cerebellum. Exp. Brain Res. 1990;82:547–565. doi: 10.1007/BF00228796. [DOI] [PubMed] [Google Scholar]
- Rousseau E, Michel PP, Hirsch EC. The iron-binding protein lactoferrin protects vulnerable dopamine neurons from degeneration by preserving mitochondrial calcium homeostasis. Mol. Pharmacol. 2013;84(6):888–898. doi: 10.1124/mol.113.087965. [DOI] [PubMed] [Google Scholar]
- Sai Y, Chen J, Ye F, Zhao Y, Zou Z, Cao J, Dong Z. Dopamine release suppression dependent on an increase of intracellular Ca(2+) contributed to rotenone-induced neurotoxicity in PC12 cells. J. Toxicol. Pathol. 2013;26(2):149–157. doi: 10.1293/tox.26.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz DS, Kaufmann WA, Saria A, Humpel C. Dopamine neurons in a simple GDNF-treated meso-striatal organotypic co-culture model. Exp. Brain Res. 1999;127:270–280. doi: 10.1007/s002210050796. [DOI] [PubMed] [Google Scholar]
- Schatz DS, Kaufmann WA, Schuligoi R, Humpel C, Saria A. 3,4-methylen-edioxymetamphetamine (Ecstasy) induces c-fos-like protein and mRNA in rat organotypic dorsal striatal slices. Synapse. 2000;36:75–83. doi: 10.1002/(SICI)1098-2396(200004)36:1<75::AID-SYN8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Smith MP, Cass WA. GDNF reduces oxidative stress in a 6-hydroxydopamine model of Parkinson’s disease. Neurosci. Lett. 2007;412(3):259–263. doi: 10.1016/j.neulet.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buch PA, Muller D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Striessnig J, Koschak A, Sinnegger-Brauns MJ, Hetzenauer A, Nguyen NK, Busquet P, Pelster G, Singewald N. Role of voltage-gated L-type Ca2+ channel isoforms for brain function. Biochem. Soc. Trans. 2006;34:903–909. doi: 10.1042/BST0340903. [DOI] [PubMed] [Google Scholar]
- Surmeier D. Calcium, ageing, and neuronal vulnerability in Parkinson’s disease. Lancet Neurol. 2007;6:933–938. doi: 10.1016/S1474-4422(07)70246-6. [DOI] [PubMed] [Google Scholar]
- Syeda K, Mohammed AM, Arora DK, Kowluru A. Glucotoxic conditions induce endoplasmic reticulum stress to cause caspase 3 mediated lamin B degradation in pancreatic β-cells: protection by nifedipine. Biochem. Pharmacol. 2013;86(9):1338–1346. doi: 10.1016/j.bcp.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich C, Daschil N, Humpel C. Organotypic vibrosections: novel whole sagittal brain cultures. J. Neurosci. Methods. 2011;201(1):131–141. doi: 10.1016/j.jneumeth.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich C, Humpel C. Rotenone induces cell death of cholinergic neurons in an organotypic co-culture brain slice model. Neurochem. Res. 2009a;34:2147–2153. doi: 10.1007/s11064-009-0014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich C, Humpel C. The pro-apoptotic substance thapsigargin selectively stimulates re-growth of brain capillaries. Curr. Neurovasc. Res. 2009b;6:171–180. doi: 10.2174/156720209788970063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis C, Marksteiner J, Humpel C. Nerve growth factor and glial cell line-derived neurotrophic factor restore the cholinergic phenotype in organotypic brain slices of the basal nucleus of Meynert. Neuroscience. 2001;102:129–138. doi: 10.1016/s0306-4522(00)00452-8. [DOI] [PubMed] [Google Scholar]
- Yao K, Ina Y, Nagashima K, Ohmori K, Ohno T. Antioxidant effects of calcium antagonists in rat brain homogenates. Biol. Pharm. Bull. 2000;23(6):766–769. doi: 10.1248/bpb.23.766. [DOI] [PubMed] [Google Scholar]