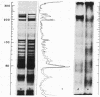
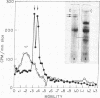
Abstract
A cell surface component has been isolated in partially purified form from cultured chick embryo and chick heart fibroblasts. This glycoprotein is similar to a protein recently reported to be present at the surface of normal cells, and missing after neoplastic transformation. It is a major cell surface glycoprotein that is synthesized by cultured fibroblasts, but is not collagen. It is shown to be markedly trypsin-sensitive, and its recovery from the cell surface is dependent on cell density. It is excluded from Sephadex G-200, but is not rapidly sedimented by ultracentrifugation, and has an apparent molecular weight of 220,000. The isolation of this cell surface glycoprotein may now provide a means of determining its function.
Keywords: transformation, proteolysis, urea, iodination, gel electrophoresis
Full text
PDF
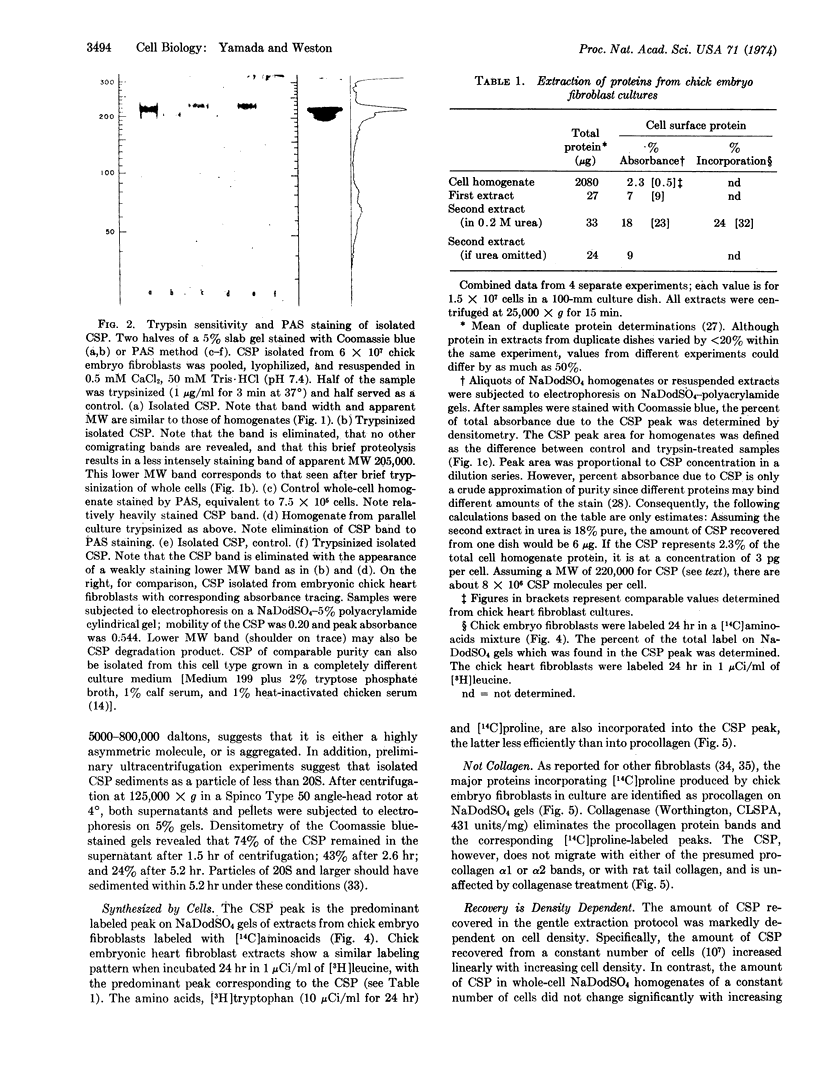
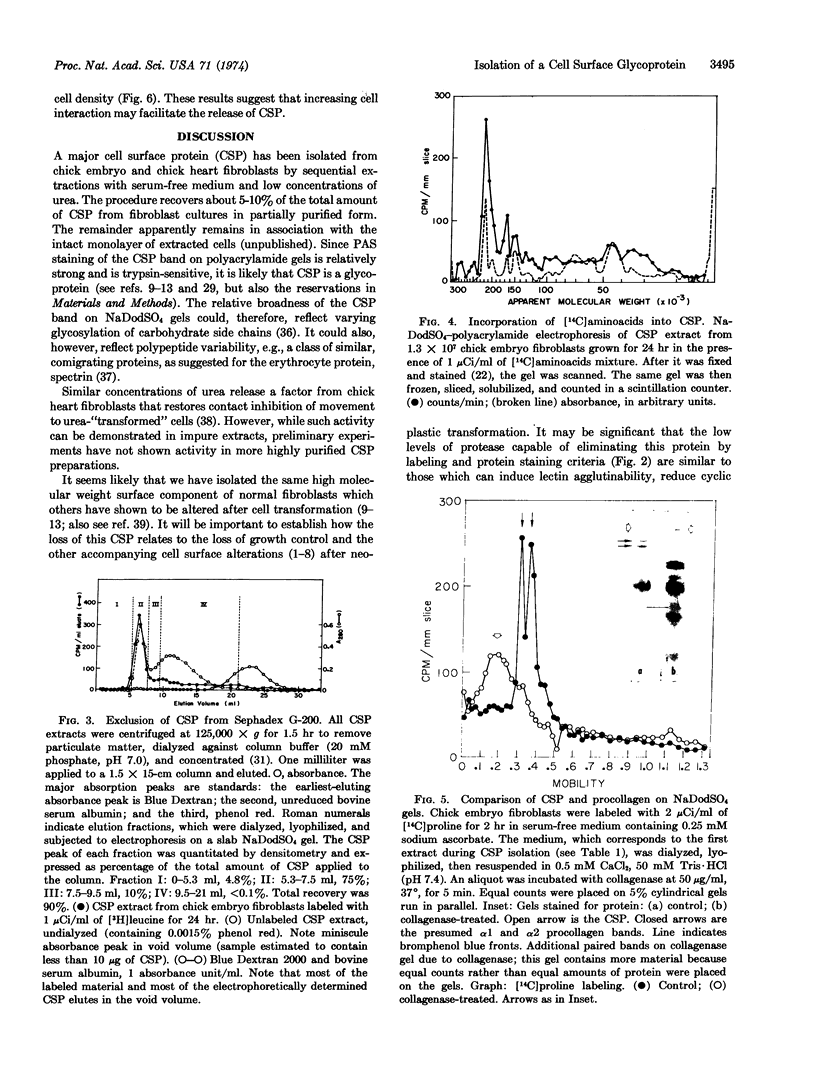
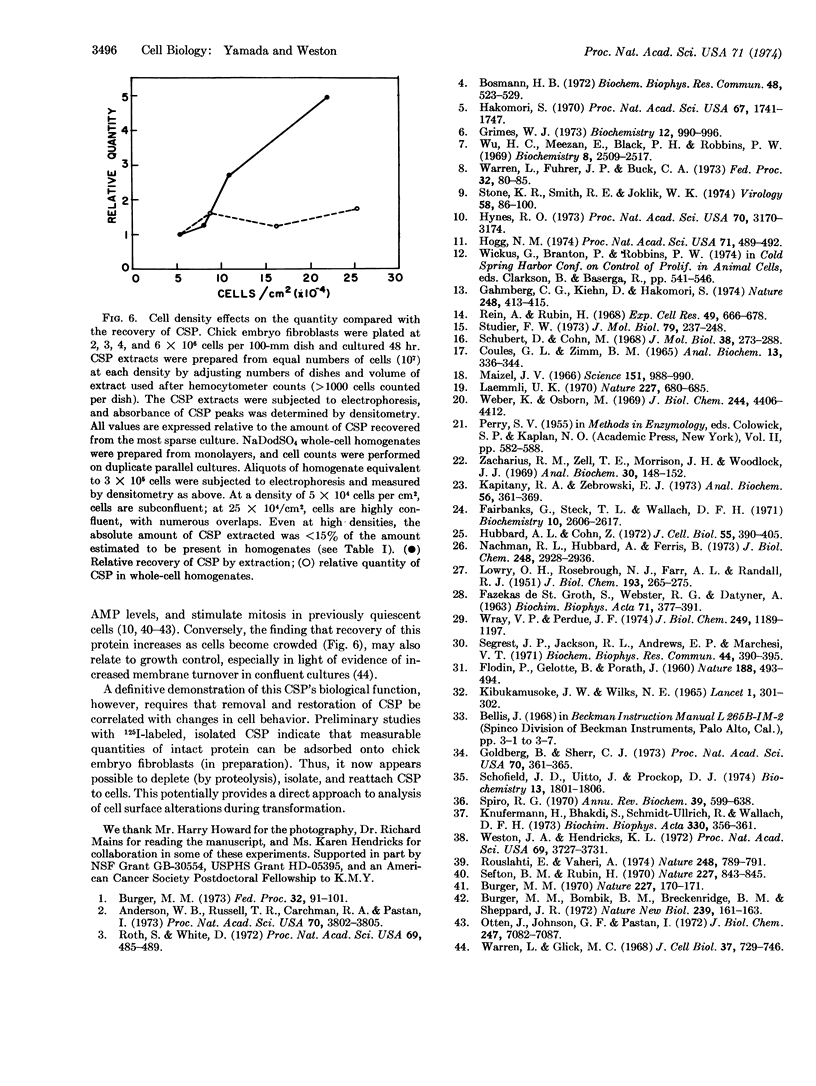
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Russell T. R., Carchman R. A., Pastan I. Interrelationship between adenylate cyclase activity, adenosine 3':5' cyclic monophosphate phosphodiesterase activity, adenosine 3':5' cyclic monophosphate levels, and growth of cells in culture. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3802–3805. doi: 10.1073/pnas.70.12.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B. Cell surface glycosyl transferases and acceptors in normal and RNA- and DNA-virus transformed fibroblasts. Biochem Biophys Res Commun. 1972 Aug 7;48(3):523–529. doi: 10.1016/0006-291x(72)90379-8. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Bombik B. M., Breckenridge B. M., Sheppard J. R. Growth control and cyclic alterations of cyclic AMP in the cell cycle. Nat New Biol. 1972 Oct 11;239(93):161–163. doi: 10.1038/newbio239161a0. [DOI] [PubMed] [Google Scholar]
- Burger M. M. Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature. 1970 Jul 11;227(5254):170–171. doi: 10.1038/227170a0. [DOI] [PubMed] [Google Scholar]
- Burger M. M. Surface changes in transformed cells detected by lectins. Fed Proc. 1973 Jan;32(1):91–101. [PubMed] [Google Scholar]
- Choules G. L., Zimm B. H. An acrylamide gel soluble in scintillation fluids: its application to electrophoresis at neutral and low pH. Anal Biochem. 1965 Nov;13(2):336–344. doi: 10.1016/0003-2697(65)90202-2. [DOI] [PubMed] [Google Scholar]
- FLODIN P., GELOTTE B., PORATH J. A method for concentrating solutes of high molecular weight. Nature. 1960 Nov 5;188:493–494. doi: 10.1038/188493a0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Kiehn D., Hakomori S. Changes in a surface-labelled galactoprotein and in glycolipid concentrations in cells transformed by a temperature-sensitive polyoma virus mutant. Nature. 1974 Mar 29;248(447):413–415. doi: 10.1038/248413a0. [DOI] [PubMed] [Google Scholar]
- Goldberg B., Sherr C. J. Secretion and extracellular processing of procollagen by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1973 Feb;70(2):361–365. doi: 10.1073/pnas.70.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes W. J. Glycosyltransferase and sialic acid levels of normal and transformed cells. Biochemistry. 1973 Feb 27;12(5):990–996. doi: 10.1021/bi00729a031. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Cell density-dependent changes of glycolipid concentrations in fibroblasts, and loss of this response in virus-transformed cells. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1741–1747. doi: 10.1073/pnas.67.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N. M. A comparison of membrane proteins of normal and transformed cells by lactoperoxidase labeling. Proc Natl Acad Sci U S A. 1974 Feb;71(2):489–492. doi: 10.1073/pnas.71.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIBUKAMUSOKE J. W., WILKS N. E. RAPID METHOD FOR URINARY PROTEIN CONCENTRATION. APPEARANCE OF MALARIA ANTIBODIES IN NEPHROTIC URINES. Lancet. 1965 Feb 6;1(7380):301–302. doi: 10.1016/s0140-6736(65)91031-7. [DOI] [PubMed] [Google Scholar]
- Kapitany R. A., Zebrowski E. J. A high resolution PAS stain for polyacrylamide gel electrophoresis. Anal Biochem. 1973 Dec;56(2):361–369. doi: 10.1016/0003-2697(73)90202-9. [DOI] [PubMed] [Google Scholar]
- Knufermann H., Bhakdi S., Schmidt-Ullrich R., Wallach D. F. N-terminal amino acid analysis reveal peptide heterogeneity in major electrophoretic protein components of erythrocyte ghosts. Biochim Biophys Acta. 1973 Dec 22;330(3):356–361. doi: 10.1016/0005-2736(73)90246-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- Nachman R. L., Hubbard A., Ferris B. Iodination of the human platelet membrane. Studies of the major surface glycoprotein. J Biol Chem. 1973 Apr 25;248(8):2928–2936. [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Regulation of cell growth by cyclic adenosine 3',5'-monophosphate. Effect of cell density and agents which alter cell growth on cyclic adenosine 3',5'-monophosphate levels in fibroblasts. J Biol Chem. 1972 Nov 10;247(21):7082–7087. [PubMed] [Google Scholar]
- Rein A., Rubin H. Effects of local cell concentrations upon the growth of chick embryo cells in tissue culture. Exp Cell Res. 1968 Mar;49(3):666–678. doi: 10.1016/0014-4827(68)90213-9. [DOI] [PubMed] [Google Scholar]
- Roth S., White D. Intercellular contact and cell-surface galactosyl transferase activity (cell culture-mouse-radioautography-contact inhibition-cis-and trans-galactosylation). Proc Natl Acad Sci U S A. 1972 Feb;69(2):485–489. doi: 10.1073/pnas.69.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A. Novel human serum protein from fibroblast plasma membrane. Nature. 1974 Apr 26;248(5451):789–791. doi: 10.1038/248789a0. [DOI] [PubMed] [Google Scholar]
- Schofield J. D., Uitto J., Prockop D. J. Formation of interchain disulfide bonds and helical structure during biosynthesis of procollagen by embryonic tendon cells. Biochemistry. 1974 Apr 23;13(9):1801–1806. doi: 10.1021/bi00706a004. [DOI] [PubMed] [Google Scholar]
- Schubert D., Cohn M. Immunoglobulin biosynthesis. 3. Blocks in defective synthesis. J Mol Biol. 1968 Dec;38(3):273–288. doi: 10.1016/0022-2836(68)90386-0. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Rubin H. Release from density dependent growth inhibition by proteolytic enzymes. Nature. 1970 Aug 22;227(5260):843–845. doi: 10.1038/227843a0. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Andrews E. P., Marchesi V. T. Human erythrocyte membrane glycoprotein: a re-evaluation of the molecular weight as determined by SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1971 Jul 16;44(2):390–395. doi: 10.1016/0006-291x(71)90612-7. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Annu Rev Biochem. 1970;39:599–638. doi: 10.1146/annurev.bi.39.070170.003123. [DOI] [PubMed] [Google Scholar]
- Stone K. R., Smith R. E., Joklik W. K. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974 Mar;58(1):86–100. doi: 10.1016/0042-6822(74)90143-3. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Warren L., Fuhrer J. P., Buck C. A. Surface glycoproteins of cells before and after transformation by oncogenic viruses. Fed Proc. 1973 Jan;32(1):80–85. [PubMed] [Google Scholar]
- Warren L., Glick M. C. Membranes of animal cells. II. The metabolism and turnover of the surface membrane. J Cell Biol. 1968 Jun;37(3):729–746. doi: 10.1083/jcb.37.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weston J. A., Hendricks K. L. Reversible transformation by urea of contact-inhibited fibroblasts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3727–3731. doi: 10.1073/pnas.69.12.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray V. P., Perdue J. F. Protein and glycoprotein subunit composition of plasma membrane from chick embryo fibroblasts. J Biol Chem. 1974 Feb 25;249(4):1189–1197. [PubMed] [Google Scholar]
- Wu H. C., Meezan E., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. I. Glucosamine-labeling patterns in 3T3, spontaneously transformed 3T3, and SV-40-transformed 3T3 cells. Biochemistry. 1969 Jun;8(6):2509–2517. doi: 10.1021/bi00834a038. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]