Abstract
Arbuscular mycorrhizal fungi have been known to increase metal uptake in plants. In this study, mesquite (Prosopis juliflora-velutina) inoculated with Glomus deserticola or amended with EDTA were grown for 30 days in soil containing Cr(III) or Cr(VI) at 0, 40, 80, and 160 mg kg−1. Total amylase activity (TAA) was monitored as a stress indicator. Element concentrations and distribution in tissue were determined using ICP-OES, electron scanning microprobe, and TEM. Inoculated Cr(VI) treated plants had 21% and 30% more Cr than uninoculated and EDTA treated roots, respectively, at 80 mg Cr kg−1 treatment. In the case of Cr(III), EDTA produced the highest Cr accumulation in roots. TAA was higher in inoculated plants grown with Cr(III) at 80 and 160 mg kg−1 and Cr(VI) at 40 and 160 mg kg−1. The X-ray mapping showed higher metal concentrations in the vascular system of inoculated plants and the TEM micrographs demonstrated the presence of G. deserticola in roots.
Keywords: Plant growth, Metal uptake, X-Ray mapping, Microprobe, TEM, Mesquite, Glomus, EDTA
Introduction
It has been documented that an excess of chromium (Cr) in soil and water poses health risks for all living organisms (1-3). There are a number of Cr impacted sites around the world, mostly linked with improper dumping and storage of mine and industrial wastes (4). Different techniques including physical and chemical methods, have been applied for the restoration and management of areas contaminated with Cr. One of the techniques has been the use of chelating agents such as EDTA to solubilize soil metals facilitating their uptake by plants (5). However, most of these techniques are expensive, labor consuming, soil disturbing, and improper for large areas (6). In the last three decades, environmental scientists have realized that plants and microorganisms can restore heavy metal contaminated soil and water (7-9). In addition, researchers have reported that the association of plants with arbuscular mycorrhizal (AM) fungi represents an excellent option for soil restoration, water use efficiency, and management of metal enriched sites (10,11). Plant-AM symbiosis is a very ancient association that includes more than 80% of land plants with fungi from the Phylum Glomeromycota (12,13). For instance, Lingua et al. (13) reported that Populus alba Villafranca and Populus nigra Jean Pourtet in association with an extraradical mycelial network of Glomus mosseae (Gerd. and Nicol.) can uptake and translocate Zn. In the plant-fungus association, the AM fungi contribute to plant health as they improve water draw, nutrition of the plant and, in most instances, the resistance against pathogen attacks (12-15). In turn, the host plant supplies sugars and carbon to the AM fungi (15,16). Furthermore, studies have demonstrated that the symbiotic interaction between plants and AM fungi may improve tolerance and uptake of heavy metals, but little is known about the responsible mechanisms of chromium uptake (11,16).
The uptake of Cr, by Prosopis sp. has been documented (17-20). Previous reports indicated that mesquite cultivated in hydroponics with the AM fungus Glomus deserticola increased the uptake of Cr (20). In the present study, mesquite plants in association with G. deserticola were grown in soil artificially contaminated with selected concentrations of Cr(III) and Cr(VI). Treatments containing EDTA without G. deserticola were also undertaken. The presence of Cr, macro, and micro elements were determined using inductively coupled plasma optical emission spectroscopy (ICP-OES). Transmission electron microscopy (TEM) and X-ray mapping were also used to examine the control and Cr-treated plants. Total amylase activity was recorded as an indicator of stress.
Materials and Methods
Soil preparation, sterilization, and characterization
Uncontaminated loamy sand (82.6% sand, 16.6% silt, 0.81% clay, and pH 7.9) soil was collected from the top 30 cm of a field site in El Paso County. No chromium contamination was detected (21). Plastic container of soil samples were taken to the lab for sterilization and analysis. Samples were sieved using a 10 mm mesh sieve, and wrapped in aluminum foil packages of approximately 1 kg. Each package was autoclaved at 1.05 kg cm−2 for 45 min at 120 °C. A representative uncontaminated soil sample was analyzed for pH, organic matter, carbon content, nitrogen, phosphorus, inorganic NO3, Ca, Mg, SO4, Na, Zn, Cu, Fe, Mn, Mo, B, cation exchange capacity, texture, and water holding capacity (21).
Soil water holding capacity and metal stabilization
To determine the water holding capacity, 100 g of oven dried soil was gently placed in a 500 mL beaker and gradually watered until soil saturation was reached. Mass of the beaker, soil, and water was recorded. Percentage of the water holding capacity was calculated dividing the weight of the water held in the sample vs. the sample dry weight times 100. This was determined to prevent leaching losses of nutrients and metals (21,22).
Soil samples were dampened with full strength Murashige and Skoog (MS) nutrient solution and Cr(III) [from CrNO3)]. The final Cr(III) concentrations in soil were at 0, 40, 80, and 160 mg kg−1. The Cr(III) spiked soil was placed in 500 mL capacity culture vessels for 30 d to allow stabilization of metals. Every 4 days the soil was carefully mixed to ensure uniform distribution of the nutrient solution and metals in the soil medium. Cr(VI) [from K2CrO4], was also used at 0, 40, 80, and 160 mg kg−1 and applied to the soil the same day of seed sowing because Cr(VI) in soil tends to be reduced to Cr(III) in a short period of time (23).
Plant germination, G. deserticola inoculation, and metal treatments application
Mesquite (Prosopis juliflora- velutina) seeds were obtained from Wild Seeds (Tempe, Arizona, USA). For germination, the seeds were sterilized in 4% sodium hypochlorite solution for 30 min, rinsed three times with sterilized deionized water (DI), and placed in sterilized paper towels dampened with MS solution as described by (24,25).
For fungus inoculation, 5 g of clay containing the G. deserticola spores provided by Reforestation Technologies International Company (Salinas, CA, USA) were ground and diluted in 5 ml of DI. An aliquot of 2 mL of the fungi-clay solution was added to the seeds in each paper towel.
When the radicle-hypocotyl axis (the axial part below the cotyledons) and the G. deserticola mycelia appeared (5 d), seedlings were placed in a 1000 mL capacity duplex culture vessels system (Magenta, Corp. Chicago, IL). The culture vessels and DI were previously sterilized at 120 °C and 1.25 kg cm−2 for 45 min to avoid fungal and microbial contamination (Market Forge Industries, Albertville, MN, USA).
The upper vessel contained 400 g of clean soil or soil contaminated with Cr(III) or Cr(VI) and the lower vessels contained 400 ml of MS nutritive solution. A capillary irrigation system (cotton thread) maintained the humidity of the soil medium. The MS solution was replenished every 10 days for 30 days.
The experimental set up included: (1) Control (plants with nutrient solution), (2) plants with nutrient solution + G. deserticola, (3) plants + Cr at 40, 80, and 160 mg kg−1, (4) plants + Cr at 40, 80, and 160 mg kg−1 + G. deserticola, and (5) plants + Cr at 40, 80, and 160 mg kg−1+ EDTA. An agar-based experiment was previously performed to determine the ability of G. deserticola to grow in the Cr concentrations chosen. Each treatment had 5 replicates and each replicate had 15 seedlings. The ambient temperature was 27°C, light/dark cycle 12/12 h, and irradiance of 53 molesm−2s−1. EDTA was used equimolar to Cr concentrations. Solutions of EDTA at 0.76, 1.5, and 3.1 mM were applied to the soil at the time of Cr application.
Growth determination, and metal and nutritive element analyses
After 30 d of growth, the seedlings and AM hyphae were carefully harvested, washed with 0.01 M HNO3, and rinsed with DI to eliminate metal deposited on the root and hyphal surfaces. Subsequently, 10 seedlings per replicate/treatment were randomly selected and measured to determine the size of plants. The roots were measured from the apex to the crown and the stems from the crown to the shoot apex. For the leaves, we measured the rachis length from the apex to the end of the petiole. The plants and associated hyphae were separated into roots, stems, and leaves, oven dried at 70 °C for 2 days (Fisher Scientific Isotemp, Pittsburg, PA, USA), weighed and digested using 6 mL of trace pure HNO3 (SCP Science, New York, NY, USA). The digestion was performed in a CEM microwave oven (CEM Corporation Mathews, NC, USA) at 125 °C for 30 min following the EPA 3051 method. Afterward, 2 mL of the digested samples were adjusted to 10 mL with DI and analyzed for Cr, micro and macroelement concentrations using inductively coupled plasma/optical emission spectroscopy (ICP-OES, Optima 4300 DV (Perkin Elmer, Shelton, CT, USA). The operation parameters of the ICP-OES were: nebulizer flow, 1.00 L min−1; power, 1400 W; peristaltic pump flow rate 1.50 mL min−1; flush time, 30 s; delay time, 30 s; read time, 20 s; replicates, 3; wash rate, 1.50; wash time, 30 s. The instrument was calibrated from 0.005 to 0.5 mg L−1 for Cr at the wavelength of 283.563 nm. The detection limits (in ppb) for the studied elements were: Cr, 5; Mn, 1; Zn, 5; Cu, 5; Fe, 5; Mo, 10; Mg, 0.5; Ca, 0.5; S, 20; and P, 50. The calibration curves obtained had correlation coefficients of 0.9999 or greater for all ICP determinations. The accuracy of instrument readings was checked every 10 samples with a Cr spiked sample of known concentration. Due to the lack of a Cr certified reference material, the results were validated with a certified reference material for zinc. A sample of 1570a standard reference material (spinach leaves, National Institute of Standards & Technology) was used to validate the digestion and analytical method. The percent recovery from the certified material and spiked samples was 95% and 99% ± 2%, respectively. Ten blanks were analyzed in order to calculate the detection limit for Cr.
The concentration of Cr in each sample was determined using the mean of three readings; in addition, the standard error was calculated for each sample value.
Cryosectioning of plant samples and electron microprobe analysis
The cryosectioning of mesquite root and stem was performed as previously described using a microtome plus (Triangle Biomedical Sciences, Durham, NC) (20). Plant samples were dissected in 1 cm sections and placed in water. The samples were attached to the sample holder with Tissue Tek (Sakura Finetek USA, Torrance, CA), frozen at −40°C and sectioned at −20°C. Then, 8 μm thick samples were mounted onto glass microprobe slides and stored at room temperature until microprobe analysis.
The electron probe micro analyzer (EPMA) has a camera SX50 (Cameca S.A. Courbevoie Cedex, France) with four wavelength dispersive detectors. The back scattered electrons (BSE) root and stem images and the elemental x-ray maps were acquired at 15 kV, 20 nA, with a focused beam condition. The dwell time was set at 3 ms and the definition of the image at 512 × 512 pixels. The Cr element was selected for 4 spectrometers and dwell time was set at 20 ms. The peak scan spectra were collected in a qualitative mode, interpreted by Quali peak mode, and re-plotted in MS Excel 97-2003. Three crystals, LIF-PET-TAP, on three spectrometers were selected to cover the most elements (from F to U).
TEM analysis
Samples were prepared using a glutaraldehyde-osmium tetroxide fixation as previously described (26). Specimens were then dehydrated and embedded in Poly Bed 812 (Polysciences, Inc., Warrington, PA). Histological samples were obtained from thick sections (1 μm) stained with Toluidine Blue and Fuschin. Ultrastructural analysis of plastic thin sections (60-90 nm) post stained with 2% UrAc and Reynolds lead citrate (27) were examined and photographed in a Zeiss EM-10 transmission electron microscope (Peabody, MA) operating at an accelerating voltage of 60 or 80 kV.
Total amylase activity determination
The total amylase activity assay was performed as described by Fuwa (1954). Briefly, 0.1-0.2 g of fresh leaves were ground and extracted with 2mM imidazole buffer solution (pH 7.). The samples and the respective standards were placed in a microplate reader and analyzed at 660 nm using a UV/VIS spectrometer (SpectraMax Plus 384, Sunnyvale, CA).
Statistical analysis
The data were analyzed using a one-way analysis of variance (ANOVA) (SPSS version 13.0, Chicago, IL). The Tukey honestly significant difference (HSD) test was used to separate treatment means. The reference to significant differences between data is based on a probability of p <0.05, unless otherwise noted.
Results and Discussion
Effect of G. deserticola on mesquite growth in Cr contaminated soil
During the growth period (30 days), qualitative observation of plant development and pH measurements were obtained. During the first 17 days, seedlings showed changes in leaf color and shape. Plants treated with Cr(VI) without G. deserticola showed more qualitative changes compared with controls and Cr(III) treated plants. After 30 days, the plants recovered from all treatments. Plants inoculated with G. deserticola showed a higher recovery rate compared to controls, possibly due to a symbiotic association of G. deserticola with mesquite which alleviated metal toxicity and helped maintain high plant biomass (26).
At the end of experimental period, Cr(VI) treatments had pH values of 6.4, 5.75, and 4.41 for the 40, 80 and 160 mg kg−1 treatments, respectively. The pH values for the Cr(III) treatments were 6.58, 6.45, and 6.0. It is hypothesized that these differences in pH values caused variations in the absorption of some elements. Acidic pH increase metal solubility and absorption.
The growth of mesquite cultivated in soil spiked with Cr(III) and Cr(VI) without/with G. deserticola or EDTA is shown in Figure 1(A-B SI, Supporting Information). In all cases, compared to controls (no AM fungus, no Cr), plants associated with G. deserticola had shorter roots. Stems and leaves were not affected. In the case of Cr(VI) treatments, none of the concentrations (with/without AM fungus or EDTA) reduced root and stem growth; however, at 80 mg kg−1 and above all treatment combinations significantly reduced leaf growth (Figure 1A, SI). This could be associated with the toxicity of Cr(VI) as has been shown by the increase in amylase activity of mesquite plants treated with Cr(VI) (Figure 5). In all Cr(III) treatments, plants inoculated with G. deserticola had shorter roots but the differences at stem and leaf levels were not conspicuous (Figure 1B, SI). The addition of EDTA did not affect plant growth in Cr(III) treatments.
Figure 5.
Total amylase activity of mesquite (Prosopis) leaves treated for 30 days in soil medium with A) chromium(VI), and B) chromium(III) at 0, 40, 80, and 160 mg kg−1. This bar ( ) indicates uninoculated plants (P) and this (
) indicates uninoculated plants (P) and this ( ) indicates plants inoculated with Glomus deserticola (P/F). Error bars stand for SE.
) indicates plants inoculated with Glomus deserticola (P/F). Error bars stand for SE.
Treatment effects on concentrations of Cr in tissues
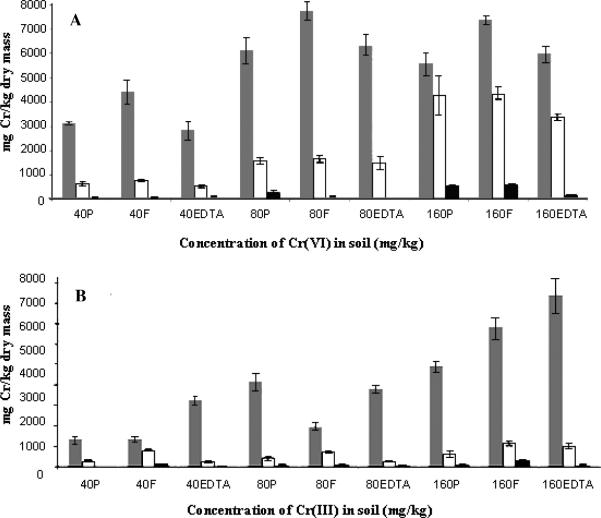
The amounts of Cr in roots, stems, and leaves of mesquite plants grown without/with G. deserticola or EDTA in Cr(III) or Cr(VI) contaminated soil are shown in Figure 1. In the case of Cr(VI), all plants associated with G. deserticola had higher metal accumulation in roots, compared to uninoculated or EDTA treated plants. The highest metal accumulation was found in roots of plants treated with 80 mg Cr(VI) kg−1 (about 8000 mg Cr kg−1 dry weight biomass). In the case of Cr(VI) treated plants, at 160 mg kg−1 all treatments had significantly more Cr in stems compared to the 40 and 80 mg kg−1 treatments; however, stems of EDTA treated plants had lower Cr compared to G. deserticola and Cr alone treated plants. In addition, leaves of plants treated with Cr(VI) at 160 mg kg−1 without/with G. deserticola, had significantly more Cr compared to the other treatments (Figure 1A). In grapevine, AM fungi were associated with increased element absorption (28,29) and in mesquite increased Cr absorption as well as the absorption and translocation of nutrients such as Ca, Fe, and Mn (Table 1-4 SI, Supporting Information). The AM fungus could also help to alleviate the Cr toxicity as indicated by plant recovery. In other plants like Helianthus annuus, Lolium perenne, and Trifolium repens the AM fungus was associated withgreater growth (26,30).
Figure 1.
Concentration of chromium in mesquite (Prosopis) roots ( ), stems (
), stems ( ), and leaves (
), and leaves ( ) of uninoculated plants (P), inoculated with Glomus deserticola (F), or treated with EDTA and grown for 30 days in soil contaminated with (A) Cr(VI), and (B) Cr(III) at 0, 40, 80, and 160 mg kg−1. Each datum is average of 5 replicates ± SE.
) of uninoculated plants (P), inoculated with Glomus deserticola (F), or treated with EDTA and grown for 30 days in soil contaminated with (A) Cr(VI), and (B) Cr(III) at 0, 40, 80, and 160 mg kg−1. Each datum is average of 5 replicates ± SE.
The absorption of Cr from Cr(III) treatments followed a different pattern. At 40 and 160 mg kg−1 EDTA treated plants accumulated the most Cr in roots, followed by G. deserticola inoculated plants (Figure 1B). At 80 mg kg−1 inoculated plants had less Cr, and the translocation to stems and leaves was numerically lower compared to Cr(VI) treated plants. However, the translocation factor (TF) was higher compared to the TF obtained with inoculated Cr(VI) treated plants (Table 5 SI). It is hypothesized that the lower stability constant of the complex EDTA-Cr(III) (32.3) allowed a higher absorption of Cr by the plants, while the higher stability constant of Cr(III)-OH (47.8) favored the precipitation of Cr in the soil medium (31). The contribution of G. deserticola in the translocation of Cr in the case of Cr(III) treatments can be seen in the amount of metal found in stems and leaves of plants grown with this AM fungus (Figure 1B). As seen in this figure, plants with G. deserticola had higher Cr concentration in stems and also in leaves for the 40 and 80 mg kg−1 treatments. This was also observed in the TF shown in Table 5 SI). More experiments need to be done to understand the response at 80 mg kg−1. The role of AM fungi in the increase and translocation of metals has been previously reported for Populus (13).
Treatment effects on translocation factors
The translocation factors (TF) for the Cr(III) and Cr(VI) treatments are shown in Table 5 SI. As seen in this table, TF for uninoculated plants were similar for both Cr ions (0.15 for Cr(III) and 0.16 for Cr(VI)) at the lowest concentration (40 mg kg−1). At higher Cr concentrations the TF increased for Cr(VI) treatments, reaching 3.3 and 3.75 fold, respectively at 80 and 160 mg kg−1. Similar results were found with EDTA treatments. Conversely, in G. deserticola inoculated plants, the TF were higher for Cr(III) at lower Cr concentrations, and decreased as the Cr concentration in soil increased. At 160 mg Cr kg−1, the TF for Cr(VI) treated plants inoculated with G. deserticola was 3.1 fold compared to inoculated Cr(III) treated plants. Although at the highest Cr(VI) concentrations the TF were high, in all cases they were < 1, even when the root concentration was between 6000 to 8000 mg kg−1 dry weight mass. This demonstrated that mesquite is capable of a well-balanced uptake and translocation when grown in a highly Cr(VI) impacted soil (32).
Treatment effects on concentrations of micro and macro nutrients
Microelement concentrations found in mesquite plants grown with/without G. deserticola or EDTA and in C r(III) or Cr(VI) contaminated soil are shown in Tables 1 and 2 SI. As seen in these tables, none of the treatments produced a definite trend in microelement uptake. In the case of Cr(VI), in roots Mn was reduced by all treatments, Cu was increased by G. deserticola in the 40 mg kg−1 treatment and EDTA in the 160 mg kg−1 treatment. In stems, Mn was increased by EDTA at the 160 mg kg−1 treatment while Mo was reduced by all Cr(VI) treatments. In leaves, Mn was reduced at all treatment combinations, Zn was not affected, Fe was increased at 160 mg kg−1 + G. deserticola and 40 mg kg−1 + EDTA treatments,with molybdenum reduced by all Cr(VI) concentrations (Table 1 SI). In Cr(III) treatments, at root level only the 40 mg kg−1 + EDTA treatment increased the concentration of Mn, Zn, Cu, Fe, and Mo. No effects were noted in stems and leaves except for the reduction of Fe in leaves at all Cr(III) treatments and in plants + G. deserticola treatment (Table 2 SI).
The data for macroelements Mg, Ca, S and P absorption are shown in Tables 3 and 4 SI. The Cr (III) and Cr(VI) treatments differentially affected the concentration of macroelements in roots, stems, and leaves of mesquite. In Cr(VI) treated plants, the absorption of Ca in roots was increased by all treatments compared to the universal control (nutrient solution added). No conspicuous changes were observed in Mg, S and P absorption and no changes were observed in stems. While in leaves, all Cr(VI) treatments (without/with G. deserticola or EDTA) increased Mg accumulation; however, all EDTACr(VI) treatments and 80 and 160 ppm of Cr(VI) + G. deserticola reduced Ca accumulation (Table 3 SI).
Cr(III) had the greatest effect on the absorption of macroelements. All plants inoculated with G. deserticola and plants grown in EDTA amended soil showed an increased in Mg accumulation in roots, but no significant changes were observed in stems. In leaves, all treatments involving G. deserticola and EDTA increased Mg accumulation; however, all Cr(III) treatments (without/with G. deserticola or EDTA) significantly reduced Ca, S, and P accumulation (Table 4 SI). Reports have indicated that heavy metals increase nutrient absorption in plants associated with AM fungi or when EDTA is applied to solubilized heavy metal compounds (18,33). Cr increased the absorption of Ca in Salsola kali (34) but reduced the absorption of Mn in bush bean (35). Ca has an important role in the preservation of plant homeostasis, thus plants may increase Ca accumulation as a detoxification strategy (36). It has been documented that G. deserticola improves plant nutritional status, sensitivity, uptake and translocation efficiency, and metal tolerance (33, 37). Results of the present research have shown that the AM fungus G. deserticola increased the concentration of macronutrients in leaves of Cr(III) and Cr(VI) treated plants.
Distribution of Cr on mesquite tissues
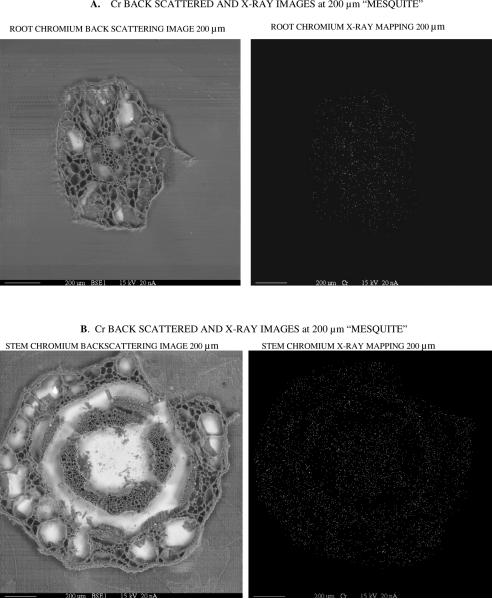
For the analysis of metal distribution in plants, only Cr(VI) treatments at the highest concentration were selected. Previous studies have shown that mesquite biotransforms Cr(VI) to Cr(III) (17), because of that it is hypothesized that most of the Cr within plants was Cr(III). In addition, there are no images of EDTA treated plants because it chelates more than one element. The back scattered images (BES) at 100, 50, and 20 μm of Cr on mesquite tissues without/with G. deserticola are shown in Figures 2-4. The distribution of Cr in root (A) and stem (B) tissues without G. deserticola is shown in Figure 2. Without G. deserticola, Cr was evenly distributed throughout the entire tissue with a greater concentration in roots. In plants inoculated with this AM fungus, Cr was more concentrated around the vascular system in both root and stem (Figures 3 and 4). In addition, the x-ray images showed that the amount of chromium in G. deserticola inoculated plants was higher compared to uninoculated plants, corroborating the data shown in Figure 1.
Figure 2.
Back scattered images at 200 μm and X-ray mapping image at 200 μm of uninoculated mesquite (Prosopis) roots (A) and stems (B) and treated with 40 mg kg−1 of Cr(VI) for 30 days in soil medium. Brighter spots in X-ray mapping image at 200 μm indicate chromium occurrence in mesquite tissues.
Figure 4.
Back scattered images at 200, & 20 μm and X-ray mapping image at 20 μm of mesquite (Prosopis) stems + fungus (Glomus deserticola) treated with Cr(VI) for 30 days in soil medium. Brighter spots in X-ray mapping image at 20 μm indicate chromium metal occurrence in mesquite phloem structure.
Figure 3.
Back scattered images at 200, 100, & 20 μm and X-ray mapping image at 200 & 20 μm of mesquite (Prosopis) roots + fungus (Glomus deserticola) treated with 40 mg kg−1 of Cr(VI) for 30 days in soil medium. Brighter spots in X-ray mapping images at 200 & 20 μm indicate chromium metal occurrence in mesquite epidermis, cortex, endodermis, xylem, and phloem structures. Brighter spots are predominant in phloem structures.
Effects of Cr on the TAA of mesquite with/without G. deserticola
Total amylolitic activity was evaluated in mesquite leaves as an indicative of changes in metabolic state in plants grown with/without G. deserticola or EDTA in Cr(III) or Cr(VI) contaminated soil (Figure 5). As shown in Figure 5, at 40 mg kg−1 treatment only plants treated with Cr(VI) and G. deserticola had conspicuous changes in TAA. At 80 mg kg−1, Cr(VI) and Cr(III) treated plants presented inverse responses. At this metal concentration, the amylase activity was lower in the presence of the fungus in Cr(VI) treated plants and higher in Cr(III) treated plants (Figure 5A, B). The differential response could be attributed to differences in metal absorption. As seen in Figure 2, Cr uptake from the 40 mg kg−1 Cr(III) treatment was the same with/without G. deserticola, while in Cr(VI) treated plants, the inoculation with G. deserticola significantly increased Cr concentration in tissues. The increase in metal absorption required an increase in energy, which could explain the increase in TAA, as has been reported for Pb in alfalfa plants (31). It is possible that the higher Cr translocation to the leaves observed in the Cr(III) treated plants inoculated with G. deserticola (Figure 1) caused the increase in TAA. In addition, Castillo-Michel et al (39) reported that in leaves of pea plants, TAA was correlated to the oxidation state of arsenic. It is possible that the oxidation state of Cr also influenced the TAA observed in mesquite. At 160 mg kg−1, in all cases TAA was higher in plants inoculated with G. deserticola. The amylase activity found in soil grown mesquite plants with/without G. deserticola was different to the response found in hydroponically grown mesquite plants (20). Metal ions are more readily available for absorption in hydroponically grown plants than in soil thus causing more stress. In addition, it is very likely that the hydroponically grown plants were under additional stress produced by the air bubbling and growth medium, compared to the soil grown plants.
Transmission electron microscopy analysis
Thin sections of mesquite roots from plants grown in Cr contaminated soil were compared by transmission electron microscopy with those of plants grown in uncontaminated soil. G. deserticola was observed in the epidermal and parenchymal cells of mesquite plants similar to results reported in hydroponically grown mesquite (20) (Figure 2 SI unpoststained control a, b and 20 ppm Cr root, c). As the concentration of Cr increased within the Prosopis roots, the amount of G. deserticola observed within the peripheral cells decreased, b). A fine black precipitate gave contrast to the cell membrane of xylem cells within a Glomus-infected mesquite root with 40 mg kg−1 Cr(III) (Fig. 3 SI). Figure 4 SI shows an example of the association G. deserticola-mesquite roots. As seen in this figure, G. deserticola effectively colonized mesquite roots.
Supplementary Material
Brief.
Glomus deserticola increases chromium absorption and allows chromium accumulation around the vascular system in both root and stem of mesquite (Prosopis).
Acknowledgements
This material is based upon work supported by the National Science Foundation and the Environmental Protection Agency under Cooperative Agreement Number EF 0830117. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation or the Environmental Protection Agency. This work has not been subjected to EPA review and no official endorsement should be inferred. J.L. Gardea-Torresdey acknowledges the USDA grant # 2008-38422-19138, the Analytical Cytology Core Facility and the Toxicology Unit of the BBRC (NIH NCRR Grant # 2G12RR008124-16A1), the NSF Grant # CHE-0840525 and the Dudley family for the Endowed Research Professorship in Chemistry. The authors also acknowledge the Reforestation Technologies International Company (Salinas, CA) and Dr. Edith Flores for her contribution in the TAA studies.
Footnotes
Supporting Information Available.
Translocation factors, micro and macronutrient concentrations, plant growth data, TEM images and root colonization by G. deserticola are presented as supporting information. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Gardea-Torresdey JL, Peralta-Videa JR, Montes M, de la Rosa G, Corral-Diaz B. Bioaccumulation of cadmium, chromium and copper by Convolvulus arvensis L.: impact on plant growth and uptake of nutritional elements. Bioresour. Technol. 2004;92:229–325. doi: 10.1016/j.biortech.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Alan M, Kara D, Fisher A. Preconcentration of heavy metals and matrix elimination using silica gel chemically modified with 2,3-dihydroxybenzaldehyde. Sep. Sci. Technol. 2007;42:879–895. [Google Scholar]
- 3.Rekha D, Suvardhan K, Kumar KS, Jayaraj B, Chiranjeevi P. Extractive spectro-photometric determination of copper(II) in water and alloy samples with 3-methoxy-4-hydroxy benzaldehyde-4-bromo phenyl hydrazone (3,4-MHBBPH). J. Servian Chem. Soc. 2007;72:299–310. [Google Scholar]
- 4. [3-9-2010];Superfund site treatment system completed. Available at http://www.epa.gov/region6/6sf/pdffiles/odessa_5_year_review_9_2006.pdf.
- 5.Meers SE, Qadir M, de Caritat P, Tack FMG, Du Laing G, Zia MH. EDTA-assisted Pb phytoextraction. Chemosphere. 2009;74:1279–1291. doi: 10.1016/j.chemosphere.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 6.French HK, van der Zee SEATM, Meju M. SoilCAM: Soil contamination: advanced integrated characterisation and time-lapse monitoring. Rev. Environ. Sci. Biotechnol. 2009;8:125–130. [Google Scholar]
- 7.Alvarez PJJ, Illman W. Bioremediation and Natural Attenuation of Groundwater Contaminants: Process Fundamentals and Mathematical Models. John Wiley and Sons; Hoboken, New Jersey: 2006. [Google Scholar]
- 8.Khan MS, Zaidi A, Wani PA, Oves M. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ. Chem. Lett. 2009;7:1–19. [Google Scholar]
- 9.Malcová R, Vosátka M, Gryndler M. Effects of inoculation with Glomus intraradices on lead uptake by Zea mays L. and Agrostis capillaris L. App. Soil Ecol. 2003;23:55–67. [Google Scholar]
- 10.Usman ARA, Mohamed HM. Effect of microbial inoculation and EDTA on the uptake and translocation of heavy metal by corn and sunflower. Chemosphere. 2009;76:893–899. doi: 10.1016/j.chemosphere.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Harrier LA. The arbuscular mycorrhizal symbiosis: a molecular review of the fungal dimension. J. Exp. Bot. 2001;52:469–478. doi: 10.1093/jexbot/52.suppl_1.469. [DOI] [PubMed] [Google Scholar]
- 12.Bonfante P, Bianciotto V, Ruiz-Lozano JM, Minerdi D, Lumini E, Perotto S. Arbuscular mycorrhizal fungi and their endobacteria. In: Seckbach J, editor. Cellular Origin, Life in Extreme Habitats and Astrobiology. Springer; Netherlands: 2006. [Google Scholar]
- 13.Lingua G, Franchin C, Todeschini V, Castiglione S, Biondi S, Burlando P, Parravicini V, Berta PTG. Arbuscular mycorrhizal fungi differentially affect the response to high zinc concentrations of two registered poplar clones. Environ. Pollut. 2008;153:137–147. doi: 10.1016/j.envpol.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Jeffries P, Gianinazzi S, Perotto S, Turnau K, Barea J-M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fert. Soils. 2003;37:1–16. [Google Scholar]
- 15.Brundrett MC. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002;154:275–304. doi: 10.1046/j.1469-8137.2002.00397.x. [DOI] [PubMed] [Google Scholar]
- 16.Takács T, Biró B, Vörös I. Arbuscular mycorrhizal effect on heavy metal uptake of ryegrass (Lolium p erenne L.) in pot culture with polluted soils. In: Horst WWJ, Sattelmacher B, Schmidhalter U, Schubert S, Wiren N. von, editors. Plant nutrition – Food security and sustainability of agro-ecosystems. Lavoisier; Paris, France: 2001. [Google Scholar]
- 17.Aldrich MV, Gardea-Torresdey JL, Peralta-Videa JR, Parsons JG. Uptake and reduction of Cr (VI) to Cr (III) by mesquite (Prosopis spp.): Chromate–plant interaction in hydroponics and solid media studied using XAS. Environ. Sci. Technol. 2003;37:1859–1864. doi: 10.1021/es0208916. [DOI] [PubMed] [Google Scholar]
- 18.Aldrich MV, Ellzey JT, Peralta-Videa JR, Gonzalez JH, Gardea-Torresdey JL. Lead uptake and effects of EDTA on lead-tissue concentrations in the desert species mesquite (Prosopis spp.). Int. J. Phytorem. 2004;6:195–207. doi: 10.1080/16226510490496357. [DOI] [PubMed] [Google Scholar]
- 19.Mokgalaka-Matlala NS, Flores-Tavizόn E, Castillo-Michel H, Peralta-Videa JR, Gardea-Torresdey JL. Arsenic tolerance in mesquite (Prosopis sp.): Low molecular weight thiols synthesis and glutathione activity in response to arsenic. Plant Physiol. Biochem. 2009;47:822–826. doi: 10.1016/j.plaphy.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Arias JA, Peralta-Videa JR, Ellzey JT, Ren M, Viveros M, Gardea-Torresdey JL. Effect of Glomus desertícola inoculation on Prosopis: enhancing chromium and lead uptake and translocation as confirmed by x-ray mapping, ICPOES and TEM techniques. Environ. Exp. Bot. 2010;68:139–148. [Google Scholar]
- 21.Rodríguez E. Ph.D. Dissertation. University of Texas at El Paso; El Paso, TX: 2006. Gold bioabsorption and reduction by Chilopsis linearis (Desert Willow): an alternative for in situ gold extraction. [Google Scholar]
- 22.Duniway MC, Herrick JE, Monger HC. The high water holding capacity of petrocalcic horizons. Soil Sci. Soc. Am. 2007;71:812–819. [Google Scholar]
- 23.Lan Y, Deng D, Kim C, Thorton EC. Influence of soil minerals on Cr(VI) reduction by sulfide under anoxic conditions. Geochem. Trans. 2007 doi: 10.1186/1467-4866-8-4. doi:10.1186/1467-4866-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrillo-Castañeda G, Juarez MJ, Peralta-Videa JR, Gomez E, Gardea-Torresdey JL. The modulation of the uptake and translocation of iron and copper from root to shoot in common beans (Phaseolus vulgaris) by siderophore producing microorganisms. J. Plant Nutr. 2005;28:1853–1865. [Google Scholar]
- 25.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plantarum. 1962;15:473–497. [Google Scholar]
- 26.Chen BD, Zhu Y-G, Duan J, Xiao XY, Smith SE. Effects of the arbuscular mycorrhizal fungus Glomus mosseae on growth and metal uptake by four plant species in copper mine tailings. Environ. Pollut. 2007;147:374–380. doi: 10.1016/j.envpol.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreiner RP, Tarara JM. Deficit irrigation promotes arbuscular colonization of fine roots by mycorrhizal fungi in grapevines (Vitis vinifera L.) in an arid climate. Mycorrhiza. 2007;17:551–562. doi: 10.1007/s00572-007-0128-3. [DOI] [PubMed] [Google Scholar]
- 29.Schreiner RP, Tarara JM, Smithyman RP. Effects of native and nonnative arbuscular mycorrhizal fungi on growth and nutrient uptake of ‘Pinot Noir’ (Vitis vinifera L.) in two soils with contrasting levels of phosphorus. Appl. Soil Ecol. 2007;36:205–215. [Google Scholar]
- 30.Davies FT, Puryear JD, Newton RJ, Egilla JN, Saraiva Grossi JA. Mycorrhizal fungi increase chromium uptake by sunflower plants: Influence on tissue mineral concentration, growth, and gas exchange. J. Plant Nutr. 2002;25:2389–2407. [Google Scholar]
- 31.Lopez ML, Peralta-Videa JR, Castillo-Michel H, Martinez-Martinez A, Gardea-Torresdey JL. Lead toxicity in alfalfa plants exposed to phytohormones and ethilenediaminetetracetic acid monitored by peroxidase, catalase and amylase activities. Environ. Toxicol. Chem. 2007;26:2717–2723. doi: 10.1897/07-302.1. [DOI] [PubMed] [Google Scholar]
- 32.Deng H, Ye ZH, Wong MH. Accumulation of lead, zinc copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environ. Pollut. 2004;132:29–40. doi: 10.1016/j.envpol.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 33.Leyval C, Turnau K, Haselwandter K. Effect of heavy metal pollution on mycorrhizal colonization and function: physiological, ecological and applied aspects. Mycorrhiza. 1997;7:139–153. [Google Scholar]
- 34.Gardea-Torresdey JL, de la Rosa G, Peralta-Videa JR, Montes M. A study of the differential uptake and transportation of trivalent and hexavalent chromium by tumbleweed (Salsola kali). Arch. Environ. Contam. Toxicol. 2005;48:225–232. doi: 10.1007/s00244-003-0162-x. [DOI] [PubMed] [Google Scholar]
- 35.Wallace A, Soufi SM, Cha JW, Romney EM. Some effects of chromium toxicity on bush bean plants grown in soil. Plant Soil. 1976;44:471–473. [Google Scholar]
- 36.de la Rosa G, Martinez-Martinez A, Pelayo HR, Peralta-Videa JR, Gardea-Torresdey JL. Tumbleweed (Salsola kali) a potential Cd hyperaccumulator: low molecular weight thiols and ions response to Cd(II) stress. Plant Physiol. Biochem. 2005;43:491–498. doi: 10.1016/j.plaphy.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Stribley DP, Tinker PB, Snellgrove RC. Effect of vesicular-arbuscular mycorrhizal fungi on the relations of plant growth, internal phosphorus concentration and soil phosphate analyses. European J. Soil Sci. 1980;31:655–672. [Google Scholar]
- 38.Joner EJ, Briones R, Leyval C. Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant Soil. 2000;226:227–234. [Google Scholar]
- 39.Castillo-Michel H, Parsons JG, Peralta-Videa JR, Martinez-Martinez A, Dokken KM, Gardea-Torresdey JL. Use of X-ray absorption spectroscopy and biochemical techniques to characterize arsenic uptake and reduction in pea (Pisum sativum) plants. Plant Physiol. Biochem. 2007;45:457–463. doi: 10.1016/j.plaphy.2007.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.