Abstract
Background
Our study examined the relative contributions of host, pharmacokinetic, and parasitological factors in determining the therapeutic response to artemether-lumefantrine (AL).
Methods
On the northwest border of Thailand, patients with uncomplicated Plasmodium falciparum malaria were enrolled in prospective studies of AL treatment (4- or 6-dose regimens) and followed up for 42 days. Plasma lumefantrine concentrations were measured by high performance liquid chromatography; malaria parasite pfmdr1 copy number was quantified using a real-time polymerase chain reaction assay (PCR), and in vitro drug susceptibility was tested.
Results
All treatments resulted in a rapid clinical response and were well tolerated. PCR-corrected failure rates at day 42 were 13% (95% confidence interval [CI], 9.6%–17%) for the 4-dose regimen and 3.2% (95% CI, 1.8%–4.6%) for the 6-dose regimen. Increased pfmdr1 copy number was associated with a 2-fold (95% CI, 1.8–2.4-fold) increase in lumefantrine inhibitory concentration50 (P = .001) and an adjusted hazard ratio for risk of treatment failure following completion of a 4-dose regimen, but not a 6-dose regimen, of 4.0 (95% CI, 1.4–11; P = .008). Patients who had lumefantrine levels below 175 ng/mL on day 7 were more likely to experience recrudescence by day 42 (adjusted hazard ratio, 17; 95% CI, 5.5–53), allowing prediction of treatment failure with 75% sensitivity and 84% specificity. The 6-dose regimen ensured that therapeutic levels were achieved in 91% of treated patients.
Conclusions
The lumefantrine plasma concentration profile is the main determinant of efficacy of artemether-lumefantrine. Amplification in pfmdr1 determines lumefantrine susceptibility and, therefore, treatment responses when plasma lumefantrine levels are subtherapeutic.
The emergence of drug resistance in Plasmodium falciparum has significantly undermined malaria-control programs in countries where it is endemic [1]. To ensure high cure rates and to combat this threat, the World Health Organization (WHO) has recommended the use of artemisinin combination therapy (ACT), although debate regarding the most suitable combination and how ACTs should be deployed and funded still continues. Artemisinins ensure rapid reduction of the initial infecting biomass, with the residual parasites being removed by a more slowly eliminated component usually included in combination therapy. The rationale supporting the use of ACT is improvement of anti-malarial efficacy, facilitation of adherence to a full treatment course, and minimization of selection of drug-resistant parasites.
Artemether-lumefantrine (AL) is currently the only combination therapy widely available that is manufactured to Good Manufacturing Practice standards in a fixed-dose preparation (each tablet contains 20 mg of artemether and 120 mg of lumefantrine). The fixed-dose regimen ensures that malaria parasites always encounter artemether and metabolites in the presence of lumefantrine and provides protection against the emergence of resistance to both components, but absorption of the lipophilic lumefantrine is erratic, and the drug needs to be administered twice per day [2]. Therapeutic levels are more reliably achieved by coadministration with a fatty meal and by extending the treatment course from 4 to 6 doses [3-5]. The WHO now advocates the higher, 6-dose regimen for the treatment of P. falciparum malaria in all areas, irrespective of the levels of host immunity or prevalence of multidrug resistance.
On the western border of Thailand, P. falciparum has become resistant to chloroquine, sulfadoxine-pyrimethamine, mefloquine, and halofantrine [6], although there have been no convincing reports of clinical resistance to the artemisinin derivatives. In studies conducted to determine the molecular basis of mefloquine resistance, we have highlighted a central role of amplification of pfmdr1 [7]. In vitro cross-resistance has been observed between lumefantrine, mefloquine, and halofantrine, and subsequent molecular studies have suggested a common mechanism of resistance [8]. We hypothesized that pfmdr1 polymorphisms would also contribute to treatment failure following AL treatment. To investigate the relative contributions of host, pharmacokinetic, and parasitological factors in determining therapeutic outcome following treatment with AL, we analyzed the cumulative AL experience at the Shoklo Malaria Research Unit (SMRU; Mae Sod, Tak Province, Thailand) between 1995 and 2002.
MATERIALS AND METHODS
Study sites
The patients studied were enrolled in 3 comparative studies [3, 5, 9] that were conducted at SMRU. Patients came from 4 Karen communities living in a malarious hill forest on the northwestern border of Thailand. The transmission of malaria in this area is low, and nearly all P. falciparum and Plasmodium vivax malaria infections are symptomatic [10]. An additional cohort of patients was also recruited at SMRU between 2000 and 2002 to monitor the clinical efficacy of the 6-dose AL regimen.
Patients
Between 1995 and 2002, symptomatic patients with microscopically confirmed uncomplicated P. falciparum malaria were recruited to participate in prospective chemotherapeutic studies, provided that they or their accompanying relatives gave fully informed consent. Pregnant women, children weighing <10 kg, and patients with signs of severe infection or concomitant disease requiring hospital admission were excluded. On admission, a full clinical examination was completed, and blood was taken for quantitation of malaria parasitemia.
AL (Coartem; Novartis) was dispensed as a fixed-dose combination tablet, each of which contained 20 mg of artemether and 120 mg of lumefantrine. The number of tablets given to each patient corresponded with each patient’s body weight, as follows: persons weighing 10–14 kg received 1 tablet per dose, persons weighing 15–24 kg received 2 tablets per dose, persons weighing 25–34 kg received 3 tablets per dose, and persons weighing >35 kg received 5 tablets per dose. Three dosing regimens were administered: AL48, a 4-dose regimen administered at admission and 8, 24, and 48 h; AL60, a 6-dose regimen administered over 3 days at admission and 8, 24, 36, 48, and 60 h; and AL96, a 6-dose regimen administered over 5 days at admission and 8, 24, 48, 72, and 96 h. Drug administration was observed in all cases, and if vomiting occurred <30 min after the dose was ingested, administration of the full dose was readministered. If vomiting occurred between 30 and 60 min after the dose was ingested, one-half of the dose was repeated. After enrollment, patients were treated and observed daily until resolution of symptoms and parasitemia, after which patients were followed up weekly for 42 days to evaluate the clinical and parasitological efficacy of the drug regimens.
All clinical and laboratory studies were approved by the Ethics Committee of the Faculty of Tropical Medicine (Mahidol University) and by the Karen Refugee Committee. Molecular studies conducted at St. George’s Hospital Medical School (University of London) were approved by the Wandsworth Local Research Ethics Committee.
Isolation of P. falciparum and drug susceptibility assay
Fresh isolates of P. falciparum were collected routinely and analyzed for drug susceptibility in vitro, both for patients recruited to participate in clinical studies of AL and other chemotherapeutic studies conducted since 2001 [11]. Assays were performed within 4–8 h after collection, without prior cryopreservation, using 3H-hypoxanthine uptake inhibition and with quality control measures described elsewhere [12].
Sample collection
Blood samples were collected on Whatman filter paper (3MM) from all patients on admission to the study and, for patients who experienced treatment failure, on the day of reappearance of parasites. Venous blood samples were also collected from patients enrolled in 2 of the studies to define the pharmacokinetic profile of lumefantrine [5, 9]. Blood was drawn by venepunture into heparinized tubes and centrifuged at 1000 g for 15 min. Thereafter, plasma was transferred immediately into polypropylene tubes and stored at −70 °C until shipment. Lumefantrine was measured in plasma using high performance liquid chromatography with UV detection, with a limit of detection of 40 ng/mL [2]. DNA was available from whole blood samples (in heparinised tubes stored at −70 °C) or from 50 μL of capillary blood on a filter spot, and it was extracted as described elsewhere [7]. Polymorphisms in MSP-1, MSP-2, and GLURP were used to assess clonality and to distinguish between reinfection and recrudescence [13].
Quantitation of pfmdr1 copy number and detection of polymorphisms
Fifty patients from both the AL48 and AL60 groups were randomly selected for analysis of pfmdr1 genotype. Because treatment failure rates were generally low, an additional 25 samples from each group were randomly selected from patients who experienced treatment failure.
Molecular analysis of blood samples was conducted at St. George’s Hospital Medical School in London, England (n = 306), and at the Southwest Foundation for Biomedical Research in San Antonio, Texas (n = 317). Pfmdr1 copy number was assessed in London by quantitative PCR (ABI sequence detector 7700; Applied Biosystems) and in Texas (ABI 7900HT real-time PCR system) using methods described elsewhere [7]. All reactions were performed in triplicate (London) or quadruplicate (Texas) and were rejected if they did not conform to exponential kinetics.
Previous studies defined the repeatability coefficient of the copy number assay as 0.64 in London (i.e., 95% of repeated estimates of pfmdr1 copy number would be expected to be within ±0.32 of the first estimate) and as 0.54 in Texas. Assay results between London and Texas were compared by running 31 samples in parallel with the derived copy number differing by <0.35 between laboratories and a repeatability coefficient of 0.82.
Single-nucleotide polymorphisms (SNPs) in pfmdr1 and pfcrt were detected by nested PCR–restriction fragment–length polymorphism or primer extension methods, as described elsewhere [11, 14, 15]. All PCRs and digests included DNA of laboratory strains 3D7, HB3, 7G8, and Dd2 as positive and negative controls.
Statistical analyses
Data were analyzed using SPSS software for Windows (SPSS). Proportions were examined using the χ2 test with Yates’ correction or by Fisher’s exact test. Thresholds for defining suboptimal lumefantrine levels on day 7 were assessed using the receiver operator curve and Youden’s index, which was calculated as sensitivity plus specificity minus 1. The relationship between genotypic data (codon mutation and gene amplification) and clinical response to treatment was evaluated by survival analysis, in which patients who had been lost to follow-up or who presented again with reinfection were included, but regarded as not being failures. Results for patients for whom PCR was not possible or indeterminant were adjusted according to the temporal probabilities of recrudescence versus reinfection that were determined using values for patients with complete data [16]. Failure rates were calculated using the cumulative incidence rate at day 42 and were compared using the Mantel-Haenszel log rank test. Cox proportional hazards modelling was used for multivariable analyses. Because age (a surrogate marker of host immunity) and baseline parasitemia have been shown to be reliable determinants of the therapeutic response in this study site [17, 18], both were always included in the model.
RESULTS
Between December 1995 and June 2002, a total of 1588 patients with uncomplicated P. falciparum malaria were enrolled in chemotherapeutic studies and received a complete course of treatment with AL (figure 1). Of these, 687 patients (43%) were recruited to participate in comparative studies, and the remaining 901 patients were enrolled in part of the routine surveillance of the treatment efficacy of AL. In total, 387 patients received a 4-dose regimen over 48 h (AL48), 1114 patients received a 6-dose regimen over 60 h (AL60), and 87 patients received a 6-dose regimen over 96 h (AL96). Overall, 1273 (80%) of 1588 patients completed at least 42 days of follow up. Baseline characteristics are shown in table 1.
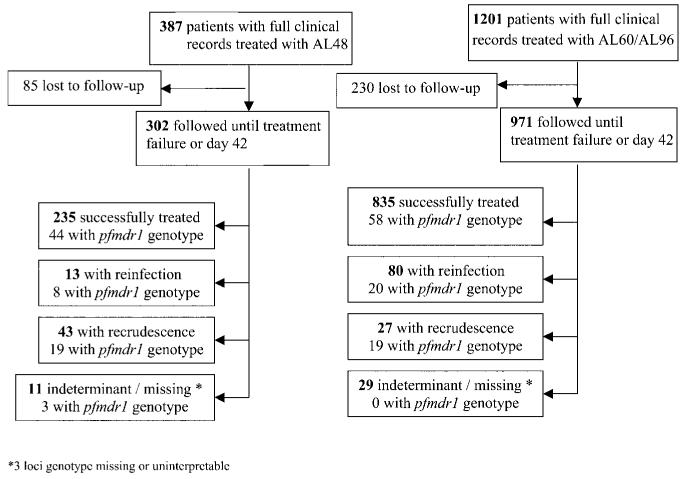
Figure 1.
Study profile. AL48, regimen of 4 doses of artemether-lumefantrine (AL) over 48 h; AL60, regimen of 6 doses of AL over 60 h; AL96, regimen of 6 doses of AL over 96 h.
Table 1. Baseline characteristics of patients assessed for in vivo responses to treatment with artemether-lumefantrine (AL).
| AL regimen |
||||
|---|---|---|---|---|
| Variable | 4 doses over 48 h | 6 doses over 60 h | 6 doses over 96 h | P |
| No. of patients | 387 | 1114 | 87 | |
| Male patients | 265 (69) | 757 (68) | 62 (71) | NS |
|
| ||||
| Age | ||||
| Median years (range) | 21 (3–75) | 23 (2–70) | 22 (5–60) | NS |
| <5 years | 2 (0.5) | 42 (3.8) | 0 | .001 |
| 5–14 years | 98 (25) | 325 (29) | 18 (21) | |
| >14 years | 287 (74) | 747 (67) | 69 (79) | |
|
| ||||
| Artemether per dose, mean mg/kg (95% CI) | 1.58 (1.56–1.61) | 1.70 (1.68–1.72) | 1.65 (1.61–1.70) | .001 |
|
| ||||
| Lumefantrine per dose, mean mg/kg (95% CI) | 9.52 (9.39–9.66) | 10.2 (10.09–10.31) | 9.92 (9.64–10.21) | .001 |
|
| ||||
| Parasite count, geometric mean μL−1 (95% CI) | 4857 (3877–6086) | 6739 (5970–7605) | 8221 (5645–11,913) | .01 |
|
| ||||
| Plasmodium falciparum and Plasmodium vivax infection | 21 (5.4) | 66 (5.9) | 4 (5) | NS |
|
| ||||
| Proportion (%) of patients with fevera | 224/387 (58) | 530/1112 (48) | 49/87 (56) | .003 |
|
| ||||
| Proportion (%) of patients with a history of vomiting at admission | 90/387 (23) | 240/1113 (22) | 13/87 (15) | NS |
|
| ||||
| Mean hematocrit ± SD | 37.3 ± 6.3 | 37.1 ± 6.1 | 37.8 ± 6.3 | NS |
|
| ||||
| Proportion (%) of patients with anaemiab | 51/384 (13) | 118/1097 (11) | 9/86 (11) | NS |
NOTE. Data are no. (%) of patients, unless otherwise indicated. NS, not significant.
>37.5°C per axilla.
Hematocrit = 30%.
All 3 treatment regimens were well tolerated, and within 48 h, 1406 (91%) of 1551 patients had achieved clearance of parasitemia, and 554 (95%) of 584 patients had experienced defervescence. On the day of admission, 18 (1.1%) of 1587 patients vomited within 1 h after their first dose of AL. The occurrence of vomiting within 1 h after ingestion of the first dose was higher in children <5 years old (4 [9.1%] of 44 children), compared with children 5–14 years old (19 [4.3%] of 441 children) and adults (8 [0.7%] of 1103 adults; P = .001). The frequency of early vomiting was also 4.1-fold higher (95% CI, 1.7–9.9-fold) among patients presenting with fever and 5-fold higher (95% CI, 2.5–10.2-fold) among patients with a history of vomiting.
Treatment failure
Overall, no patients experienced early therapeutic failure or reappearance of parasitemia within 7 days of treatment. The cumulative risk of failure by day 42 was 20% (95% CI, 15%–24%) in the AL48 group, 13.1% (95% CI, 11%–15%) in the AL60 group, and 3.9% (95% CI, 0%–9.6%) in the AL96 group (P = .001) (table 2). Three loci genotyping was successfully performed on 163 (80%) of 203 paired isolates (figure 1). PCR-corrected failure rates by day 42 were 13.3% (95% CI, 9.6%–17%) following completion of the AL48 regimen, 3.2% (95% CI, 1.8%–4.6%) following completion of the AL60 regimen, and 0% following completion of the AL96 regimen (overall, P < .001) (table 2). The median time to recrudescence was 21 days (range, 14–42 days) after completion of a 4-dose regimen and 27 days (range, 14–43 days) after completion of a 6-dose regimen (P = .008). The cumulative rate of reinfection within 42 days after completion of a regimen was 10.5% (95% CI, 8.7%–12.3%) but did not differ significantly between treatment groups (P = .07).
Table 2. Day 42 treatment failure rates following completion of artemether-lumefantrine (AL) therapy.
| AL regimen |
||||
|---|---|---|---|---|
| Rate | 4 doses over 48 h | 6 doses over 60 h | 6 doses over 96 h | P |
| Day 42 uncorrected rate of recurrent parasitemia, % (95% CI) | 20 (15–24)a | 13 (11–15)b | 3.9 (0–9.6) | .001 |
|
| ||||
| Day 42 rate of recrudescence, % (95% CI) | 13.3 (9.6–17)c | 3.2 (1.8–4.6)d | 0 | <.001 |
|
| ||||
| Day 42 rate of reinfection, % (95% CI) | 7.7 (4.8–10.6)e | 11.7 (9.6–13.7)f | 3.9 (0–9.6) | .07 |
48-h regimen vs. 60-h regimen, P = .005.
60-h regimen vs. 96-h regimen, P = .01.
48-h regimen vs. 60-h regimen, P< .001.
60-h regimen vs. 96-h regimen, P = .17.
48-h regimen vs. 60-h regimen, P = .14.
60-h regimen vs. 96-h regimen, P = .08.
Clinical risk factors for recrudescence
After controlling for age and parasitemia, the only clinical factor at admission associated significantly with an increased risk of subsequent recrudescence after completion of the AL48 regimen was a history of vomiting (adjusted hazard ratio [AHR], 2.2; 95% CI, 1.2–4.1; P = .012). Although the trend was similar among patients receiving a 6-dose regimen, it did not reach statistical significance. After controlling for these confounding factors, the AHR for recrudescence following a 4-dose regimen was 6.2 (95% CI, 3.8–9.9), compared with that for patients receiving a 6-dose regimen (P < .001).
Lumefantrine levels
Plasma lumefantrine levels were available for 407 patients (131 patients following completion of the AL48 regimen, 201 patients following completion of the AL60 regimen, and 75 patients following completion of the AL96 regimen). Day 7 levels were significantly lower following completion of the 4-dose regimen, for which the median level (range) was 237 ng/mL (0–1828 ng/mL), compared with 528 ng/mL (49–5175 ng/mL) among patients who completed the AL60 and 932 ng/mL (127–3094 ng/mL) among patients who completed the AL96 regimen (P < .001 for the overall comparison and the comparison between the 60-h and 96-h regimens).
Day 7 levels were 17% lower among patients who were febrile at admission, compared with patients who were afebrile at admission (median level, 427 ng/mL [interquartile range {IQR}, 196–696 ng/mL] and 515 ng/mL [IQR, 366–979 ng/mL], respectively; P = .001), and 14% lower among patients with a history of vomiting at admission (429 ng/mL [IQR, 187–917 ng/mL]; P = .03). For both of these factors, the differences were only apparent in patients receiving the AL48 and AL60 regimens, but not in patients receiving the AL96 regimen. Day 7 levels were not affected significantly by other baseline characteristics or by early therapeutic response.
Patients with recrudescent infections had median plasma lumefantrine levels on day 7 of 93 ng/mL (IQR, 13–205 ng/mL), compared with 484 ng/mL (IQR, 248–855 ng/mL) among patients treated successfully (P < .001). Using Youden’s index, the best cutoff for day 7 lumefantrine levels predicting recrudescence was 175 ng/mL. Plasma levels <175 ng/mL were present in 43 (36%) of 119 patients treated with the AL48 regimen, 15 (8.6%) of 175 patients treated with the AL60 regimen, and 1 (1.6%) of 63 patients treated with the AL96 regimen (P < .001). Overall, 24% (95% CI, 12%–36%) of patients with levels <175 ng/mL experienced treatment failure, compared with 1.1% (95% CI, 0%–2.3%) of patients with day 7 lumefantrine levels ≥175 ng/mL (AHR, 17; 95% CI, 5.5–33; P < .001). The cutoff value of 175 ng/mL predicted recrudescence with 75% sensitivity and 84% specificity.
Pfmdr1 SNPs and copy number
In total, 207 isolates from patients treated with AL were genotyped for pfmdr1 polymorphisms; copy number could be determined reliably for 171 isolates (83%) (figure 1). A single copy number of pfmdr1 was observed in 92 isolates (52%); 55 isolates (31%) had 2 copies; 26 isolates (15%) had 3 copies; 1 isolate had 4 copies; and 2 isolates had 5 copies. SNPs were detected in 4 (5%) of 78 isolates at codon 86, in 19 (24%) of 78 isolates at codon 184, and in 3 (3.8%) of 78 isolates at codon 1042, but in 0 of 50 isolates at codon 1034 and 0 of 13 isolates at codon 1246. All isolates with increased copy number had a wild-type allele at codons 86 and 1042.
The complete sample selection from which parasites were genotyped was deliberately biased to increase the number of isolates associated with subsequent recrudescence. Parasites successfully genotyped were also significantly more likely to come from patients who were febrile (115 [65%] of 176 patients vs. 688 [49%] of 1410 patients; P = .001) and who had a higher level of parasitemia (geometric mean, 17,712 parasites/μL [95% CI, 13,292–23,602] vs. 5528 parasites/μL [95% CI, 4958–6162]; P < .001), compared with the overall cohort. There was no significant difference in any of the other baseline factors assessed.
In vivo molecular correlates
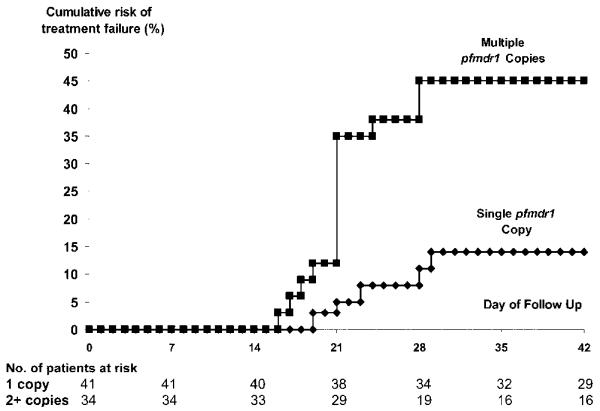
SNPs were not correlated significantly with the therapeutic response. However, following therapy with the AL48 regimen, the recrudescence rate for isolates with an increased pfmdr1 copy number was 43% (95% CI, 26%–60%), compared with 13% (95% CI, 2.5%–24%) for isolates with a single copy (AHR, 4.0; 95% CI, 1.4–11; P = .008) (figure 2). The AHR did not change after controlling for baseline parasitemia, and history of vomiting in a multivariable model. There was no difference in recrudescence rates among patients treated with the 6-dose regimen. The prevalence of isolates with an increased copy number in a random cohort of patients was 30% (12 of 40 patients); thus, the population attributable risk of patients infected with isolates with increased pfmdr1 copy number was 47%.
Figure 2.
Cumulative percentage of patients with recrudescence (PCR-corrected) following completion of a 4-dose regimen of artemether-lumefantrine. Overall, P = .004.
In vitro molecular correlates
The relationship between pfmdr1 polymorphisms and in vitro response was assessed in 493 isolates with susceptibility profiles for either artesunate or lumefantrine (61 isolates from patients treated with AL and 432 isolates from patients participating in other clinical studies). Pfmdr1 genotyping was successful in 490 isolates (99%).
The influence of pfmdr1 SNPs was assessed in the 294 isolates (60%) with a single copy number. Isolates with the N1042D mutation had a significantly lower lumefantrine IC50 than did isolates with wild-type pfmdr1 (geometric mean, 7.8 ng/mL [95% CI, 5.1–11.9 ng/mL] and 16.9 ng/mL [95% CI, 14.9–19.1], respectively; P < .001). The presence of N86Y did not significantly alter in vitro lumefantrine susceptibility. Pfmdr1 codon mutations at any of the loci tested did not significantly influence the in vitro susceptibility of artesunate.
Pfmdr1 amplification occurred in 196 (40%) of 490 isolates. An increase in pfmdr1 copy number from 1 (wild type) to >1 was associated with a 2-fold (95% CI, 1.8–2.4-fold) increase in the lumefantrine IC50 (P = .0001). There was no significant difference in the IC50 of lumefantrine between isolates with 2 copies and isolates with >2 copies (table 3). Isolates with a single copy of pfmdr1 had a geometric mean artesunate IC50 of 0.84 ng/mL (95% CI, 0.76–0.92 ng/mL), compared with 1.37 ng/mL (95% CI, 1.19–1.57 ng/mL) for isolates with 2 copies and 1.87 ng/mL (95% CI, 1.51–2.32 ng/mL) for isolates with >2 copies. The differences in artesunate IC50 between isolates with 1 and 2 copies and between isolates with 2 and >2 copies were both significant (P < .001 and P = .012, respectively).
Table 3. IC50 values for lumefantrine, by pfmdr1 copy number.
| Pfmdr1 copy number | Pfmdr1 point mutation | No. of isolates | IC50, geometric mean ng/mL (95% CI) | P a |
|---|---|---|---|---|
| 1 | 86N/1042D | 20 | 7.8 (5.1–11.9) | <.001 |
| 1 | 86Y/1042N | 16 | 12.6 (7.6–20.8) | .19 |
| 1 | 86Y/1042D | 1 | 35.6 (…) | … |
| 1 | 86N/1042N | 187 | 16.9 (14.9–19.1) | … |
| 2 | 86N/1042N | 94 | 31.9 (27.2–37.5) | <.001 |
| ≥3 | 86N/1042N | 32 | 34.5 (27.5–43.3) | <.001 |
NOTE. Wild-type pfmdr1 refers to 86N/1042N. 86Y and 1042D are observed mutations.
Compared with single-copy wild-type Pfmdr1.
DISCUSSION
Artemisinin combination therapy is currently the treatment of choice for uncomplicated P. falciparum malaria. The combination achieves its antimalarial effect through an initial rapid reduction in parasite biomass attributable to the short-acting but highly potent artemether, with the subsequent removal of the remaining parasites by the intrinsically less-active but more slowly eliminated lumefantrine. Overall cure rates depend on there being sufficient lumefantrine to remove the residual parasite biomass left by artemether [19, 20].
The initially recommended 4-dose regimen was associated with unacceptably high recrudescence rates [9, 21]; thus, dosing recommendations subsequently were changed to 6 doses over 60 h, a regimen that cures illness in >95% of patients [5, 22, 23]. In these studies, the area under the plasma lumefantrine concentration time curve (AUC) was shown to be the most important pharmacokinetic determinant of therapeutic response, and the day 7 plasma lumefantrine level proved to be a good surrogate of the AUC [24].
In the current study, we have pooled our large experience of the use of AL for uncomplicated malaria to characterize the relationship between clinical, pharmacological, and molecular determinants of treatment failure. After controlling for age and baseline parasitemia, the only clinical parameter associated with subsequent failure was a history of vomiting (AHR, 2.2; 95% CI, 1.2–4.1), which was associated with a 14% reduction in day 7 plasma lumefantrine levels. Although a 6-dose regimen was more reliable at achieving adequate lumefantrine concentrations, recrudescence rates increased from 1.2% to 13% (P < .001) when day 7 levels were <175 ng/mL.
The relationship between drug concentrations and therapeutic response depends on the drug susceptibility of the infecting parasites. Conventionally, the latter has been measured using in vitro susceptibility testing, although such assays are often difficult to perform and interpret. Molecular genotyping is becoming increasingly important as an epidemiological tool for assessing resistance. The P-glycoprotein pump encoded by pfmdr1 has been shown to regulate the in vitro and in vivo response to mefloquine, halofantrine, quinine, and artesunate [7, 14, 25]. Whereas attention initially was focussed on the role of SNPs and chlorquine resistance, gene amplification of pfmdr1 is the main determinant of mefloquine resistance. In the current study, parasites with point mutations at codon 86 or 1042 were more susceptible in vitro to lumefantrine than isolates with wild-type single copy pfmdr1; however, the number of isolates with mutations was small and only reached significance for codon 1042 (table 3). Increased pfmdr1 copy number was present in almost one-half of the 200 isolates tested (all of which were wild type at codons 86 and 1042) and accounted for ~47% of recrudescence cases following completion of a 4-dose regimen of AL. Reassuringly, pfmdr1 amplification did not increase the risk of treatment failure after the 6-dose regimen, which retained an efficacy of >95%. The longer course of artemether presumably reduced the initial parasite biomass to sufficient levels for the infection to be eliminated by reliably higher lumefantrine levels. Thus, the 6-dose regimen provides a sufficient level of drug to overcome even partially resistant parasites, whereas the 4-dose regimen does not reliably provide sufficient lumefantrine levels even to overcome susceptible parasites.
Recent studies from Zanzibar [26] have raised concerns that the use of AL in an area with endemicity may select for the 86N allele of pfmdr1 [27]. However, it is generally believed that the presence of asparagine at codon 86 is, in fact, the wild type and that the tyrosine substitution (86Y) is selected by chloroquine drug pressure [28, 29]. In our study of Thai isolates, there was no significant difference in the in vitro response and polymorphisms at codon 86, although a larger series would be necessary to detect small differences. The major molecular determinant of artemether-lumefantrine efficacy in Thailand is an increase in the pfmdr1 copy number, and although drug pressure selects for this polymorphism, the resistance conferred can be overcome clinically when the patient is re-treated with a 6-dose regimen.
In summary, the 6-dose regimen of coartemether is a safe and effective treatment of P. falciparum malaria, even in areas where multidrug-resistant isolates predominate. Although low lumefantrine levels at day 7 were associated with an increased risk of treatment failure, these levels occurred in <10% of patients receiving a 6-dose regimen, ensuring that cure rates exceeded 96%.
Acknowledgments
We thank the staff of the Shoklo Malaria Research Unit for their excellent work. The field studies were part of the Wellcome Trust-Mahidol University Oxford Tropical Medicine Research Programme supported by the Wellcome Trust of Great Britain. Gilbert Lefevre (Novartis Pharma) processed the lumefantrine drug levels.
Financial support. Wellcome Trust Career Development Fellowship through the Oxford-Wellcome Thailand Unit (grant 074637 to R.P.). The molecular work performed at St. George’s Hospital Medical School (London) was funded by the Wellcome Trust (grant 066201). Molecular analysis conducted at the Southwest Foundation for Biomedical Research (San Antonio, TX) was funded by the National Institutes of Health (grants RO1 AI48071 and C06 RR013556).
Footnotes
Potential conflicts of interest. R.P., M.v.V., and F.N. have received reimbursement of travel expenses to attend meetings by Novartis, who manufactures Coartem. All other authors: no conflicts.
References
- 1.Price RN, Nosten F. Drug resistant falciparum malaria: clinical consequences and strategies for prevention. Drug Resist Updat. 2001;4:187–96. doi: 10.1054/drup.2001.0195. [DOI] [PubMed] [Google Scholar]
- 2.Ezzet F, van Vugt M, Nosten F, Looareesuwan S, White NJ. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob Agents Chemother. 2000;44:697–704. doi: 10.1128/aac.44.3.697-704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vugt MV, Wilairatana P, Gemperli B, et al. Efficacy of six doses of artemether-lumefantrine (benflumetol) in multidrug-resistant Plasmodium falciparum malaria. Am J Trop Med Hyg. 1999;60:936–42. doi: 10.4269/ajtmh.1999.60.936. [DOI] [PubMed] [Google Scholar]
- 4.Lefevre G, Looareesuwan S, Treeprasertsuk S, et al. A clinical and pharmacokinetic trial of six doses of artemether-lumefantrine for multidrug-resistant Plasmodium falciparum malaria in Thailand. Am J Trop Med Hyg. 2001;64:247–56. doi: 10.4269/ajtmh.2001.64.247. [DOI] [PubMed] [Google Scholar]
- 5.van Vugt M, Looareesuwan S, Wilairatana P, et al. Artemether-lumefantrine for the treatment of multidrug-resistant falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94:545–8. doi: 10.1016/s0035-9203(00)90082-8. [DOI] [PubMed] [Google Scholar]
- 6.ter Kuile FO, Dolan G, Nosten F, et al. Halofantrine versus mefloquine in treatment of multidrug-resistant falciparum malaria. Lancet. 1993;341:1044–9. doi: 10.1016/0140-6736(93)92409-m. [DOI] [PubMed] [Google Scholar]
- 7.Price RN, Uhlemann AC, Brockman A, et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364:438–47. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duraisingh MT, Roper C, Walliker D, Warhurst DC. Increased sensitivity to the antimalarials mefloquine and artemisinin is conferred by mutations in the pfmdr1 gene of Plasmodium falciparum. Mol Microbiol. 2000;36:955–61. doi: 10.1046/j.1365-2958.2000.01914.x. [DOI] [PubMed] [Google Scholar]
- 9.van Vugt M, Brockman A, Gemperli B, et al. Randomized comparison of artemether-benflumetol and artesunate-mefloquine in treatment of multidrug-resistant falciparum malaria. Antimicrob Agents Chemother. 1998;42:135–9. doi: 10.1128/aac.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luxemburger C, Thwai KL, White NJ, et al. The epidemiology of malaria in a Karen population on the western border of Thailand. Trans R Soc Trop Med Hyg. 1996;90:105–11. doi: 10.1016/s0035-9203(96)90102-9. [DOI] [PubMed] [Google Scholar]
- 11.Anderson TJ, Nair S, Qin H, et al. Are transporter genes other than the chloroquine resistance locus (pfcrt) and multidrug resistance gene (pfmdr) associated with antimalarial drug resistance? Antimicrob Agents Chemother. 2005;49:2180–8. doi: 10.1128/AAC.49.6.2180-2188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brockman A, Price RN, van Vugt M, et al. Plasmodium falciparum antimalarial drug susceptibility on the north-western border of Thailand during five years of extensive use of artesunate-mefloquine. Trans R Soc Trop Med Hyg. 2000;94:537–44. doi: 10.1016/s0035-9203(00)90080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brockman A, Paul RE, Anderson TJ, et al. Application of genetic markers to the identification of recrudescent Plasmodium falciparum infections on the northwestern border of Thailand. Am J Trop Med Hyg. 1999;60:14–21. doi: 10.4269/ajtmh.1999.60.14. [DOI] [PubMed] [Google Scholar]
- 14.Price RN, Cassar C, Brockman A, et al. The pfmdr1 gene is associated with a multidrug-resistant phenotype in Plasmodium falciparum from the western border of Thailand. Antimicrob Agents Chemother. 1999;43:2943–9. doi: 10.1128/aac.43.12.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duraisingh MT, Jones P, Sambou I, von Seidlein L, Pinder M, Warhurst DC. The tyrosine-86 allele of the pfmdr1 gene of Plasmodium falciparum is associated with increased sensitivity to the anti-malarials mefloquine and artemisinin. Mol Biochem Parasitol. 2000;108:13–23. doi: 10.1016/s0166-6851(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 16.Stepniewska K, White NJ. Design and interpretation of antimalarial drug trials in uncomplicated falciparum malaria. Antimicrob Agents Chemother. doi: 10.1186/1475-2875-5-127. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price RN, Nosten F, Luxemburger C, et al. Artesunate/mefloquine treatment of multi-drug resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:574–7. doi: 10.1016/s0035-9203(97)90032-8. [DOI] [PubMed] [Google Scholar]
- 18.ter Kuile FO, Nosten F, Luxemburger C, et al. Mefloquine treatment of acute falciparum malaria: a prospective study of non-serious adverse effects in 3673 patients. Bull World Health Organ. 1995;73:631–42. [PMC free article] [PubMed] [Google Scholar]
- 19.White NJ. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–22. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White NJ, van Vugt M, Ezzet F. Clinical pharmacokinetics and pharmacodynamics and pharmacodynamics of artemether-lumefantrine. Clin Pharmacokinet. 1999;37:105–25. doi: 10.2165/00003088-199937020-00002. [DOI] [PubMed] [Google Scholar]
- 21.van Agtmael M, Bouchaud O, Malvy D, et al. The comparative efficacy and tolerability of CGP 56697 (artemether + lumefantrine) versus halofantrine in the treatment of uncomplicated falciparum malaria in travellers returning from the Tropics to The Netherlands and France. Int J Antimicrob Agents. 1999;12:159–69. doi: 10.1016/s0924-8579(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 22.Mutabingwa TK, Anthony D, Heller A, et al. Amodiaquine alone, amodiaquine+sulfadoxine-pyrimethamine, amodiaquine+artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomised effectiveness trial. Lancet. 2005;365:1474–80. doi: 10.1016/S0140-6736(05)66417-3. [DOI] [PubMed] [Google Scholar]
- 23.Piola P, Fogg C, Bajunirwe F, et al. Supervised versus unsupervised intake of six-dose artemether-lumefantrine for treatment of acute, uncomplicated Plasmodium falciparum malaria in Mbarara, Uganda: a randomised trial. Lancet. 2005;365:1467–73. doi: 10.1016/S0140-6736(05)66416-1. [DOI] [PubMed] [Google Scholar]
- 24.Ezzet F, Mull R, Karbwang J. Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients. Br J Clin Pharmacol. 1998;46:553–61. doi: 10.1046/j.1365-2125.1998.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson AL, Purfield A, McDaniel P, et al. pfmdr1 Genotyping and in vivo mefloquine resistance on the Thai-Myanmar border. Am J Trop Med Hyg. 2005;72:586–92. [PubMed] [Google Scholar]
- 26.Martensson A, Stromberg J, Sisowath C, et al. Efficacy of artesunate plus amodiaquine versus that of artemether-lumefantrine for the treatment of uncomplicated childhood Plasmodium falciparum malaria in Zanzibar, Tanzania. Clin Infect Dis. 2005;41:1079–86. doi: 10.1086/444460. [DOI] [PubMed] [Google Scholar]
- 27.Hastings IM, Ward SA. Coartem (artemether-lumefantrine) in Africa: the beginning of the end? J Infect Dis. 2005;192:1303–4. doi: 10.1086/432554. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland CJ, Alloueche A, Curtis J, et al. Gambian children successfully treated with chloroquine can harbor and transmit Plasmodium falciparum gametocytes carrying resistance genes. Am J Trop Med Hyg. 2002;67:578–85. doi: 10.4269/ajtmh.2002.67.578. [DOI] [PubMed] [Google Scholar]
- 29.Kublin JG, Cortese JF, Njunju EM, et al. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J Infect Dis. 2003;187:1870–5. doi: 10.1086/375419. [DOI] [PubMed] [Google Scholar]