Abstract
Objectives
Despite evidence that radiation therapy (RT) improves outcome in multiple malignancies, some patients with strong clinical indications still refuse RT. Data on factors associated with RT refusal are limited. Furthermore, the effect of RT refusal on outcome has not been clearly defined.
Methods
Patients with nonmetastatic cancer, diagnosed between 1988 and 2005, were identified in the Surveillance, Epidemiology, and End Results database. Univariate and multivariate methods were used to identify factors associated with RT refusal and the impact of refusal on outcomes.
Results
On univariate analysis, age, sex, marital status, tumor site, and tumor stage were associated with RT refusal (P < 0.001). On multivariate analysis, sex and tumor stage were not found to be associated with RT refusal. In contrast, age, race, marital status, and tumor location were significantly associated with RT refusal. The median survival of compliant patients was 171 months compared with just 96 months among patients who refused RT.
Conclusions
A significant percentage of patients continue to refuse RT despite medical advice and evidence. Subgroups at particular risk of RT refusal include elderly, black and widowed patients. RT refusal is associated with markedly worse clinical outcomes.
Keywords: radiation, refusal, SEER
Modern cancer management is increasingly multimodal and radiation therapy (RT) is recommended as a component of care in many patients with nonmetastatic, solid malignancies. Despite considerable clinical evidence that RT improves outcomes in an array of cancers, some patients with strong clinical indications continue to refuse RT. Limited evidence is available to permit rigorous assessment of factors associated with the refusal of RT. Such data may prove useful to clinicians as they approach patients reluctant to undergo recommended RT.
Prior efforts to characterize factors associated with RT refusal have, in general, been limited by small patient numbers and selection of specific tumor types, tumor stages or patient subpopulations. Furthermore, much of the limited available evidence focuses on tumor characteristics. There are only scattered studies analyzing the socioeconomic and demographic factors associated with refusing RT. Detailed analyses of socioeconomic and demographic factors predictive of RT refusal may permit identification of particularly “high-risk” groups. This may in turn prompt focused investigations in such groups and heighten awareness of clinicians to the need for diligent patient counseling in those most likely to refuse recommended therapy. To define clinical, socioeconomic and demographic factors predictive of RT refusal, we used the Surveillance, Epidemiology, and End Results (SEER) database to evaluate radiation use and refusal with respect to patient, tumor, and treatment characteristics.
MATERIALS AND METHODS
Study Population
The SEER-17 registries data set of the US National Cancer Institute (April 2008 release) was used to identify all patients diagnosed with various, nonmetastatic cancers from January 1, 1988, to December 31, 2005 for whom RT was recommended.1 The sites of disease included were lung, head and neck, breast, prostate, skin, gastrointestinal (esophagus, stomach, pancreas, colon, rectum/rectosigmoid), cervix, and uterus. Additional inclusion criteria included known age, sex, race, marital status, RT use, and known tumor stage. A total of 309,278 patients met these inclusion criteria. Informed consent by the study participants and approval of an ethics committee were unnecessary to perform the analyses in this study since all of the information from the SEER database is deidentified.
Statistical Analysis
RT use and refusal were evaluated with respect to categoric variables corresponding to multiple patient, tumor, and treatment characteristics (age, sex, race, marriage status, sites of disease, and SEER stage [localized vs. regional]). Unadjusted associations of variables of interest were evaluated using the Pearson χ2 test. Multivariate analysis was used to establish 95% confidence intervals (CI) to quantify the use of radiation therapy across different subgroups. For multivariate analyses of RT refusal, binary logistic regression models were constructed in a forward stepwise fashion using specified variables of interest (age, race, sex, marital status, disease site, tumor stage, year of diagnosis, SEER registry). Variables were entered into the model with P < 0.05 and removed if the significance of that variable subsequently exceeded P = 0.10. These models were also used to assign a probability score to each patient regarding the likelihood of having refused RT. The resulting propensity scores for each patient could range from zero to one, with scores closer to unity suggesting a propensity toward adjuvant radiation. These values were then collapsed into deciles and used as a stratification variable to allow for propensity score correction for additional multivariate survival analyses.
Overall survival was assessed using Kaplan-Meier survival analysis. Patients were censored at either death or date of last follow-up. The median survival of patients either receiving or refusing RT was determined from the Kaplan-Meier curves. Two-sided Mantel-Cox log-rank tests were used to assess the significance of differences between survival curves. Cox proportional hazards models were also used to determine the hazard ratios associated with refusing RT. In both cases, propensity score correction was performed by stratifying patients by propensity score decile.
SEER*STAT software version 6.4.4 (Surveillance Research Program, NCI, Bethesda, MD) was used to extract case level data from the SEER public-use database. All analyses were conducted using the Statistical Package for the Social Sciences (SPSS, V14.0).
RESULTS
The study population consisted of 309,278 patients diagnosed with nonmetastatic cancer. Patient characteristics, primary site of disease, and SEER stage for this population are shown in Table 1. Breast cancer represented 60.8% of the cases, whereas head & neck cancer, lung cancer, and prostate cancer represented a significantly lower percentage of the cases. Table 2 shows RT refusal by patient and tumor characteristics. Of all 309,278 patients, 2.2% refused RT. Refusal remained fairly stable over the examined time period with annual rates ranging between 1.3% and 2.8% (Fig. 1). Patients who were older, male and unmarried (particularly widowed) were more likely to refuse RT. Patients with prostate and colon cancer were more likely to refuse RT than patients with other common malignancies.
TABLE 1.
Patient and Tumor Characteristics
| No. Patients (%) | |
|---|---|
| No. patients, total | 309,278 |
| Age (yr) | |
| <40 | 5.9 |
| 40–54 | 29.4 |
| 55–64 | 26.1 |
| 65–74 | 24.3 |
| ≥75 | 14.4 |
| Sex | |
| Male | 21.4 |
| Female | 78.6 |
| Race | |
| White | 84.5 |
| Black | 8.1 |
| Asian/Pacific Islander | 6.9 |
| American Indian/Alaska Native | 0.4 |
| Marriage status | |
| Single (never married) | 11.8 |
| Married | 62.8 |
| Separated/divorced | 11.3 |
| Widowed | 14.1 |
| Site of disease | |
| Lung | 5.1 |
| Head and neck | 8.5 |
| Breast | 60.8 |
| Prostate | 3.8 |
| Skin | 0.6 |
| Gastrointestinal | |
| Esophagus | 1.0 |
| Stomach | 1.7 |
| Pancreas | 1.1 |
| Colon | 1.0 |
| Rectum/rectosigmoid | 7.9 |
| Gynecologic | |
| Cervix | 2.2 |
| Uterus | 6.2 |
| SEER stage | |
| Localized | 56.8 |
| Regional | 43.2 |
TABLE 2.
RT Use by Patient and Tumor Characteristics
| RT Refusal (%) | P* | |
|---|---|---|
| All patients | 2.2 | |
| Age (yr) | <0.001 | |
| <40 | 1.2 | |
| 40–54 | 1.4 | |
| 55–64 | 1.7 | |
| 65–74 | 2.2 | |
| ≥75 | 5.1 | |
| Sex | <0.001 | |
| Male | 3.0 | |
| Female | 2.0 | |
| Race | 0.138 | |
| White | 2.2 | |
| Black | 2.1 | |
| Asian/Pacific Islander | 2.3 | |
| American Indian/Alaska Native | 3.1 | |
| Marriage status | <0.001 | |
| Single (never married) | 2.3 | |
| Married | 1.8 | |
| Separated/divorced | 2.3 | |
| Widowed | 4.0 | |
| Site of disease | <0.001 | |
| Lung | 2.0 | |
| Head and neck | 1.6 | |
| Breast | 1.9 | |
| Prostate | 7.6 | |
| Skin | 3.1 | |
| Gastrointestinal | ||
| Esophagus | 2.1 | |
| Stomach | 3.8 | |
| Pancreas | 2.5 | |
| Colon | 8.4 | |
| Rectum/rectosigmoid | 2.0 | |
| Gynecologic | ||
| Cervix | 1.6 | |
| Uterus | 2.3 | |
| SEER stage | <0.001 | |
| Localized | 2.4 | |
| Regional | 2.0 | |
Pearson χ2 value.
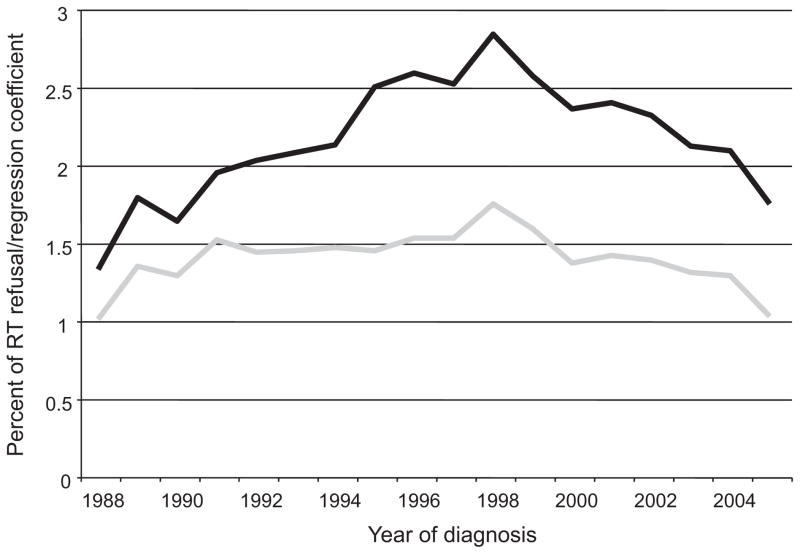
FIGURE 1.
Rate of RT refusal by year of diagnosis (black line) and associated regression coefficient (gray line). The latter value represents the coefficient associated with each year of diagnosis for a binary logistic regression model of RT refusal (1988 equals the reference category). See Table 3 for regression coefficients of other significant variables in the final model.
Logistic regression analysis of RT refusal and patient characteristics is shown in Table 3. On multivariate analysis, age remained an important factor in RT refusal, particularly with patients aged 75 years and older. Interestingly, multivariate analysis revealed that black patients were significantly more likely to refuse RT than white patients. Compared with single (never married) patients, married patients were more willing to accept RT whereas widowed patients were more likely to refuse it. Again, patients with prostate and colon cancer were most likely to refuse RT when offered.
TABLE 3.
Logistic Regression Model of RT Refusal by Tumor and Patient Characteristics*
| Regression Coefficient (95% CI) | P | |
|---|---|---|
| Age (yr) | <0.001 | |
| <40 | 1.00 (ref) | |
| 40–54 | 1.16 (1.00–1.34) | |
| 55–64 | 1.25 (1.08–1.45) | |
| 65–74 | 1.47 (1.27–1.70) | |
| ≥75 | 3.38 (2.92–3.91) | |
| Race | <0.001 | |
| White | 1.00 (ref) | |
| Black | 1.35 (1.23–1.49) | |
| Asian/Pacific Islander | 1.08 (0.97–1.20) | |
| American Indian/Alaska Native | 1.14 (0.79–1.63) | |
| Marriage status | <0.001 | |
| Single (never married) | 1.00 (ref) | |
| Married | 0.65 (0.60–0.71) | |
| Separated/divorced | 0.91 (0.82–1.00) | |
| Widowed | 1.15 (1.05–1.26) | |
| Site of disease | <0.001 | |
| Lung | 1.00 (ref) | |
| Head and neck | 0.76 (0.66–0.88) | |
| Breast | 0.85 (0.75–0.95) | |
| Prostate | 4.33 (3.79–4.95) | |
| Skin | 1.16 (0.87–1.54) | |
| Gastrointestinal | ||
| Esophagus | 1.20 (0.91–1.57) | |
| Stomach | 2.02 (1.68–2.43) | |
| Pancreas | 1.44 (1.13–1.84) | |
| Colon | 4.24 (3.57–5.04) | |
| Rectum/rectosigmoid | 0.95 (0.83–1.10) | |
| Gynecologic | ||
| Cervix | 0.84 (0.67–1.05) | |
| Uterus | 0.99 (0.85–1.15) |
In addition to the variables shown, year of diagnosis and geographic region (SEER registry) were statistically significant and were included in the final model (P < 0.001 for both variables). The variables SEER stage and patient sex did not reach statistical significance (P > 0.05) and were not included in the final model.
Ref indicates reference category.
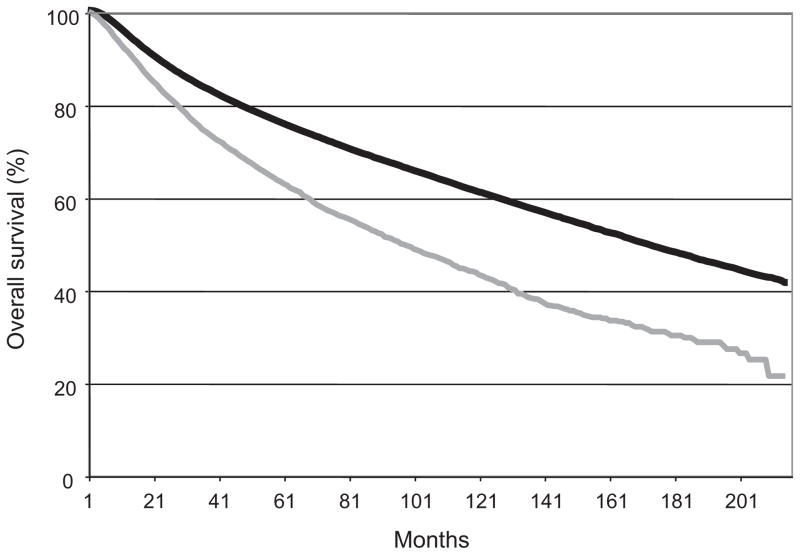
The overall survival in both the group of patients who accepted RT and those who refused are shown in Table 4 and Figure 2. The median survival for the patients who were compliant with RT recommendations was 171 months whereas the median survival for the patients who refused RT was only 96 months. The hazard ratio for the group who refused RT was 1.39 (95% CI: 1.34–1.45) after propensity score correction to account for other variables of interest.
TABLE 4.
Overall Survival of Patients Receiving and Refusing RT
| RT Given | RT Refused | P | |
|---|---|---|---|
| Median survival (months [±SE]) | 171 (±1) | 96 (±3) | <0.001* |
| Hazard ratio (95% CI)† | 1.00 (ref) | 1.39 (1.34–1.45) | <0.001 |
Log-rank test of Kaplan Meier survival curves, stratified for propensity score decile regarding likelihood of RT refusal.
Univariate hazard ratio from Cox model, with stratification by propensity score decile regarding likelihood of RT refusal.
FIGURE 2.
Overall survival for patients who received recommended RT (black line) and for patients who refused RT (gray line).
DISCUSSION
A high percentage of patients accept RT when recommended as part of their cancer treatment plan. However, the 2.2% of patients that decline recommended RT represents a large absolute number of patients who are refusing standard therapy. Interestingly, the rates of refusal did not change significantly over the past several decades (Fig. 1). One might expect that improvements in radiotherapy techniques, and subsequent toxicity reductions, would result in decreased rates of refusal. This lack of change in refusal rate substantiates the complexity of the reasons behind patient refusal and highlights the need for studies focusing on this issue to ensure radiation therapy is provided to all patients who may benefit. The particular reasons for which a patient might refuse therapy are not clear and may be attributable to a combination of socioeconomic, cultural, and health-related factors.
Ma et al analyzed radiation compliance in 855 lumpectomy patients with either ductal carcinoma in situ, infiltrating ductal cancer, or infiltrating lobular cancer.2 The thirty patients (4%) who refused RT were older and were more likely to have small tumors and noninvasive disease. Sociodemographic factors associated with RT refusal were not reported. The local recurrence for patients who refused radiation was more than double that observed in irradiated patients. However, given the small sample size, this did not achieve statistical significance. Similarly, we found that older patients were more likely to refuse RT. It is tempting to speculate on potential causative factors for this observation that could include reduced RT access, concerns about toxicities, limited social support, age-related changes in perspectives regarding health and end of life issues or even the aggressiveness of physician recommendations. However, we caution that this data does not address causation. Our results identify the elderly as an important group for further study regarding factors causally related to RT refusal but further interpretation may be compromised by bias.
As with previous studies, we found that blacks were more likely to refuse RT than whites and Asians. Merrill et al assessed the influence of race, age, stage, grade, and other disease characteristics on not receiving either surgery or RT in patients with invasive cervical cancer.3 Of 8119 patients, 8% of whites and 12% of blacks received no cancer-directed therapy. Of the women refusing RT, 54% of whites and 83% of blacks also refused surgery. Among patients refusing surgery, 23% of whites and 50% of blacks also refused radiation. Our data suggests blacks are approximately 30% more likely to refuse recommended RT. As discussed above, we caution against speculation regarding the causative factors associated with this disparity. However, further investigation is certainly merited as our data demonstrate inferior outcomes among patients who refuse RT and a higher rate of mortality has been demonstrated for black cancer patients in a number of large series.4 – 6
The influence of marital status on the acceptance of RT, and other related endpoints, has been explored. Kamer et al demonstrated that marital status influenced anxiety levels during intracavitary brachytherapy for gynecologic malignancies.7 Voti et al demonstrated that married women were 23% more likely to receive breast-conserving surgery with adjuvant RT compared with unmarried women.8 Gold et al demonstrated that among stage I breast cancer patients, being married was associated with both decreased probability of subsequent breast events and overall mortality. However, marital status was not significantly associated with the probability of RT delays or failure to complete RT.9 Chang and Barker demonstrated that married glioblastoma patients were more likely to undergo tumor resection and receive RT than unmarried patients.10 Our data largely conform with these observations and demonstrate that married patients were less likely to refuse RT than unmarried patients. Our results further demonstrate that widowed patients were most likely to refuse RT. This observation identifies a subgroup at significantly higher risk of RT refusal and merits further exploration. Additionally, as the relative risk of developing a mood or anxiety disorder is 3.5- to 9.8-fold higher among the widowed, radiation oncologists should pay careful attention to potential comorbidities that may affect decisions to accept recommended RT in this patient group.11
Rates of RT refusal for most disease sites did not exceed 2.5% and exceeded 4% only for colon cancer and prostate cancer patients. The imperfectly defined role for RT in nonmetastatic colon cancer may contribute to this high rate of RT refusal.12 Furthermore, the acceptability of close surveillance in prostate cancer management in certain clinical scenarios may explain the rates of RT refusal in this disease.13
This study has several limitations. The SEER database provides unequalled patient numbers for the examination of uncommon events, such as RT refusal. It does not provide all information that may impact the decision of physicians to offer RT or patients to decline RT. For example, margin status profoundly impacts RT recommendations but is not captured in the SEER database. It is due to such limitations that we recommend caution in interpreting the observation that patients who refuse RT fare worse with respect to overall survival. This effect remained significant on multivariate analysis that accounted for other variables of interest. Nevertheless, this observation may be related to unknown factors in addition to the loss of potential benefit from RT itself when a patient decides to forego treatment. Furthermore, as mentioned above, our analysis provides correlates with RT refusal but does not address potential causative links between the examined factors and RT refusal. Thus, our results should predominantly be interpreted as hypothesis generating and subjected to further investigation.
RT is a critical element in the management of most nonmetastatic, solid cancers. This study demonstrates a high rate of RT compliance when recommended. However, older, black and unmarried patients are significantly more likely to refuse RT. Sensitivity to these correlates of RT refusal may assist clinicians in patient counseling. Additionally, these results may guide the design of additional studies designed to clarify the factors causally linked to RT refusal.
Supplementary Material
References
- 1.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 17 Regs Limited-Use + Hurricane Katrina Impacted Louisiana Cases, Nov 2007 Sub (1973–2005 varying)—Linked To County Attributes—Total US, 1969–2005 Counties. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; [Accessed March 6, 2009]. Available at: http//:www.seer.cancer.gov. [Google Scholar]
- 2.Ma AM, Barone J, Wallis AE, et al. Noncompliance with adjuvant radiation, chemotherapy, or hormonal therapy in breast cancer patients. Am J Surg. 2008;196:500–504. doi: 10.1016/j.amjsurg.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 3.Merrill RM, Merrill AV, Mayer LS. Factors associated with no surgery or radiation therapy for invasive cervical cancer in Black and White women. Ethn Dis. 2000;10:248–256. [PubMed] [Google Scholar]
- 4.Wojcik BE, Spinks MK, Optenberg SA. Breast carcinoma survival analysis for African American and white women in an equal-access health care system. Cancer. 1998;82:1310–1318. doi: 10.1002/(sici)1097-0142(19980401)82:7<1310::aid-cncr14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Yood MU, Johnson CC, Blount A, et al. Race and differences in breast cancer survival in a managed care population. J Natl Cancer Inst. 1999;91:1487–1491. doi: 10.1093/jnci/91.17.1487. [DOI] [PubMed] [Google Scholar]
- 6.DeLancey JO, Thun MJ, Jemal A, et al. Recent trends in Black-White disparities in cancer mortality. Cancer Epidemiol Biomarkers Prev. 2008;17:2908–2912. doi: 10.1158/1055-9965.EPI-08-0131. [DOI] [PubMed] [Google Scholar]
- 7.Kamer SA, Ozsaran Z, Celik O, et al. Evaluation of anxiety levels during intracavitary brachytherapy applications in women with gynecological malignancies. Eur J Gynaecol Oncol. 2007;28:121–124. [PubMed] [Google Scholar]
- 8.Voti L, Richardson LC, Reis IM, et al. Treatment of local breast carcinoma in Florida: the role of the distance to radiation therapy facilities. Cancer. 2006;106:201–207. doi: 10.1002/cncr.21557. [DOI] [PubMed] [Google Scholar]
- 9.Gold HT, Do HT, Dick AW. Correlates and effect of suboptimal radiotherapy in women with ductal carcinoma in situ or early invasive breast cancer. Cancer. 2008;113:3108–3115. doi: 10.1002/cncr.23923. [DOI] [PubMed] [Google Scholar]
- 10.Chang SM, Barker FG., II Marital status, treatment, and survival in patients with glioblastoma multiforme: a population based study. Cancer. 2005;104:1975–1984. doi: 10.1002/cncr.21399. [DOI] [PubMed] [Google Scholar]
- 11.Onrust SA, Cuijpers P. Mood and anxiety disorders in widowhood: a systematic review. Aging Ment Health. 2006;10:327–334. doi: 10.1080/13607860600638529. [DOI] [PubMed] [Google Scholar]
- 12.Dunn EF, Kozak KR, Moody JS. External beam radiotherapy for colon cancer: patterns of care. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2009.03.014. In press. [DOI] [PubMed] [Google Scholar]
- 13.Dall’Era MA, Cooperberg MR, Chan JM, et al. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer. 2008;112:1650–1659. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.