Abstract
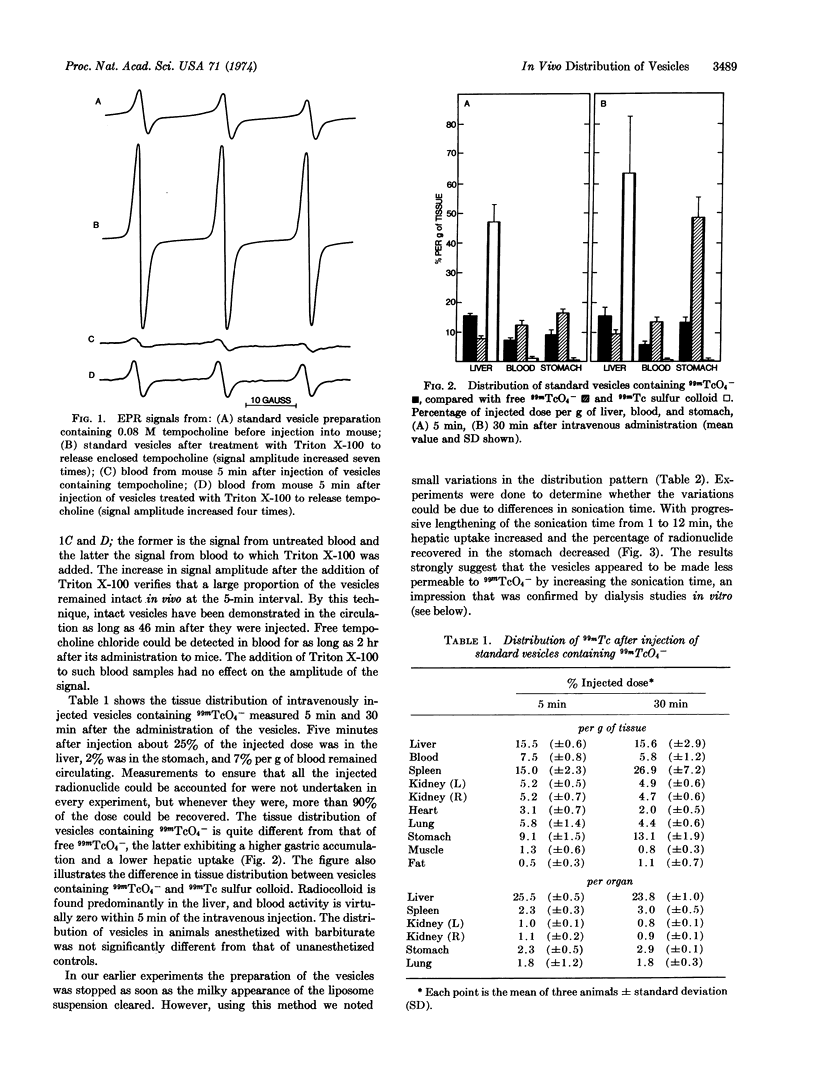
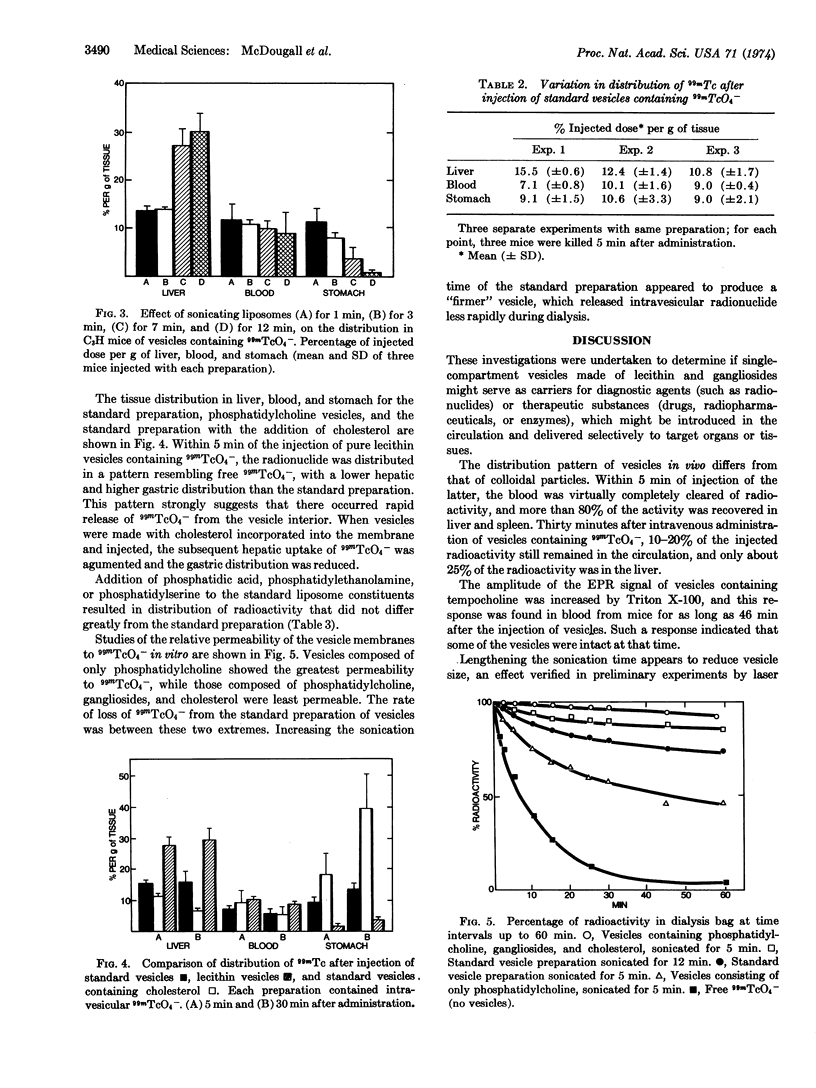
Single compartmental spherules of various lipid constituents (vesicles), enclosing 99mTcO4- as a radioactive marker, were injected intravenously into C3H mice, and the distribution of radioactivity was studied. About 25% of the administered radioactivity was present in the liver 5 min and 30 min after the injection of vesicles composed of phosphatidylcholine and gangliosides, which were sonicated for 5 min (standard preparation). About 10-20% of the radioactivity remained in the circulation. By use of a nonradioactive spin label (tempocholine) enclosed within vesicles, intact vesicles were demonstrated in the circulation for 46 min after intravenous injection. The distribution of radioactivity from 99mTcO4- inside vesicles is very different from that of free 99mTcO4- or of 99mTc sulfur colloid.
Increase in the length of sonication or incorporation of cholesterol into the wall of the vesicles enhanced hepatic levels and reduced blood levels of radioactivity. These same manipulations also slowed the rate of transfer of 99mTcO4- out of vesicles in dialysis experiments in vitro. Addition of phosphatidic acid, phosphatidylethanolamine, or phosphatidylserine to the standard constituents did not greatly alter the distribution of radioactivity in vivo but did increase the number and type of active coupling sites on the outside of the vesicle. The results indicate that vesicles might be valuable as carriers of diagnostic or therapeutic agents.
Keywords: liposomes, tempocholine, pertechnetate
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boggs J. M., Hsia J. C. Effect of cholesterol and water on the rigidity and order of phosphatidylcholine bilayers. Biochim Biophys Acta. 1972 Dec 1;290(1):32–42. doi: 10.1016/0005-2736(72)90049-1. [DOI] [PubMed] [Google Scholar]
- Finer E. G., Flook A. G., Hauser H. Mechanism of sonication of aqueous egg yolk lecithin dispersions and nature of the resultant particles. Biochim Biophys Acta. 1972 Jan 27;260(1):49–58. doi: 10.1016/0005-2760(72)90073-2. [DOI] [PubMed] [Google Scholar]
- Finkelstein A. Thin lipid membranes. A model for cell membranes. Arch Intern Med. 1972 Feb;129(2):229–240. [PubMed] [Google Scholar]
- Gregoriadis G., Buckland R. A. Enzyme-containing liposomes alleviate a model for storage disease. Nature. 1973 Jul 20;244(5412):170–172. doi: 10.1038/244170a0. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Ryman B. E. Fate of protein-containing liposomes injected into rats. An approach to the treatment of storage diseases. Eur J Biochem. 1972 Jan 21;24(3):485–491. doi: 10.1111/j.1432-1033.1972.tb19710.x. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Ryman B. E. Lysosomal localization of -fructofuranosidase-containing liposomes injected into rats. Biochem J. 1972 Aug;129(1):123–133. doi: 10.1042/bj1290123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H. O. The effect of ultrasonic irradiation on the chemical structure of egg lecithin. Biochem Biophys Res Commun. 1971 Nov;45(4):1049–1055. doi: 10.1016/0006-291x(71)90443-8. [DOI] [PubMed] [Google Scholar]
- Hauser H., Barratt M. D. Effect of chain length on the stability of lecithin bilayers. Biochem Biophys Res Commun. 1973 Jul 17;53(2):399–405. doi: 10.1016/0006-291x(73)90675-x. [DOI] [PubMed] [Google Scholar]
- Hsia J. C., Long R. A., Hruska F. E., Gesser H. D. Steroid-phosphatidylcholine interactions in oriented multibilayers--a spin label study. Biochim Biophys Acta. 1972 Dec 1;290(1):22–31. doi: 10.1016/0005-2736(72)90048-x. [DOI] [PubMed] [Google Scholar]
- Huang C., Charlton J. P. Studies on the state of phosphatidylcholine molecules before and after ultrasonic and gel-filtration treatments. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1660–1666. doi: 10.1016/0006-291x(72)90800-5. [DOI] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Johnson S. M., Bangham A. D., Hill M. W., Korn E. D. Single bilayer liposomes. Biochim Biophys Acta. 1971 Jun 1;233(3):820–826. doi: 10.1016/0005-2736(71)90184-2. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., McConnell H. M. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry. 1971 Mar 30;10(7):1111–1120. doi: 10.1021/bi00783a003. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., McNamee M. G., McConnell H. M. Measurement of transmembrane potentials in phospholipid vesicles. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1508–1513. doi: 10.1073/pnas.69.6.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae J., Sugar R. M., Shipley B., Hook G. R. Alterations in tissue distribution of 99mTc-pertechnetate in rats given stannous tin. J Nucl Med. 1974 Mar;15(3):151–155. [PubMed] [Google Scholar]
- Oldfield E., Chapman D. Dynamics of lipids in membranes: Heterogeneity and the role of cholesterol. FEBS Lett. 1972 Jul 1;23(3):285–297. doi: 10.1016/0014-5793(72)80300-4. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Cowden M., Kimelberg H. Role of cholesterol in membranes. Effects on phospholipid-protein interactions, membrane permeability and enzymatic activity. Biochim Biophys Acta. 1973 Nov 30;330(1):8–26. doi: 10.1016/0005-2736(73)90280-0. [DOI] [PubMed] [Google Scholar]
- Patton D. D., Garcia E. N., Webber M. M. Simplified preparation of technetium 99m sulfide colloid for liver scanning. Am J Roentgenol Radium Ther Nucl Med. 1966 Aug;97(4):880–885. doi: 10.2214/ajr.97.4.880. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]



