Summary
Background
The borders of Thailand harbour the world’s most multidrug resistant Plasmodium falciparum parasites. In 1984 mefloquine was introduced as treatment for uncomplicated falciparum malaria, but substantial resistance developed within 6 years. A combination of artesunate with mefloquine now cures more than 95% of acute infections. For both treatment regimens, the underlying mechanisms of resistance are not known.
Methods
The relation between polymorphisms in the P falciparum multidrug resistant gene 1 (pfmdr1) and the in-vitro and in-vivo responses to mefloquine were assessed in 618 samples from patients with falciparum malaria studied prospectively over 12 years. pfmdr1 copy number was assessed by a robust real-time PCR assay. Single nucleotide polymorphisms of pfmdr1, P falciparum chloroquine resistance transporter gene (pfcrt) and P falciparum Ca2+ ATPase gene (pfATP6) were assessed by PCR-restriction fragment length polymorphism.
Findings
Increased copy number of pfmdr1 was the most important determinant of in-vitro and in-vivo resistance to mefloquine, and also to reduced artesunate sensitivity in vitro. In a Cox regression model with control for known confounders, increased pfmdr1 copy number was associated with an attributable hazard ratio (AHR) for treatment failure of 6·3 (95% CI 2·9–13·8, p<0·001) after mefloquine monotherapy and 5·4 (2·0-14·6, p=0·001) after artesunate-mefloquine therapy. Single nucleotide polymorphisms in pfmdr1 were associated with increased mefloquine susceptibility in vitro, but not in vivo.
Interpretation
Amplification in pfmdr1 is the main cause of resistance to mefloquine in falciparum malaria.
Relevance to practice
Multidrug resistant P falciparum malaria is common in southeast Asia, but difficult to identify and treat. Genes that encode parasite transport proteins maybe involved in export of drugs and so cause resistance. In this study we show that increase in copy number of pfmdr1, a gene encoding a parasite transport protein, is the best overall predictor of treatment failure with mefloquine. Increase in pfmdr1 copy number predicts failure even after chemotherapy with the highly effective combination of mefloquine and 3 days’ artesunate. Monitoring of pfmdr1 copy number will be useful in epidemiological surveys of drug resistance in P falciparum, and potentially for predicting treatment failure in individual patients.
Introduction
The emergence of drug resistant malaria is a serious threat to tropical communities.1,2 Early detection of failing treatment regimens for malaria is important for guiding public health measures in areas where the disease is endemic. Frequently, such decision making relies on results of clinical studies that assess the therapeutic efficacy of antimalarials, sometimes supported by in-vitro sensitivity testing. However, clinical studies with adequate periods of patient follow-up (≥28 days) are difficult to implement, and few malaria-endemic areas have the infrastructure needed to do parasite cultures. More recently, molecular genotyping of parasites has proved useful in assessing resistance to the antifolate, sulphonamide,3 and hydroxynaphthaquinone classes4 of drugs, since point mutations in the genes that encode their drug targets cause resistance. Chloroquine (a 4-aminoquinoline) resistance in vitro and in vivo is associated with mutations in pfmdr1 (Plasmodium falciparum multidrug resistance gene 1) and pfcrt (P falciparum chloroquine resistance transporter gene) a putative transporter that modulates intraparasitic drug concentrations.5
Southeast Asia is the epicentre of P falciparum resistance to antimalarial drugs. Chloroquine resistance emerged there more than 40 years ago, and within 20 years chloroquine became largely ineffective. Chloroquine is now reserved for P vivax, P malariae, or P ovale in this region. In Thailand, mefloquine was introduced as first-line treatment for falciparum malaria in November, 1984, but despite careful regulation of its use, significant resistance developed within 6 years. Resistance has also emerged in adjacent countries, such as Burma and Cambodia. Artemisinin derivatives have since been added to mefloquine in 3-day combination regimens (ACT),6 which remain highly effective. In these areas of southeast Asia the molecular basis of drug resistance in P falciparum is multifactorial and not completely understood.
In preliminary studies, multidrug resistance in southeast Asia was linked both to mutations in pfmdr1 and to increases in this gene’s copy number.7-9 Further studies have been hampered by the technical difficulties of assessing copy number in clinical samples.7-12
We have developed a robust assay to quantitate the copy number of pfmdr1 using TaqMan Real-time PCR (TaqMan, Wellington, VA, USA), and combined this assay with analysis of single nucleotide polymorphisms in pfmdr1, pfcrt, and P falciparum Ca2+ ATPase (pfATP6) (recently identified as an encoder of the artemisinins receptor), to assess the determinants of mefloquine resistance in vitro and in vivo.
Materials and methods
Study site
The field studies for this project took place in a Karen community living in malarious hill forest on the northwestern border of Thailand. Prospective studies were undertaken to determine antimalarial efficacy of different drug regimens against a backdrop of increasing drug resistance. The transmission of malaria is low and seasonal with a frequency of about one P vivax, and one P falciparum infection every 2 years per person. In this region, almost all falciparum malaria infections result in symptoms.13
Patients
Between 1990 and 2002, more than 10 000 patients with microscopically confirmed uncomplicated falciparum malaria were recruited into prospective chemotherapeutic studies. We excluded pregnant women, children weighing less than 5 kg, or patients with signs of severity, or concomitant disease that necessitated hospital admission. Patients presenting without a previous episode of malaria in the preceding 63 days were defined as having primary infections.
On admission, a full clinical examination was completed and blood was taken for parasite counting. After randomisation, patients were treated and seen daily until resolution of symptoms and parasitaemia, and then received follow-up once a week for between 28–63 days to assess the clinical and parasitological efficacy of the drug regimens. Before 1995, cure rates were defined as the cumulative percentages of patients who remained aparasitaemic for 28 days after treatment. In these drug studies, re-appearance of parasites could be the consequence of either treatment failure or re-infection, although cure rates were adjusted based on coincident epidemiological data of infection rates. After 1995, parasite genotyping allowed more reliable differentiation between treatment failure and reinfection.14
All clinical and laboratory studies were approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, and by the Karen Refugee Committee. Molecular studies were approved by the Wandsworth Local Research Ethics Committee (UK). Patients or their accompanying relatives gave verbal informed consent.
Isolation of P falciparum and drug sensitivity assay
From 1995 onwards, fresh isolates of P falciparum were collected routinely and analysed for drug susceptibility in vitro. We did assays within 4–8 h of collection, without previous cryopreservation, using 3H-hypoxanthine uptake inhibition and with quality control measures that are described elsewhere.15 If more than 90% of parasites were at the ring stage and parasitaemia was more than 0·5% infected red blood cells the drug susceptibility of the isolate was tested. If not, infected erythrocytes were cultured until a target parasitaemia of 0·5% was achieved. Mefloquine, artesunate and quinine sensitivities were assessed in all specimens. We assessed dihydroartemisinin sensitivity separately in 100 isolates. Halofantrine and chloroquine sensitivities were assessed in isolates collected in 1995.
Sample collection and DNA extraction
Before 1995, venous blood or capillary samples collected from patients for pharmacokinetic studies or to assess haematological profiles were used for DNA extraction.16 After 1995, DNA was extracted mainly from isolates undergoing susceptibility assays in vitro and parasite genotyping.14,15 In-vitro susceptibility testing was successful in 1077 of 1420 (76%) isolates tested. DNA was available in 920 of these isolates, from which 189 samples were selected for molecular typing without prior knowledge of parasites’ inhibitory concentration of 50% (IC50) values or clinical responses.
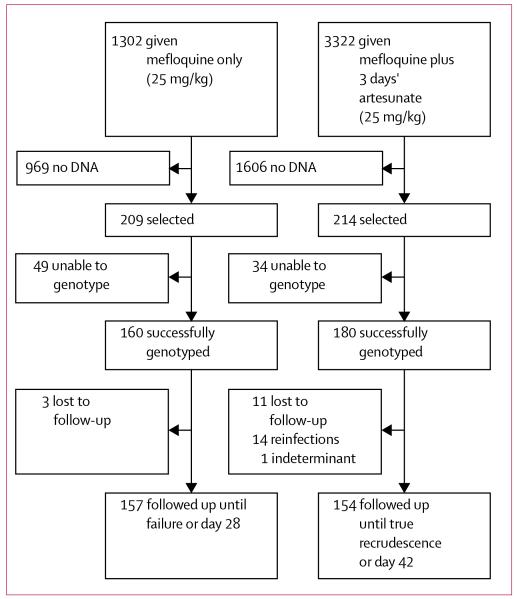
During the course of the study, 4624 patients were enrolled into trials, randomly allocated either mefloquine monotherapy (25 mg/kg) or mefloquine plus 3 days artesunate 4 mg/kg/day (MAS3). Treatment response was documented. From these 4624 patients, 423 isolates were selected for molecular typing: 209 treated with mefloquine monotherapy and 214 treated with mefloquine plus 3 days’ artesunate (figure 1). 34 of these 423 isolates had also undergone in-vitro sensitivity testing and are, therefore, included in the 189 samples mentioned previously (ie, those that were also included in in-vitro tests). To investigate changes in genotypes over time, a further 40 isolates were selected without knowledge of treatment outcomes from 2347 patients, with primary infections treated with regimens that did not include mefloquine. Hence, 618 isolates were selected for genotyping (189+423−34+40).
Figure 1. Samples analysed.
DNA was available from whole blood (in edetic acid [EDTA]) or heparinised tubes, stored at −70 °C), or 50 μL of capillary blood transferred to filter paper (Whatman, Maidstone, UK). DNA from haemolysates (100 μL) was purified using QIAamp Blood Kit (Qiagen, Crawley, UK) or 96 well plate Wizard DNA purification system (Promega, Southampton, UK). DNA from filter paper was extracted by using PrepMan Ultra (400 μL, 10 min at 95 °C; Applied Biotech, Warrington, UK). Samples were centrifuged, DNA was then precipitated from the supernatant with use of standard procedures and resuspended in water (100 μL).
Quantitation of pfmdr1 copy number by TaqMan real-time PCR
Pfmdr1 copy number was assessed by TaqMan real-time PCR (ABI sequence detector 7700; Applied Biosystems, Warrington, UK). Primers and probes were designed with Prism 7700 sequence-detection software (Primer Express, Applied Biosystems) and are summarised in table 1. The pfmdr1 probe was FAM (6-carboxyfluorescein) labelled at the 5′-end, and the β-tubulin probe was VIC labelled. Both probes had a TAMRA (6-carboxytetramethylrhodamine) label at the 3′-end.
Table 1. Primer sequences.
| Primer | Sequence | |
|---|---|---|
| TaqMan | pfmdr1–1F | 5′-TGC ATC TAT AAA ACG ATC AGA CAA A |
| pfmdr1–1R | 5′-TCG TGT GTT CCA TGT GAC TGT | |
| pfmdr1-probe | 5′-TTT AAT AAC CCT GAT CGA AAT GGA ACC TTT G | |
| β-tubulin-1F | 5′-TGA TGT GCG CAA GTG ATC C | |
| β-tubulin-1R | 5′-TCC TTT GTG GAC ATT CTT CCT C | |
| β-tubulin-probe | 5′-TAG CAC ATG CCG TTA AAT ATC TTC CAT GTC T | |
| pfATP6 89 | pfATP6–1OF | 5′-GGA AGA GGT TAT TAA GAA TGC |
| pfATP6–1OR | 5′-GCT TCA ACA TTT CCT TCA TC | |
| pfATP6–1NF | 5′-TAT TAG ATA TGA AAC ATA AAA ATC | |
| pfATP6–1NR | 5′-GGA GTT TTA TTA CCA ACA CTC AAT TCA |
Amplification reactions were done as multiplex PCR in MicroAmp 96 well plates (Applied Biosystems) in 25 μL, containing TaqMan buffer (8% glycerol, 0·625 U DNA polymerase, 5·5 mmol/L MgCl2, 300 μmol/L dNTP, 600 nmol/L passive reference dye ROX (5-carboxy-X-rhodamine), pH 8·3), 300 nmol/L of each forward and reverse primer, 100 nmol/L of each probe, and 5 μL of template DNA. 50 cycles were performed (95 °C for 15 s and at 58 °C for 1 min).
Fluorescence data were expressed as normalised reporter signal, calculated by dividing the amount of reporter signal by the passive reference signal. The detection threshold was set above the mean baseline value for fluorescence of the first 15 cycles. The threshold cycle (Ct) is when the increase in reporter signal is first detected above baseline. Results were analysed by a comparative Ct method, based on the tested assumption that the target (pfmdr1) and reference (β tubulin) amplify with the same efficiency within an appropriate range of DNA concentrations.
For each starting concentration of genomic DNA, ΔCt=CtR−CtG, where CtR is the reference Ct, and CtG is the target. This value was plotted against the log of initial DNA concentration. The efficiencies of gene amplification were sufficiently close to obviate the need for a correction factor. Therefore, we applied the comparative ΔΔCt method: ΔΔCt=CtE−CtB, where CtE denotes the experimental Ct and CtB the baseline Ct. Every Taqman run contained three reference DNA samples from clones 3D7, K1, and Fac8 having pfmdr1 copy numbers, of 1, 1, and 3, respectively. Relative expression was then calculated as 2−ΔΔCt to account for the exponential properties of PCR. All reactions were performed in triplicate and were rejected if they did not conform to exponential kinetics. Assays were repeated if one of the following three results was obtained: ΔΔCt spread >1·5; Ct values >35; or copy number 1·3-1·6.
Assessment of clonality and detection of polymorphisms in pfmdr1, pfcrt, and pfATP6
Polymorphisms in MSP-1, MSP-2, and GLURP were used to assess clonality and distinguish between reinfection and recrudescence as described previously.14 Single nucleotide polymorphisms in pfmdr1 and pfcrt were detected by nested PCR-restriction fragment length polymorphism methods, also described elsewhere.8, 17 Similar methods were designed for analysis of polymorphism in pfATP6 with use of primers given in table 1, followed by digestion of PCR products with XbaI. All PCRs and digests included as positive and negative controls the DNA of laboratory strains 3D7, HB3, 7G8, and Dd2.
Statistical analysis
Data were analysed with SPSS for Windows. The Mann-Whitney U test or Kruskal-Wallis method were used for non-parametric comparisons, and Student’s t test or one-way analysis of variance for parametric comparisons. For categorical variables, percentages and corresponding 95% CI were calculated. We assessed proportions using the χ2 test with Yates’ correction or by Fisher’s exact test.
For polyclonal infections, the assessment of copy number generates a figure representing a weighted composite of the copy numbers of genes in individual parasite clones. Therefore, we assessed the relation between copy number and drug susceptibility using a continuous variable for copy number as well as an ordinal variable in which copy number was rounded to the nearest integer. The repeatability coefficient for copy number assay was calculated as described elsewhere.18
The relation between genotypic data (codon mutation and gene amplification) and clinical response to treatment (parasite clearances times and overall cure rates) was assessed by survival analysis. Cumulative incidences were compared by the Mantel-Haenszel log-rank test. Previously identified risks for failing mefloquine treatment (fever and vomiting, baseline parasitaemia)19 and mefloquine with artesunate treatment (age, presenting parasitaemia, and single species P falciparum infections20), were included in Cox’s regression analysis when assessing the association of pfmdr1 polymorphisms and treatment response. Linear regression analysis was used to assess the relation between the prevalence in parasites with increased pfmdr1 copy number and declining mefloquine efficacy.
Role of the funding source
The sponsors of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between 1990, and June, 1994, 1302 patients with uncomplicated falciparum malaria were enrolled into chemotherapeutic studies. They were given mefloquine monotherapy and then followed-up for between 28 and 63 days (figure 1). By June, 1994, the cumulative cure rate had fallen from 95% to 63%, with 13% high grade failures (ie, failures by 7 days after treatment). Hence, the first-line treatment was changed to mefloquine with 3 days’ artesunate (MAS3). By 2002, 3322 patients had been treated with MAS3 and the therapeutic response documented. The efficacy of MAS3 remained high (cure rate greater than 95% at 42 days).
Validation of TaqMan assay
Repeated assay of reference laboratory isolates with known pfmdr1 gene copy numbers: 3D7 (1 copy), D10 (1 copy) and Fac8 (3 copies)21-23 confirmed that the real-time PCR assay had high precision and accuracy (table 2). In 105 clinical samples, the repeatability coefficient when assayed twice was 0·64 (viz 95% of repeated estimates of pfmdr1 copy number were + or −0·32 of the first). This coefficient was affected by copy number: for 23 isolates with a copy number of 1 the repeatability coefficient was 0·26, compared with 0·68 in the 23 isolates with a copy number of 2, and 0·58 in the 24 isolates with a copy number of three or more.
Table 2. Taqman validation with laboratory isolates.
| Strain | Copy | Repeats | Mean estimate | SD (range) |
|---|---|---|---|---|
| 3D7 | 1 | 55 | 1·04 | 0·13 (0·73–1·35) |
| D10 | 1 | 50 | 0·98 | 0·16 (0·71–1·51) |
| K1 | 1 | 18 | 1·06 | 0·14 (0·74–1·34) |
| Fac8 | 3 | 30 | 2·96 | 0·31 (2·31–3·47) |
Pfmdr1 copy number was ascertained in 532 (86%) samples assessed. Samples older than 5 years were more difficult to assay, (85 (20%) of these were not informative compared with 1 of 186 more recent samples; p<0·001), as were infections with a baseline parasitaemia less than 10 000/μL (69 [30%] failed assays vs 17 [6%], respectively; p<0·001) and samples from mixed P falciparum and P vivax infections (37 [74%] vs 46 unmixed P falciparum infections [9·6%]; p<0·001).
Overall, copy number was not related to baseline parasitaemia, mixed (P falciparum and P vivax) infections, clonality of infection, age, sex, presence of fever or vomiting, or recrudescent infections.
Pfmdr1 copy number and in-vitro drug sensitivities
Of the 189 isolates with results from in-vitro sensitivity testing, genotyping was successful in 187 (99%) cases. When rounded to the nearest integer, 85 (46%) isolates had a pfmdr1 copy number of one, 55 (29%) had two copies, 39 (21%) had three copies, six (3%) had four copies and two (1%) had five copies. Overall, 34 (23%) isolates were polyclonal (30 infections having two clones, three having three clones, and one having four clones).
Increased pfmdr1 copy number was associated with reduced antimalarial drug susceptibility; parasites with an increased copy number had significantly higher median IC50 values for mefloquine, artesunate, halofantrine, quinine, and dihydroartemisinin (table 3). There was no such association for in-vitro sensitivity to chloroquine (table 3). In those isolates with multiple copies of pfmdr1, copy number was correlated positively with IC50 to mefloquine (rs=0·28, p=0·008), but not with any of the other antimalarials tested. With a cutoff for mefloquine resistance of 45 ng/mL,8 increased copy number predicted in-vitro mefloquine resistance with a sensitivity of 81% (95% CI 71–88) and specificity of 71% (61–79). Overall, 97 (53·3%) isolates assayed for in-vitro mefloquine susceptibility had an increase in pfmdr1 copy number with a relative risk of in vitro resistance of 3·6 (2·3–5·7, p<0·001). Therefore, the population-attributable risk of in-vitro mefloquine resistance associated with increased copy number was 58%.
Table 3. IC50 values for antimalarials in relation to pfmdr1 copy number.
| Single pfmdr1 copy number |
Increased pfmdr1 copy number |
p* |
|||
|---|---|---|---|---|---|
| Number IC50 (ng/mL) | Number | IC50 (ng/mL) | |||
| Mefloquine | 82 | 18·7 (4·3–174·9) | 91 | 64·3 (6·9–214·6) | <0·001 |
|
| |||||
| Artesunate | 83 | 1·36 (0·18–23·47) | 95 | 2·38 (0·2–17·48) | <0·001 |
|
| |||||
| Dihydroartemisinin | 46 | 1·04 (0·17–4·66) | 54 | 1·75 (0·1–6·08) | 0·02 |
|
| |||||
| Quinine | 81 | 292·5 (34·1–1350·0) | 94 | 556·8 (55·8–1615·5) | <0·001 |
|
| |||||
| Chloroquine | 53 | 170·0 (39·1–731·0) | 72 | 127·9 (36·6–683·5) | NS |
|
| |||||
| Halofantrine | 62 | 3·2 (0·5–33·7) | 59 | 13·2 (0·5–56·5) | <0·001 |
NS=not significant. Data are median (range)
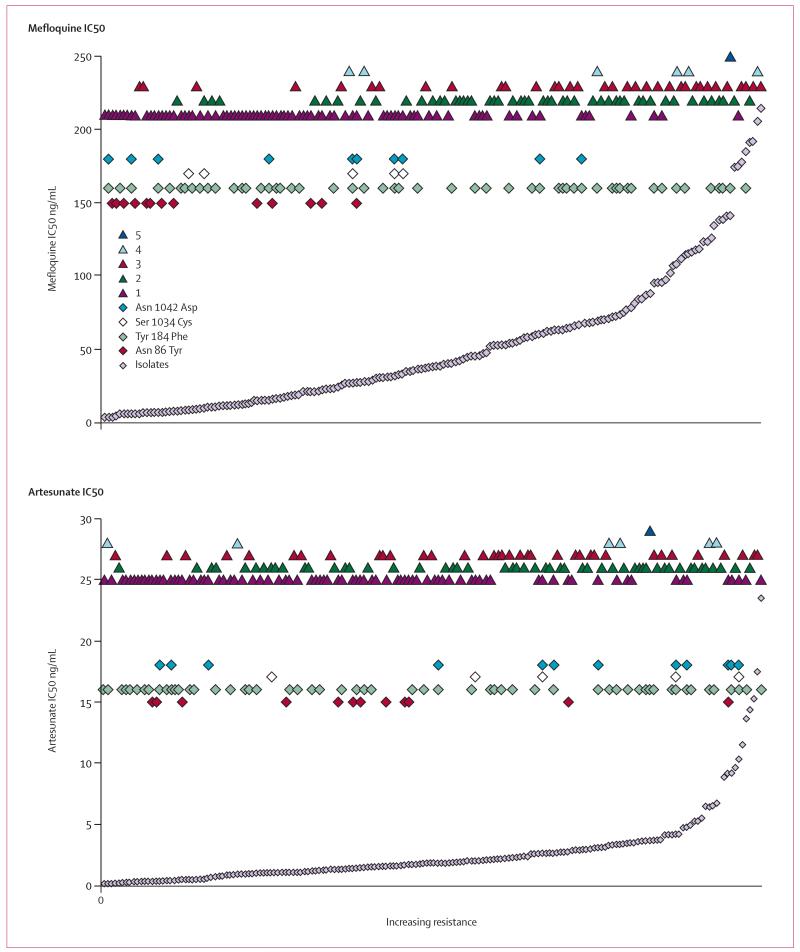
Polymorphisms in pfmdr1 were associated with increased mefloquine susceptibility in vitro and single pfmdr1 copy number (figure 2). In those isolates with single copies of pfmdr1, the N86Y mutation was associated with lower IC50s to mefloquine than were the wild genotype (median IC50 8·0 ng/mL [range 4·4–28·7] vs 20·8 ng/mL [4·3–175]; p=0·002). By contrast, the presence of either the S1034C mutation or the N1042D mutation in isolates with single copies of pfmdr1 was associated with a higher artesunate IC50s than were those with wildtype at both these loci (2·69 ng/mL [0·04–10·3] vs 1·27 ng/mL [0·18–23·5]; p=0·004). There was no significant difference in the IC50 values of wild and mutated genotypes of pfmdr1 at any loci for any of the other drugs tested.
Figure 2. Distribution of mefloquine IC50 and artesunate IC50 by copy number (triangles) and codon mutations (diamonds).
Isolates (grey diamonds) are arranged in ascending order of mefloquine resistance or artesunate sensitivity.
25 (29%) isolates with single pfmdr1 copy number were mutated at either codon 86 1034, or 1042 compared with only one isolate out of 102 samples with increased copy number that carried a single mutation at codon 1034 (p<0·001, figure 2). We noted Y184F mutation in 36 (43%) isolates with a single copy of pfmdr1 compared with 20 (20%) cases with multiple pfmdr1 copies (p=0·01).
All 127 isolates tested contained the K76T polymorphism in pfcrt that is associated with chloroquine resistance. Position 1246 in pfmdr1 was not polymorphic in these 127 isolates, so neither site was assessed further.
Polymorphism in pfATP6
PfATP6 is a SERCA-type Ca2+ ATPase with 10 transmembrane helices, and is proposed to be the primary parasite target for artemisinins.24 As there was a large range of IC50 values for artesunate in our parasite isolates (figure 2), we fully sequenced pfATP6 from seven samples with high IC50 values for artesunate (median 14·4 ng/mL [range 9·6–23·5]). Two samples had nonsynonymous mutations at position 266 from T to C (codon 89) between M1 and M2 (membrane spanning regions) resulting in an I89T substitution (accession numbers pending). A polymorphism in tandem repeats in the large cytosolic domain of pfATP6 was not assessed further. Using these sequence data, we designed an oligonucleotide-based method to examine position 89 in a large numbers of parasites. In 119 samples, 11 (9·2%) carried the mutation and 2 (1·6%) had a mixed infection with both mutant and wild genotypes. Polymorphisms in pfATP6 were not associated with changes in drug susceptibility. There was no significant difference in IC50 values of wild type (median=2·1 ng/mL [0·26–23·5]) and polymorphic (1·3 ng/mL [0·31–11·5]) genotypes of pfATP6 for artesunate, or any other drugs (not shown).
Pfmdr1 copy number and in-vivo therapeutic response
To investigate the relationship between pfmdr1 and the treatment response in vivo, we genotyped parasites from patients with acute falciparum malaria who had received either mefloquine alone or mefloquine plus three days artesunate (figure 1). pfmdr1 copy number could be assessed reliably in 160 (76·6%) of 209 mefloquine treated patients, and 180 (84·1%) of 214 patients treated with mefloquine combined with artesunate (table 4).
Table 4. Baseline characteristics of all patients and those assessed for in-vivo responses to treatment and pfmdr1 copy number.
| Mefloquine |
Mefloquine+3 days’ artesunate |
|||
|---|---|---|---|---|
| Complete cohort | Selection genotyped | Complete cohort | Selection genotyped | |
| Patients (n) | 1302 | 160 | 3322 | 180 |
|
| ||||
| Men | 746 (57%) | 101 (63%) | 2012/3322 (61%) | 118/180 (66%) |
|
| ||||
| Median age (range) (years) | 14 (0·4–88) | 16 (1–60) | 12 (0·24–72) | 16 (0·64–60) |
|
| ||||
| Geometric mean (range) parasite count (μL–1) | 3383 (17–351 512) | 6818 (17–351 512)* | 5953 (7–822 415) | 26334 (100–479 261)* |
|
| ||||
| Baseline parasitaemia >10000/μL (%) | 404/1282 (32%) | 61/160 (38%) | 1400/3301 (42%) | 135/174 (78%)* |
|
| ||||
| Mixed P falciparum and P vivax infection (n) (%) | 185/1271 (15%) | 6/152 (4%) * | 532/3299 (16%) | 4/174 (2%)* |
|
| ||||
| Infections failing treatment before entry to study | 251/1293 (19%) | 28/160 (18%) | 44/3317 (1%) | 2/180 (1%) |
|
| ||||
| Fever† | 850/1294 (66%) | 119/160 (74%) | 1712/3281 (52%) | 90/155 (58%) |
|
| ||||
| Vomiting and febrile | 223/1272 (18%) | 37/160 (23%) | 461/3151 (15%) | 34/151 (23%)* |
|
| ||||
| Anaemia (packed cell volume <0·30) | 162/829 (20%) | 33/148 (22%) | 424/3126 (14%) | 19/152 (13%) |
|
| ||||
| Day 28 cure rate | 802/1068 (75%) | 126/157 (80%) | 2686/2885 (93%) | 146/175 (83%)* |
|
| ||||
| Day 42 cure rate (PCR corrected)‡ | NA | NA | 2323/2364 (98%) | 128/154 (83%)* |
NA=not available. Data are n (%) unless otherwise stated.
p<0·01 for comparison between selection and complete cohort.
>37·5°C oral or >38°C per axilla.
Polymorphisms at MSP-1, MSP-2, and GLURP were assessed to distinguish between reinfection and recrudescence.
Mefloquine monotherapy
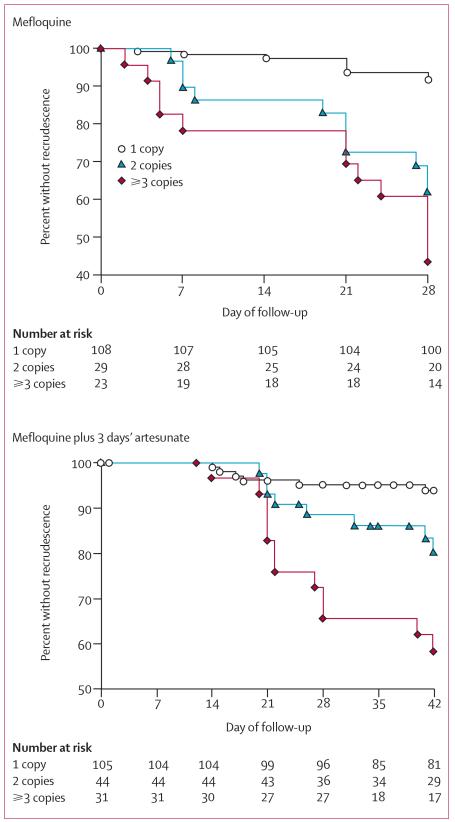
Pfmdr1 copy number was increased in 52 (32·5%) cases treated with mefloquine. Of these patients, 31 (19·7%) failed early (within 7 days) or recrudesced by day 28, with another five cases recrudescing by day 63 (figure 3). The median (range) time to failure of therapy was 21 days (2–51). Genotyping was successfully carried out in 9 (42·8%) treatment failures and showed that all were due to recrudescence rather than reinfection. Increased pfmdr1 copy number correlated with delayed parasite clearance. By day 3, 15 (30%) patients infected with parasites having more than one copy of pfmdr1 were still parasitaemic, compared with 12 (11·8%) with parasites having single copies (RR=2·6 [95% CI 1·3–5·0], p=0·01).
Figure 3. Cumulative percentage of patients free from malaria after treatment with mefloquine monotherapy or mefloquine and 3 days’ artesunate.
For mefloquine monotherapy, overall p< 0·001; p=0·2 for 2 vs ≥3 copies.
For mefloquine plus artesunate, overall p<0·001; p=0·042 vs ≥3.
The median pfmdr1 copy number in patients successfully treated by day 28 with mefloquine was 1·03 (range 0·46–4·13) compared with a copy number of 2·10 (range 0·54–3·84) in those who had treatment failure (p=0·001). In total, 9 (8·4%) patients infected with parasites carrying single copies of pfmdr1 had treatment failure by day 28 compared with 22 (44%) of those infected with parasites with increased pfmdr1 copy number (RR=5·2 [95% CI 2·6–11]; p<0·001) (figure 3). Increased pfmdr1 copy number, therefore, predicted failure at day 28 with a sensitivity of 0·71 (95% CI 0·53-0·84), a specificity of 0·78 (0·70–0·84) and a positive predictive value of 0·44 (0·31–0·58). Multiple pfmdr1 copy number was also associated with an increased risk of high-grade treatment failures. Only 2 (1·9%) patients infected with single copies had treatment failure within 7 days, compared with 4 (13·8%) of those with two copies and 5 (21·7%) of those with three or more pfmdr1 copies (p<0·001).
Pfmdr1 codon mutations at positions 86, 1034, and 1042 were present in 8 (5·2%), 5 (3·2%), and 45 parasites (29·2%) respectively, and mixed infection with both mutant and wild genotypes in 5 (3·2%), 5 (3·2%), and 7 parasites (4·5%), respectively. After stratifying by pfmdr1 copy number there was no significant relation between codon mutation and risk of failure by day 28.
Previous multivariable analysis in this population had identified baseline parasitaemia, fever, or vomiting as risk factors for failure of antimalarial treatment with mefloquine at day 28.19 After controlling for these variables in a Cox regression model, pfmdr1 copy number (any increased compared to single copy) was the most important predictor of failure (AHR 6·3 [95% CI 2·9–13·8], p<0·001). After stratifying by pfmdr1 copy number, the inclusion of pfmdr1 codon mutations did not improve the model. Overall, 32·5% (52/160) of patients treated with mefloquine monotherapy were infected with isolates with an increase in pfmdr1 copy number. Therefore, the population attributable risk of treatment failure by day 28 associated with increased pfmdr1 copy number was 63%.
Mefloquine-artesunate treatment
Of the 180 patients treated with mefloquine-artesunate therapy, 169 (94%) were followed-up until recurrence of parasitaemia (46 patients) or for at least 42 days (figure 3). PCR-corrected recrudescence was confirmed in 26 cases (ie, the rate of PCR confirmed recrudescence in this selected group was 26 [17%]). A further five infections recrudesced after day 42. The median (range) time to parasite clearance was 2 days (1–3) and to recrudescence was 22 days (14–54). Increased pfmdr1 copy number was not associated with a delay in fever or parasite clearance times.
Pfmdr1 copy number was increased in 75 (42%) isolates. Of those with single copies 6 (7%) failed by day 42 compared with 20 (31%) in those infected with parasites with increased pfmdr1 copy number (RR 4·6 [95% CI 1·9–10·7]; p<0·001) (figure 3). The failure rate at day 42 was higher in those patients harbouring isolates with three or more copies of pfmdr1 compared with two copies (figure 3; p=0·04). Increased pfmdr1 copy number predicted failure of mefloquine-artesunate treatment with a sensitivity of 0·77 (95% CI 0·58–0·89) and a specificity of 0·65 (0·56–0·73).
Codon mutations at positions 86, 1034, and 1042 were present in 14 (8·1%), 0 of 171, and 16 (9·4%) of cases, respectively, and mixed infection with both mutant and wild genotypes in 3 (1·8%), 1 (0·6%), and 3 (1·7%), respectively. After stratifying by pfmdr1 copy number, there was no significant association between the presence of codon mutation and recrudescence of parasites by day 42.
After controlling for age, baseline parasitaemia, and pure P falciparum infections in a Cox’s regression model,20 pfmdr1 copy number was the most important predictor of failure (AHR 5·4 [95% CI 2·0–14·6], p=0·001). The addition of pfmdr1 codon mutations did not improve this model.
Temporal trends in mefloquine efficacy
Between 1990 and 1994, 861 patients with primary infections were treated with mefloquine (25 mg/kg) and followed-up until day 28 or until parasite recrudescence. The cure rate fell from 95·2% in 1990 to 63% in 1994 (table 5). Of the 164 DNA samples successfully genotyped for pfmdr1 during these years, full clinical records, with day 28 failure rates were available for 136 (83%) patients. After stratifying by year of enrolment, there was no significant difference between the day 28 cure rates or baseline characteristics in the selected samples and those noted in the whole cohort of patients. The rise in the proportion of genotyped isolates with increased copies of pfmdr1 was correlated significantly with the day 28 cure rates for mefloquine in the population (table 5), p<0·001. In a linear regression model, the clinical efficacy at day 28 remained above 90% until the proportion of isolates with multiple copies of pfmdr1 rose to 28% (r2 0·97, p<0·001).
Table 5. Temporal trends of the efficacy of mefloquine monotherapy and parasite genotype in primary infections.
| 1990 | 1991 | 1992 | 1993 | 1994* | |
|---|---|---|---|---|---|
| Day 28 failure rate (complete cohort) | 3/63 (5%) | 41/314 (13%) | 56/231 (24%) | 65/180 (36%) | 27/73 (37%) |
| Day 7 failure rate (complete cohort) | 0/79 (0%) | 12/363 (3%) | 19/273 (7%) | 30/194 (16%) | 10/77 (13%) |
| Isolates with 1 copy of pfmdr1 | 32/42 (76%) | 17/60 (72%) | 28/51 (55%) | 4/11 (36%) | NA |
| Isolates with 2 copies of pfmdr1 | 6/42 (14%) | 8/60 (13%) | 14/51 (28%) | 5/11 (45%) | NA |
| Isolates with ≥3 copies of pfmdr1 | 4/42 (10%) | 9/60 (15%) | 9/51 (18%) | 2/11 (18%) | NA |
| N86Y | 3/38 (8%) | 4/60 (7%) | 1/48 (2%) | 0/11 (0%) | NA |
| S1034C | 2/39 (5%) | 2/60 (3%) | 1/47 (2%) | 0/11 (0%) | NA |
| N1042D | 7/39 (18%) | 23/39 (39%) | 7/47 (15%) | 2/11 (18%) | NA |
NA=not available.
Until July 1994.
The efficacy of MAS3 therapy for primary infections has not changed in the past 8 years. After correcting for reinfections, the cure rate at day 42 was 97% (1262 of 1301). Overall, 114 (45%) isolates had increased copy number. However, isolates selected for genotyping during these years had higher treatment failure rates than did the population as a whole (table 4), precluding assessment of any temporal trend.
Discussion
Molecular genotyping to identify drug resistant P falciparum parasites is of increasing importance in characterising the epidemiology of malaria and informing the choice of antimalarial treatment regimens. Genotyping for point mutations in the genes encoding dihydrofolate reductase and dihydropteroate synthase, the targets of antifols and sulphonamides respectively, is of established value in predicting susceptibility to these drugs in vivo and in vitro. More recently, single nucleotide polymorphisms in pfcrt, a putative intraparasitic transporter, have also been found to correlate with reduced sensitivity to chloroquine in vitro25 and to predict treatment failure.5
The P-glycoprotein pump encoded by pfmdr1 affects the intraparasitic concentrations of several important antimalarial drugs. Single nucleotide polymorphisms in pfmdr1 that alter sensitivity in vitro to structurally unrelated antimalarials26,27 have been identified in the past 15 years. However, the epidemiological significance of these single nucleotide polymorphisms is uncertain. Gene amplification of pfmdr1 was first suggested as a possible cause of antimalarial drug resistance 14 years ago.21 Few studies have attempted to discern the relation between amplification in pfmdr1 copy number and multidrug resistance in vitro from isolates derived from the field.7-12 No studies have related these findings to drug response in-vivo. With use of a reliable, sensitive, and rapid assay to quantify pfmdr1 copy number in large numbers of samples, we studied more than 600 parasite isolates from the western border of Thailand, where highly multidrug resistant falciparum malaria first emerged and is now established.
Increase in pfmdr1 copy number was associated with up to a 40-fold decrease in the in-vitro susceptibility to mefloquine. As expected, there were similar increases in IC50 values for structurally related antimalarials such as halofantrine and quinine. In-vitro mefloquine resistance (IC50 >45 ng/mL) arose in almost a fifth (16/85) of isolates, without any increase in pfmdr1 copy number. Mefloquine resistance with single copies of wild-type pfmdr1 may arise, therefore, in a small number of cases through other, as yet undefined, molecular mechanisms.
Sensitivity to artemisinins, which are unrelated to mefloquine in their mode of action24 but commonly used together with it in combinations, was also associated with increased pfmdr1 copy number through a 25-fold range of IC50 values. A target of the artemisinin derivatives has recently been shown to be pfATP6.24 Analysis of polymorphisms of this gene were not associated with any variation in IC50s to artesunate. This finding leaves pfmdr1 copy number as the main apparent modulator of sensitivity to these increasingly important drugs, and explains the correlations observed in susceptibility in vitro. It also explains why susceptibility to artemisinin and derivatives declined, albeit within a very low range, as mefloquine resistance worsened on the western border. This decline is not attributed to primary selection of artemisinin resistance.9 Fortunately, the concentrations of dihydroartemisinin, the main biologically active metabolite of artesunate in blood, far exceed the highest IC50 value observed in our in-vitro assays,28 suggesting that these higher IC50 values are unlikely to be of clinical relevance. The early therapeutic response after treatment with a regimen including artesunate is attributable to the artesunate component. Residual parasites still present after 4 days (two asexual cycles) are eliminated by mefloquine, which is intrinsically less active than artesunate but has a significantly longer elimination half life (about 14 days compared with 45 min for artesunate’s metabolite).1 This finding explains why in these in-vivo studies, increased pfmdr1 copy number was associated with a significant delay in parasite clearance after mefloquine monotherapy, but not in patients treated with MAS3. 3 days of treatment with an artemisinin derivative is insufficient to eradicate all the parasites in the body, and so the cure rate is determined also by susceptibility to mefloquine.
Previous studies from this region have shown that clinical features (age, fever, and vomiting) and admission parasitaemia predict the patients’ responses after treatments containing mefloquine.19,20 These variables, which reflect lower immunity and lower drug absorption, accounted for between 49% and 62% of treatment failures. In our study, pfmdr1 copy number was the most important predictor of failure after both mefloquine and MAS3 treatments in a multivariable analysis incorporating these other known variables. Infections with parasites with multiple pfmdr1 copy numbers increased the risk of treatment failure six-fold, with this variable alone accounting for more than 63% of mefloquine treatment failures. Our prediction of failure is improved to 67% if clinical variables are included in modelling and may be improved further if pharmacokinetic variables are available. However, amplification of pfmdr1 copy number explains most failures, even after combination treatment. Thus drug resistance, rather than host or disease factors, is the main determinant of treatment response in this area of relatively low antimalarial immunity.
These data, taken together with previous work, confirm the importance of genetic variation in parasite encoded transporters in altering susceptibility to various drugs. In African isolates, where drug pressure has resulted mainly from 4-aminoquinoline use and not mefloquine, the N86Y genotype of pfmdr1 might occur infrequently by itself, or more frequently (>50%) in association with K76T in pfcrt.5,29,30 This combined genotype (N86Y+K76T) is strongly associated with resistance to chloroquine. In Thailand, chloroquine has not been used to treat falciparum infections for more than 20 years, although it is still widely used to treat the other three malaria species that account for half of all infections. The K76T mutation is at fixation (>95%) (ie, reached the limit of its frequency in a population). However, the frequency of N86Y has declined substantially in this population, in effect dissociating it from K76T. This finding may be because considerable additional drug selection pressure with mefloquine has been exerted since the mid 1980s.
Our results show that although the N86Y mutation was associated with increased mefloquine susceptibility in vitro, it did not alter responses significantly in vivo. This finding is in contrast with increases in pfmdr1 copy number, which, in the absence of variation at codon 86, resulted in a greater than four-fold increase in risk of failure after antimalarial treatment. Furthermore, in all the field isolates assessed, increases in pfmdr1 copy number arose only with N86 and not with the N86Y variant. These findings provide strong support for pfmdr1 copy number being the main factor that determines in-vivo response, and suggest that the N86Y mutation is a negative marker for increased copy number.
Between 1990 and 1994, the efficacy of mefloquine monotherapy fell dramatically, a fall that was associated with a three-fold rise in the prevalence of parasites with increased pfmdr1 copy number (table 5). Extrapolation of these findings would suggest that, in a community in which host immunity to malaria is low, the day 28 cure rate of mefloquine will exceed 90% until the prevalence of infections with increased pfmdr1 copy number rises above 28%. Although these findings need to be tested in other endemic areas, our findings suggest that pfmdr1 copy number will become a useful tool in the population-based surveillance of drug resistance.
Acknowledgments
We thank the staff of the Shoklo Malaria Research Unit for their excellent work. The field studies were part of the Wellcome-Trust Mahidol University Oxford Tropical Medicine Research Programme supported by the Wellcome Trust of Great Britain. The molecular work was done at St Georges Hospital Medical School, London, and was funded by the Wellcome Trust (Grant 066201).
Glossary
- pfmdr1
This gene encodes a P-glycoprotein transporter belonging to the family of ATP-dependent transporters that also mediate multidrug resistance in some human tumours. It is localised to the parasite’s food vacuole.
- pfcrt
utations in this protein cause chloroquine resistance.
- Real-time PCR
This technique monitors products of DNA amplification by fluorescence techniques as they are generated. Comparision of results with controls allows estimation of gene copy number.
- PfATP6
This is an ATP-dependent calcium pump, similar to mammalian SERCA (sarcoplasmic/endoplasmic reticulum type calcium ATPase) pumps and is a receptor for artemisinin antimalarials.
Footnotes
Conflict of interest statement
None declared.
References
- 1.White NJ. Antimalarial drug resistance and combination chemotherapy. Philos Trans R Soc Lond B Biol Sci B. 1999;354:739–49. doi: 10.1098/rstb.1999.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korenromp EL, Williams BG, Gouws E, Dye C, Snow RW. Measurement of trends in childhood malaria mortality in Africa: an assessment of progress toward targets based on verbal autopsy. Lancet Infect Dis. 2003;3:349–58. doi: 10.1016/s1473-3099(03)00657-1. [DOI] [PubMed] [Google Scholar]
- 3.Plowe CV. Folate antagonists and mechanisms of resistance. In: Rosenthal PJ, editor. Antimalarial chemotherapy. Humana Press; Totowa: 2001. pp. 173–90. [Google Scholar]
- 4.Vaidya AB. Atovaquone-proguanil combination. In: Rosenthal PJ, editor. Antimalarial chemotherapy. Humana Press; Totowa: 2001. p. 396. [Google Scholar]
- 5.Djimde A, Doumbo OK, Cortese JF, et al. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001;344:257–63. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 6.Nosten F, van Vugt M, Price R, et al. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet. 2000;356:297–302. doi: 10.1016/s0140-6736(00)02505-8. [DOI] [PubMed] [Google Scholar]
- 7.Wilson CM, Volkman SK, Thaithong S, et al. Amplification of pfmdr1 associated with mefloquine and halofantrine resistance in Plasmodium falciparum from Thailand. Mol Biochem Parasitol. 1993;57:151–60. doi: 10.1016/0166-6851(93)90252-s. [DOI] [PubMed] [Google Scholar]
- 8.Price R, Cassar CA, Brockman A, et al. Pfmdr1 gene amplification and multidrug resistant Plasmodium falciparum on the North West border of Thailand. Antimicrob Agents Chemother. 1999;43:2943–49. doi: 10.1128/aac.43.12.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickard AL, Wongsrichanalai C, Purfield A, et al. Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob Agents Chemother. 2003;47:2418–23. doi: 10.1128/AAC.47.8.2418-2423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basco LK, Le Bras J, Rhoades Z, Wilson CM. Analysis of pfmdr1 and drug susceptibility in fresh isolates of Plasmodium falciparum from subsaharan Africa. Mol Biochem Parasitol. 1995;74:157–66. doi: 10.1016/0166-6851(95)02492-1. [DOI] [PubMed] [Google Scholar]
- 11.Basco LK, de Pecoulas PE, Le Bras J, Wilson CM. Plasmodium falciparum: molecular characterization of multidrug-resistant Cambodian isolates. Exp Parasitol. 1996;82:97–103. doi: 10.1006/expr.1996.0013. [DOI] [PubMed] [Google Scholar]
- 12.Zalis MG, Pang L, Silveira MS, Milhous WK, Wirth DF. Characterization of Plasmodium falciparum isolated from the Amazon region of Brazil: evidence for quinine resistance. Am J Trop Med Hyg. 1998;58:630–37. doi: 10.4269/ajtmh.1998.58.630. [DOI] [PubMed] [Google Scholar]
- 13.Luxemburger C, Thwai KL, White NJ, et al. The epidemiology of malaria in a Karen population on the western border of Thailand. Trans R Soc Trop Med Hyg. 1996;90:105–11. doi: 10.1016/s0035-9203(96)90102-9. [DOI] [PubMed] [Google Scholar]
- 14.Brockman A, Paul RE, Anderson TJ, et al. Application of genetic markers to the identification of recrudescent Plasmodium falciparum infections on the northwestern border of Thailand. Am J Trop Med Hyg. 1999;60:14–21. doi: 10.4269/ajtmh.1999.60.14. [DOI] [PubMed] [Google Scholar]
- 15.Brockman A, Price RN, van Vugt M, et al. Plasmodium falciparum antimalarial drug susceptibility on the north-western border of Thailand during five years of extensive use of artesunate-mefloquine. Trans R Soc Trop Med Hyg. 2000;94:537–44. doi: 10.1016/s0035-9203(00)90080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson JA, Price R, ter Kuile F, et al. Population pharmacokinetics of mefloquine in patients with acute falciparum malaria. Clin Pharmacol Ther. 1999;66:472–84. doi: 10.1016/S0009-9236(99)70010-X. [DOI] [PubMed] [Google Scholar]
- 17.Duraisingh MT, Jones P, Sambou I, von Seidlein L, Pinder M, Warhurst DC. The tyrosine-86 allele of the pfmdr1 gene of Plasmodium falciparum is associated with increased sensitivity to the anti-malarials mefloquine and artemisinin. Mol Biochem Parasitol. 2000;108:13–23. doi: 10.1016/s0166-6851(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 18.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 19.ter Kuile FO, Luxemburger C, Nosten F, et al. Predictors of mefloquine treatment failure: a prospective study of 1590 patients with uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg. 1995;89:660–64. doi: 10.1016/0035-9203(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 20.Price RN, Nosten F, Luxemburger C, et al. Artesunate/mefloquine treatment of multi-drug resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:574–77. doi: 10.1016/s0035-9203(97)90032-8. [DOI] [PubMed] [Google Scholar]
- 21.Foote SJ, Thompson JK, Cowman AF, Kemp DJ. Amplification of the multidrug resistance gene in some chloroquine-resistant isolates of P falciparum. Cell. 1989;57:921–30. doi: 10.1016/0092-8674(89)90330-9. [DOI] [PubMed] [Google Scholar]
- 22.Price R, Robinson G, Brockman A, Cowman A, Krishna S. Assessment of pfmdr1 gene copy number by tandem competitive polymerase chain reaction. Mol Biochem Parasitol. 1997;85:161–69. doi: 10.1016/s0166-6851(96)02822-8. [DOI] [PubMed] [Google Scholar]
- 23.Barnes DA, Foote SJ, Galatis D, Kemp DJ, Cowman AF. Selection for high-level chloroquine resistance results in deamplification of the pfmdr1 gene and increased sensitivity to mefloquine in Plasmodium falciparum. Embo J. 1992;11:3067–75. doi: 10.1002/j.1460-2075.1992.tb05378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckstein-Ludwig U, Webb RJ, Van Goethem ID, et al. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 25.Fidock DA, Nomura T, Talley AK, et al. Mutations in the P falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6:861–71. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. PgH1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403:906–09. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 27.Foote SJ, Cowman AF. The mode of action and the mechanism of resistance to antimalarial drugs. Acta Tropica. 1994;56:157–71. doi: 10.1016/0001-706x(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 28.Krishna S, Planche T, Agbenyega T, et al. Bioavailability and preliminary clinical efficacy of intrarectal artesunate in Ghanaian children with moderate malaria. Antimicrob Agents Chemother. 2001;45:509–16. doi: 10.1128/AAC.45.2.509-516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adagu IS, Warhurst DC. Plasmodium falciparum: linkage disequilibrium between loci in chromosomes 7 and 5 and chloroquine selective pressure in Northern Nigeria. Parasitology. 2001;123:219–24. doi: 10.1017/s0031182001008344. [DOI] [PubMed] [Google Scholar]
- 30.Thomas SM, Ndir O, Dieng T, et al. In vitro chloroquine susceptibility and PCR analysis of pfcrt and pfmdr1 polymorphisms in Plasmodium falciparum isolates from Senegal. Am J Trop Med Hyg. 2002;66:474–80. doi: 10.4269/ajtmh.2002.66.474. [DOI] [PubMed] [Google Scholar]