Abstract
Morphine, a preferential μ opioid receptor agonist, alters astroglial development by inhibiting cell proliferation and by promoting cellular differentiation. Although morphine affects cellular differentiation through a Ca2+-dependent mechanism, few studies have examined whether Ca2+ mediates the effect of opioids on cell proliferation, or whether a particular Ca2+ signal transduction pathway mediates opioid actions. Moreover, it is uncertain whether one or more opioid receptor types mediates the developmental effects of opioids. To address these questions, the present study examined the role of μ opioid receptors and Ca2+ mobilization in morphine-induced astrocyte development. Morphine (1 μM) and non-morphine exposed cultures enriched in murine astrocytes were incubated in Ca2+-free media supplemented with < 0.005, 0.3, 1.0, or 3.0 mM Ca2+ ([Ca2+]o), or in unmodified media containing Ca2+ ionophore (A23187), nifedipine (1 μM), dantrolene (10 μM), thapsigargin (100 nM), or L-glutamate (100 μM) for 0–72 h. μ-Opioid receptor expression was examined immunocytochemically using specific (MOR1) antibodies. Intracellular Ca2+ ([Ca2+]i) was measured by microfluorometric analysis using fura-2. Astrocyte morphology and bromodeoxyuridine (BrdU) incorporation (DNA synthesis) were assessed in glial fibrillary acidic protein (GFAP) immunoreactive astrocytes. The results showed that morphine inhibited astroglial growth by activating μ opioid receptors. Astrocytes expressed MOR1 immunoreactivity and morphine’s actions were mimicked by the selective μ agonist PL017. In addition, morphine inhibited DNA synthesis by mobilizing [Ca2+]i in developing astroglia. At normal [Ca2+]o, morphine attenuated DNA synthesis by increasing [Ca2+]i; low [Ca2+]o (0.3 mM) blocked this effect, while treatment with Ca2+ ionophore or glutamate mimicked morphine’s actions. At extremely low [Ca2+]o (<0.005 mM), morphine paradoxically increased BrdU incorporation. Although opioids can increase [Ca2+]i in astrocytes through several pathways, not all affect DNA synthesis or cellular morphology. Nifedipine (which blocks L-type Ca2+ channels) did not prevent morphine-induced reductions in BrdU incorporation or cellular differentiation, while thapsigargin (which depletes IP3-sensitive Ca2+ stores) severely affected inhibited DNA synthesis and cellular differentiation—irrespective of morphine treatment. However, dantrolene (an inhibitor of Ca2+-dependent Ca2+ release) selectively blocked the effects of morphine. Collectively, the findings suggest that opioids suppress astroglial DNA synthesis and promote cellular hypertrophy by inhibiting Ca2+-dependent Ca2+ release from dantrolene-sensitive intracellular stores. This implies a fundamental mechanism by which opioids affect central nervous system maturation.
Keywords: Cell division, Endogenous opioid system, μ-Opioid receptors, Neural development, Intracellular calcium, Dantrolene, Nifedipine, Thapsigargin, fura-2
1. Introduction
Endogenous opioid peptides and opioid receptors are present during development and can modify nervous system maturation through both direct and indirect mechanisms 24,51. Opioids can profoundly inhibit the growth of neurons and glia.
Astrocytes are important in mediating opioid-induced maturational changes in the nervous system. Not only do astrocytes themselves express endogenous opioid peptides during development 22,36,49,50,53, but opioids inhibit astrocyte proliferation and promote premature morphologic differentiation in vitro 22,55,57 and in vivo 45,64. Opioid drugs with abuse liability, such as heroin or morphine, affect glial development by disrupting the normal interactions between endogenous opioids and opioid receptors. Depending on the parameter measured, morphine is as potent as the endogenous opioid Met-enkephalin in affecting astrocyte growth 23,55. Morphine is particularly efficacious at inducing changes in morphologic differentiation including increases in astrocyte area and cell processes 55,57. Recently, diffuse, reactive astrocytosis, with regressive astrocytic changes, has been noted in the postmortem brains of chronic (non-HIV-infected) i.v. heroin abusers 19. Despite findings that astroglia are a target for opioid action, little is known about the mechanisms by which opioids affect astroglial function.
Although opioids are predominately inhibitory in their action 12, it has been shown that opioids can also be excitatory 11,63. Crain and Shen report that nanomolar amounts of opioids are excitatory, while micromolar concentrations of the same opioids are inhibitory in modulating the action potential of dorsal root ganglia neurons 11 Opioid actions are complex and differ depending on the particular opioid receptor type and its coupling to particular intracellular effectors 12,25–27,40. μ- And δ-opioid receptors are reported to preferentially mediate potassium conductance, whereas κ opioid receptors preferentially affect Ca2+ conductance in this system 11,37,62.
There is emerging evidence that opioid receptors are “promiscuous” in their interactions with particular intracellular effectors, especially in developing cells. Both excitatory and inhibitory opioid actions have been directly or indirectly linked to changes in intracellular Ca2+ ([Ca2+]i). Opioid receptor activation may increase [Ca2+]i through several pathways. For example, in neuroblastoma × glioma (NG108-15) hybrid cell lines, δ opioid receptor agonists can increase [Ca2+]i through the G-protein coupled phospholipase C-dependent production of inositol 1,4,5-trisphosphate (IP3), or through L-type or ω-conotoxin GVIa-sensitive voltage-gated Ca2+ channels 25,26. Opioids elevate [Ca2+]i in neuroblastoma X dorsal root ganglia hybrid cells through activation of Gαi2 58.
In astrocytes or C6 glioma cells, opioids appear to increase [Ca2+]i by two separate pathways 4,15,57. The ability of the dihydropyridine, nifedipine, to block κ opioid agonist-induced increases in [Ca2+]i has implicated L-type Ca2+ channels as mediating κ opioid receptor increases in [Ca2+]i in type 1 astrocytes15. Alternatively, the endogenous κ opioid ligand, dynorphin A (1–17), attenuates vasopressin-induced mobilization of [Ca2+]i through an IP3-dependent process in pituicytes (GFAP-immunoreactive cells in the neurohypophysis) 6. Moreover, in a Xenopus oocyte translation system functionally expressing μ, δ, or κ opioid receptors, all three opioid receptor types can mobilize [Ca2+]i by signaling Ca2+ release from internal stores 28. Despite some advances, the mechanism(s) by which opioids mobilize intracellular calcium in astrocytes during development are incompletely understood.
We previously reported that continuous morphine exposure (72 h) causes Ca2+-dependent increases in astrocyte size and shape which are similar to reactive changes/cellular hypertrophy57. The importance of Ca2+ in cell proliferation additionally prompted us to explore whether opioids inhibit astroglial DNA synthesis by affecting Ca2+ homeostasis. Our results suggest that Ca2+-dependent Ca2+ release from dantrolene sensitive intracellular stores mediates the effects of opioids on astrocyte development.
2. Materials and Methods
2.1. Drugs and compounds
Morphine sulfate was obtained from Sigma Chemical Co. (St. Louis, MO) and (−)-naloxone was obtained from E.I. Dupont (Wilmington, DE). H-Tyr-Pro-Phe (N-Me)-D-Pro-NH2 (PL017) was obtained from Chiron (Chiron Mimotopes Peptide Systems, San Diego, CA). Dantrolene; thapsigargin; Ca2+ ionophore, A23187; L-glutamate; and nifedipine were obtained from Sigma.
2.2. Cell culture
Primary cultures, enriched in astrocytes were obtained from 1- to 2-day-old Swiss-Webster mice (ICR strain, Harlen Sprague Dawley, IN) as previously described 55,57. Briefly, using aseptic technique, cells were isolated from the cerebral hemispheres of mouse pups killed by ether anesthesia and decapitation. Sterile 16 mm diameter, plain glass coverslips were coated with poly-L-lysine, placed into 22 mm diameter wells, and seeded with 5 × 105 cells in 1 mL of culture media. Culture media consisted of Dulbecco’s modified Eagle’s medium (DMEM) (1.8 mM CaCl2) supplemented with 0.5% glucose, 0.06% Na2CO3, and 5% fetal bovine serum (FBS) (KC Biological, Lenexa, KS). Cultures were incubated at 35°C in 5% CO2/95% air at high humidity and examined at 6 to 9 days in vitro. The serum-containing culture medium supports the growth of flat, polyhedral (type 1) astrocytes; this astroglial type is developmentally sensitive to opioids 23. In these cultures, the greatest number of type 1 astroglia express μ, δ and κ opioid receptors after day 5 in vitro (Hauser, unpublished), and that the inhibitory effect of opioids on astroglial division is maximal at 4–9 days in vitro. Typically, the cells in these cultures reach confluence on day 12.
2.3 Pharmacological manipulation of opioids and Ca2+
Independent preparations of cells were distributed across treatment groups. In acute fura-2 studies, the agonists, morphine or PL017, were added to the cultures where indicated. Naloxone was added 20 min prior to the addition of agonist. Extracellular Ca2+ was removed by 4-fold-rinses with media containing 0 [Ca2+] and 1 mM EGTA, 5 min prior to the addition of an agonist. In studies where astrocyte development was assessed, the media was replaced with basal media containing Ca2+ (<0.005, 0.3, 1.0, or 3.0 mM) with or without morphine sulfate (1 μM) 72 h before harvesting. in addition, some cultures were continuously treated 6 or 48 h prior to harvesting with culture media plus: Ca2+ ionophore A23187 (1.0 μM), glutamate (100 μM), nifedipine (1 μM), dantrolene (10 μM); or thapsigargin (100 nM) 9. Nifedipine and thapsigargin are initially dissolved in DMSO and serially diluted to reduce the final concentration of DMSO in the media to < 0.1% as previously described 9; nifedipine is protected from exposure to light. Media and drugs were changed daily.
2.4. μ-Opioid receptor immunocytochemistry
Cultures were fixed for 10 min in 3% paraformaldehyde in Sorenson’s phosphate buffer, pH 7.2, at 4 °C. Rabbit-anti-μ-opioid receptor (MOR1) antibodies 1 were diluted 1:5000 in phosphate buffered saline (PBS, pH 7.2) containing 0.1% Triton-X 100 and 0.1% crystalline bovine serum albumin (Calbiochem, San Diego, CA). Cultures were incubated with MOR1 antibodies for 24 hr at 4 °C on an orbital shaker at 40–60 rpm. Secondary, biotinylated goat-anti-rabbit antibodies conjugated to an avidin-peroxidase (Vectastain ABC kit, Vector Laboratories, Burlingame, CA) were used as directed to detect MOR1 primary antibodies. Nickel-intensified DAB consisting of 2.5% nickel ammonium sulfate, 0.35% DAB, and 0.012% H2O2 in 0.1 M sodium acetate (pH 6.0) was used as a substrate for peroxidase. Preabsorbed controls were included to assure specificity of the MOR1 antibodies.
2.5. Intracellular Ca2+
The procedures for fura-2 fluorescence imaging have been described before 57. Briefly, cells were grown on 35 mm diameter plastic dishes with glass bottoms (MatTek Co., MA). The cells were loaded at 37°C for 45 min with 4 or 10 μM fura-2/AM (Molecular Probes, Eugene, OR) in culture media containing 2% DMSO. The loaded cells were then washed 3 times with DMEM at 35°C and incubated for about 45 min in 5% CO2/95% air at 35°C to allow for complete hydrolysis of the fura-2/AM. Determinations of free [Ca2+]i were made in 1 mL of DMEM (or Ca2+-free DMEM with 1 mM EGTA), containing 10 or 25 mM Hepes buffer at pH 7.2: Repeated measures of [Ca2+]i levels were made at intervals from 1 to 20 seconds in the same cells before and at varied intervals after the addition of opioids. Opioids and/or Ca2+ agents were dissolved in DMEM before adding to the cultures. To block drug effects, cells were pretreated for 20 min (unless noted otherwise) with the opioid antagonist naloxone, nifedipine, thapsigargin, or 0 [Ca2+]o + 1 mM EGTA. In some experiments, cells were grown on 25 mm diameter glass coverslips and imaged in a perfusion chamber (RC-21; Warner Instrument Corp., Hamden, CT) which allowed continuous monitoring of cells while exchanging drug solutions. Cells were perfused at a rate of about 1 mL/min (100 μL bath volume) using a syringe pump (MD-1001; Bioanalytical Systems, W. Lafayette, IN). The cultures were viewed using an inverted Nikon microscope with an oil immersion, fluoro 40x, N.A. 1.3 objective and an intensified (Hamamatsu C2400, Hamamatsu City, Japan) CCD camera (Dage 72, Dage-MTI, Michigan City, IN). Intracellular Ca2+ levels were determined from the ratio of the fluorescence using two different excitation wavelengths (340 nm and 380 nm) as described before 20. A computer-controlled optical filter wheel (Lambda 10; Sutter Instrument Co./Axon Instruments, Novato, CA) was used to change excitation filters. Imaging Research Corporation M1 or M4 imaging systems (St. Catharines, Ontario) with fura-2 ratiometric software were used to acquire and process the images. Measurements were taken from the glial cytoplasm, away from peripheral processes but not over the nucleus 57. Values represent the average [Ca2+]i level therein. Cultures were processed for GFAP immunocytochemistry (see below) and re-examined to confirm that the cells were GFAP-immunoreactive (astrocytes).
2.6. Combined BrdU and GFAP Immunocytochemistry
To determine whether opioids affect DNA synthesis, cultures were treated with 5-bromodeoxyuridine (BrdU; Sigma) (50 mg/mL) for 6 h immediately before harvesting. At 4 or 6 days in vitro, cells were fixed in situ on the coverslips on which they were grown for 30 min in ice-cold Zamboni’s fixative with 3% paraformaldehyde and then post-fixed in 70% ethanol at 4°C overnight. A sequential double-labeling immunocytochemical procedure was used to detect BrdU and GFAP in the same cells.
To detect incorporated BrdU, double-stranded DNA was partially denatured by treating cultures with 0.07 M NaOH for 1 h. Mouse-anti-BrdU monoclonal antibodies (Chemicon, Temecula, CA) were diluted 1:1000 (w/v) in PBS (phosphate buffered saline) at pH 7.4 in the presence of 0.1% Triton-X 100 and 1% crystalline grade BSA (Calbiochem). Tissues were incubated with anti-BrdU antibodies for 24 h at 4°C on an orbital shaker at 40–60 rpm. Biotinylated donkey-anti-mouse secondary antibodies conjugated to avidin-peroxidase were used to detect BrdU antibodies (Vectastain ABC kit). The reaction yields an insoluble, blue-black product within the cell nucleus by incubating for 4–6 min in nickel-intensified DAB.
Rabbit-anti-GFAP antibodies (Chemicon, Temecula, CA) were used at a 1:1000 dilution overnight at 4°C to detect GFAP. Biotinylated, goat-anti-rabbit secondary antibodies conjugated to avidin-peroxidase were used to detect GFAP antibodies as directed (Vectastain ABC kit). Peroxidase-conjugated GFAP antibody complexes were visualized by the brown reaction with 0.06% DAB, 0.02% hydrogen peroxide in 50 mM Tris buffer, pH 7.6. The DAB reaction was observed using an inverted microscope and stopped when signal-to-noise is optimal (e.g., 4–8 min). The black BrdU product in the nucleus and brown GFAP product in the cytoplasm did not overlap and were discernible in the same cell. To demonstrate the BrdU and GFAP immunoproducts in the same cells using black and white photography, a blue-green acetate filter (#858 Roscolene; Rosco, Port Chester, NY) was used to enhance the GFAP product, while a dark copper-brown filter (#819 Roscolene) was used to attenuate the GFAP product compared to the BrdU product. There was no specific immunoreactivity when primary BrdU or GFAP antibodies were eliminated from the reaction.
2.7. Astroglial size and shape
The area, perimeter, and form factor of individual flat, polyhedral (type 1) GFAP-positive cells were determined using computer-assisted image analysis (M1; Imaging Research Corporation) and an Olympus Vanox microscope with a 40x objective as previously described 57. Form factor is an index of cell shape [(4π)(area)/(perimeter2)]; its value is highest for circles, but decreases with complex shapes (increased size/number of processes). Glia pooled from 2–3 mice were maintained as independent preparations of cells (n = 1) and distributed across treatment groups as separate cultures. Twenty cells were randomly sampled from each of at least n = 6 independent preparations of cells (at least 120 cells per group).
2.8. Percentage of astroglia incorporating BrdU
Individual astrocytes were manually counted using an Olympus Vanox microscope with a 40x objective and a calibrated 10 X 10 square-lattice eyepiece reticle. About 600 randomly selected, type 1 astrocytes were sampled from defined areas (μm2) in each culture with the observer unaware of treatment history. Astrocytes in multi-cell clusters were not counted. Flat, polyhedral (type 1) astroglia were identified by using morphologic and immunological criteria. The labeling index was determined by the formula: [BrdU labeled astrocytes/(BrdU-labeled astrocytes + non-BrdU-labeled astrocytes)]. Glia pooled from 2–3 mice were maintained as independent preparations of cells (n = 1) and distributed across treatment groups. Determinations were made from at least n = 6 independent preparations of cells in each experiment, and all experiments were replicated at least twice.
2.9. Statistics
Data were reported as the mean ± SE. Experimental differences were tested using analysis of variance (ANOVA) and subsequent post hoc comparisons were made using Newman-Keuls test (General ANOVA programs, Statistica, StatSoft, Tulsa, OK). Student’s t test was used to assess the effect of manipulating Ca2+ with or without morphine (or L-glutamate). Differences were considered significant if P < 0.05.
3. Results
3.1. μ-Opioid receptor immunocytochemistry
At 6 to 9 days in vitro, 95–97% of the flat, polyhedral cells in our cultures are GFAP immunoreactive identifying them as type 1 astrocytes (Hauser and Stiene-Martin, unpublished). Using MOR1 antibodies, one-third to one-half of the type 1 astrocytes expressed μ-opioid receptor immunoreactivity (Fig. 1). Punctate patterns of MOR1 immunoreactivity were associated with the cytoplasm and/or plasma membrane, and were especially prominent in the juxtanuclear cytoplasm which may be associated with the endoplasmic reticulum or with the endosomal compartment. Fainter, more diffuse patterns of immunoreactivity occasionally outlined the plasma membrane or adjacent cytoplasm of some astrocytes. The levels of immunoreactivity differed greatly among type 1 astrocytes, some cells contained high levels of MOR1 immunoreactivity, whereas others lacked immunoproduct. Immunoreactive product was absent in preabsorbed controls.
Fig. 1.
Brightfield photomicrographs of μ-opioid receptor (MOR1) immunoreactive type 1 astrocytes at 6 days in vitro. MOR1 immunoreactivity was typically punctate with some faint, diffuse immunoreactivity occasionally outlining the plasma membrane and/or adjacent cytoplasm (a,c) Intense immunostaining was often associated with the juxtanuclear cytoplasm (c). The intensity of MOR1 immunoreactivity varied among individual astrocytes; some cells lacked immunoproduct (not clearly shown here). MOR1 immunoreactivity was absent in preabsorbed controls (b). Greater than 95% of the flat, polyhedral cells were glial fibrillary acidic protein-immunoreactive (d). Scale bars: 10 μm (a and b); 25 μm (c), 25 μm (d). Unmodified brightfield optics (a,b,d); differential interference contrast optics (c). Arrows = astrocytes.
3.2. Intracellular Ca2+
Morphine (100 nM) treatment increased [Ca2+]i in a subpopulation of astrocytes (Fig. 2a). [Ca2+]i increases were similarly observed following the addition of the selective μ opioid receptor agonist PL017 (Fig. 2b), and prolonged increases in [Ca2+]i were seen with higher agonist concentrations or prolonged exposure. Pretreatment with equimolar concentrations of naloxone prevented PL017 or morphine (data not shown) induced increases in [Ca2+]i, suggesting that the effect was mediated through opioid receptors (2c). Morphine, a less-selective μ agonist, typically activated [Ca2+]i in the same astrocytes as PL017, a highly selective μ agonist, suggesting that both drugs affected μ sites (2d).
Fig. 2.
Effect of morphine and/or PL017 on [Ca2+]i in individual type 1 astrocytes (a–e). Morphine (1 μM) (a), or the selective μ opioid receptor agonist PL017 (b), increased [Ca2+]i in some astrocytes, while naloxone pretreatment prevented PL017-induced increases in [Ca2+]i (c). Morphine and PL017 increased [Ca2+]i in the same cells (d). PL017 treatment increased [Ca2+]i in the absence of extracellular Ca2+ (0 [Ca2+]o and 1 mM EGTA) in some cells (e). Drugs were added at 100 nM concentrations, except where noted. Responsive astrocyte (------), non-responsive astrocyte (-------). The scale is identical in a–b, and in c,d–e.
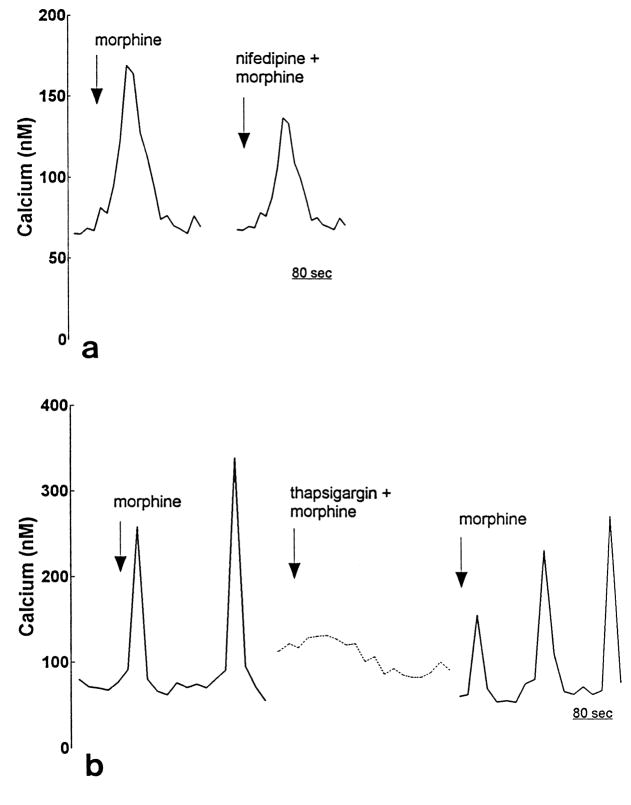
In a subset of astrocytes, morphine-induced increases in [Ca2+]i were caused by Ca2+ release from IP3-sensitive intracellular stores, rather than Ca2+ influx across the plasma membrane. When Ca2+ influx was prevented by removing [Ca2+]o, PL017 treatment increased [Ca2+]i (2e). Blocking L-type Ca2+ channels with nifedipine also did not prevent morphine-induced increases in [Ca2+]i (Fig. 3a). However, morphine-induced increases in [Ca2+]i were prevented when IP3-sensitive intracellular Ca2+ stores were depleted by thapsigargin pretreatment (3b).
Fig. 3.
Effect of nifedipine or thapsigargin on morphine-induced increases in [Ca2+]i in individual type 1 astrocytes. Nifedipine (1 μM) treatment did not prevent morphine-induced increases in [Ca2+]i in some astrocytes (a). In contrast, thapsigargin treatment reversibly blocked morphine-induced increases in [Ca2+]i (b). Drug concentrations were 100 nM—except where noted. Responsive astrocyte (-------), non-responsive astrocyte (-------).
3.3. Astroglial size and shape
We have previously shown that morphine-induced elevations in [Ca2+]i cause increases in astrocyte size and in the elaboration of cytoplasmic processes 57. To explore the role of particular Ca2+ activation pathways in mediating these changes, Ca2+ was manipulated pharmacologically and morphine-induced changes were assessed morphometrically (Figs. 4; 5a,b).
Fig. 4.
Brightfield photomicrographs showing the effects of morphine, and/or drugs affecting [Ca2+]i, on astrocyte morphology at 48 h following continuous drug exposure (a–f). Compared to untreated controls (a), morphine treatment caused cellular hypertrophy (b). Dantrolene treatment prevented morphine-induced cellular hypertrophy (c), while nifedipine did not prevent morphine-dependent changes in the appearance of individual cells (d). Thapsigargin treatment caused astrocytes to assume an abnormal morphology—irrespective of morphine treatment (e,f); a blue-green filter was used to enhance photographic contrast; scale bar = 25 μm.
Fig. 5.
Effects of morphine, and/or drugs affecting [Ca2+]i, on the area (a) and form factor (b) of astrocytes at 48 h following continuous drug exposure. Compared to untreated controls, morphine treatment caused significant increases in the area (a) and in the abundance of cytoplasmic processes (indicated by significant decreases in form factor) (b). Measurements of form factor decrease with increasingly complex shape; i.e., form factor is greatest for circles. Treatment with dantrolene (Dantrl; 10 μM), but not nifedipine (Nifdpn; 1 μM), prevented morphine-induced changes in astrocyte morphology. Thapsigargin treatment (Thapsgn; 100 nM) caused significant decreases in area and form factor compared to untreated controls—irrespective of morphine treatment. *P < 0.05 vs. non-morphine treated controls.
Although morphine can increase [Ca2+]i through several different mechanisms, only the activation of some Ca2+ pathways resulted in changes in cellular morphology at 48 h (Figs. 4; 5a,b). For example, morphine alone caused a significant 94% increase in astrocyte area (P < 0.01), while significantly reducing form factor by 26% (P < 0.05). Dantrolene treatment prevented morphine-induced alterations in astroglial morphology, while dantrolene alone had no effect on cell size or shape compared to controls. In contrast, morphine-induced increases in astroglial morphology were unaffected by nifedipine. Lastly, thapsigargin treatment caused significant decreases in astrocyte area (44% without morphine; 47% with morphine) and marked reductions in form factor (37% without morphine; 47% with morphine) compared to control values--irrespective of whether morphine was co-administered.
3.4. Percentage of astroglia incorporating BrdU
BrdU and GFAP immunoproducts were readily discernible in the same cell (Fig. 6).
Fig. 6.

Brightfield photomicrographs showing combined bromodeoxyuridine (BrdU) and glial fibrillary acidic protein (GFAP) immunoreactivity in the same cells. To better distinguish the two products with black and white photography, a blue-green filter is used to enhance the copper-brown (DAB) GFAP immunoproduct (a); whereas a copper-brown filter is used to attenuate the GFAP immunoproduct relative to the black-blue BrdU immunoproduct (b). (a) and (b) are the same field. Scale bar = 25 μm.
In the presence of unmodified cell culture medium, morphine caused a 24% reduction in BrdU incorporation by astrocytes compared to control values. In media containing 1.0 or 3.0 mM [Ca2+]o, morphine also caused a significant suppression in the proportion of astroglia incorporating BrdU (Fig. 7). However, at low [Ca2+]o (0.3 mM), morphine did not suppress BrdU incorporation (Fig. 7). Treatment with Ca2+ ionophore A23187 significantly reduced BrdU incorporation by 26–27%--irrespective of morphine treatment; whereas treatment with glutamate (100 μM), which also increases intracellular Ca2+ in type 1-like astrocytes (Fig. 7) 10,18, significantly inhibited BrdU incorporation by 13% compared to non-glutamate-treated cultures. At extremely low <0.005 mM [Ca2+]o (0 Ca2+), DNA synthesis was reduced compared to astrocytes in unmodified culture medium. Surprisingly, at <0.005 mM [Ca2+]o, morphine treatment caused a paradoxical 52% increase in BrdU incorporation compared to non-morphine treated astrocytes (Fig. 7). Growth could not be assessed in the complete absence of extracellular Ca2+ (0 [Ca2+]o + 1 mM EGTA), because without [Ca2+]o astrocytes detach from the cell culture dish within 30–40 min.
Fig. 7.

Effect of manipulating Ca2+ on morphine-induced suppression of bromodeoxyuridine (BrdU) incorporation by type 1 astrocytes. In the presence of unmodified culture medium (Control), or medium with near-normal (1.0 mM) or high (3.0 mM) [Ca2+]o, morphine significantly decreased BrdU incorporation into astrocytic DNA. In contrast, at low (0.3 mM) [Ca2+]o, or in the presence of 1.0 mM [Ca2+]o and Ca2+ ionophore A23187 (1 μM), morphine no longer affected BrdU incorporation. Paradoxically, when [Ca2+]o levels were exceedingly low (< 0.005 mM), morphine treatment significantly increased the proportion of astrocytes incorporating BrdU compared to non-morphine treated controls. Treatment with glutamate (100 μM), which elevates [Ca2+]i in astroglia, also significantly reduced BrdU incorporation. The ability of morphine to inhibit BrdU incorporation by astrocytes was dependent on [Ca2+]o. *P < 0.05 vs. non-morphine (or non-glutamate) treated controls.
To assess how opioid-induced changes in [Ca2+]i affected DNA synthesis, particular Ca2+ activation pathways were blocked and morphine-induced changes in BrdU incorporation were assessed at 6 and 48 h (Fig. 8a,b).
Fig. 8.
Effect of manipulating intracellular calcium ([Ca2+]i) on morphine-dependent decreases in bromodeoxyuridine (BrdU) incorporation in type 1 astrocytes at 6 h (a) and 48 h (b). At 6 h, continuous treatment with morphine alone, or drugs affecting [Ca2+]i, did not affect the percentage of astrocytes incorporating BrdU (a). In contrast, at 48 h following continuous exposure, dantrolene treatment (Dantrl; 10 μM) prevented morphine-induced decreases in BrdU synthesis compared to control values. Dantrolene alone did not affect on the rate of BrdU synthesis. Nifedipine treatment (Nifdpn; 1 μM) did not prevent morphine-induced declines in BrdU incorporation compared to control values, while nifedipine alone had no affect on BrdU uptake. Thapsigargin treatment (Thapsgn; 100 nM) alone, or in the presence of morphine, significantly reduced the proportion of astrocytes incorporating BrdU. *P < 0.05 vs. non-morphine treated control.
At 6 h, morphine treatment did not affect BrdU incorporation by type 1 astrocytes (Fig. 8a). In addition, neither dantrolene-induced inhibition of Ca2+ -dependent Ca2+ release nor thapsigargin-induced depletion of IP3-dependent Ca2+ stores affected BrdU incorporation by astroglia at 6 h following exposure (Fig. 8a). Furthermore, BrdU incorporation was unaffected by blocking L-type Ca2+ channels with nifedipine (Fig. 8a).
In contrast to 6 h, at 48 h, the manipulation of some Ca2+ pathways significantly affected the ability of morphine to reduce BrdU incorporation in type 1 astrocytes (P < 0.05) (Fig. 8b). Most notably, dantrolene treatment prevented morphine-induced decreases in BrdU incorporation by astroglia, while dantrolene alone had no effect on BrdU incorporation compared to untreated controls. DNA synthesis was also unaffected by administering nifedipine alone. Notably, however, nifedipine did not prevent morphine-induced reductions in the proportion of astroglia incorporating BrdU. When co-administered with nifedipine, morphine significantly reduced BrdU incorporation by 34% compared to cultures treated with nifedipine alone (P < 0.05) or by 44% compared to untreated controls (P < 0.01). Although nifedipine appeared to enhance the inhibitory effect of morphine, combined treatment with morphine and nifedipine did not differ markedly from treatment with morphine alone. Lastly, irrespective of whether morphine was given, prolonged (48 h) thapsigargin treatment caused a near cessation of BrdU incorporation by astroglia (P < 0.025).
4. Discussion
Identifying the particular opioid receptor types that affect cellular growth is of fundamental importance toward understanding the mechanisms by which opioids affect neural maturation. Previous studies have shown that morphine inhibits astroglial proliferation while causing marked cellular hypertrophy 23,55,57. More recent evidence shows that the cellular hypertrophy is caused by increased [Ca2+]i and is likely to be mediated by the activation of μ- or κ-opioid receptors 57. The present study further supports a role for μ opioid receptors in mediating the effects of morphine on cellular development by showing that (i) μ opioid receptors are expressed by astrocytes, (ii) μ receptor activation mobilizes [Ca2+]i, and (iii) the mobilization of [Ca2+]i inhibits DNA synthesis and causes cellular hypertrophy. In addition, the present study identifies a particular Ca2+-dependent pathway, i.e., Ca2+-dependent Ca2+-release, as a critical step in mediating the developmental perturbations associated with opioid exposure. This suggests a sequence of events by which opioids inhibit cellular growth consisting of the activation of μ opioid receptors, mobilization of [Ca2+]i, and amplification of that signal through Ca2+-dependent Ca2+ release.
The present study provides immunocytochemical and functional evidence that astrocytes express μ opioid receptors. However, there has been controversy regarding whether astrocytes express opioid receptors. Recent evidence suggests that primary astrocytes can express multiple opioid receptor types in vitro 13–15,21,31,43,60. Using ribonuclease protection and/or reverse transcriptase-PCR assays, astrocytes have been shown to express μ, δ, and κ opioid receptor mRNAs 43,60. In contrast, several earlier studies failed to identify either μ opioid receptors, or other non-μ opioid receptor types, in cultured astrocytes 13,14,46,61. The reason for these inconsistencies is uncertain, but probably result from differing culture conditions, including differences in the availability of cytokines and/or growth factors, e.g., interleukin-1β, which can induce opioid receptor expression in astrocytes 44, or differences in opioid receptor expression among astrocytes derived from different brain regions and times during development 43,54. In studies examining glial proliferation, we have noted that astroglia gradually lose their response to opioids with progressive development in vitro. The loss occurs irrespective of whether astroglia are subcultured to a lower density to promote growth, and presumably coincides with the loss of functional opioid receptors 23 (Hauser, unpublished). Therefore, when assessing the opioid receptor expression by astrocytes it is important to consider the potential impact of species (and strain) differences, regional and developmental differences in the brain, and varied culture conditions.
Although μ opioids affect astrocyte development in the present study, we cannot exclude the possibility that other opioid receptor types might also affect astrocyte development through a similar Ca2+-dependent (or alternative) mechanism. This suggestion is prompted by findings that δ or κ opioid receptor activation increases [Ca2+]i in subsets of astrocytes 15,21,60, and by findings that selective κ opioid agonists can inhibit astrocyte proliferation 21. In addition, because only 30–50% of the astrocytes in our cultures appear to express μ opioid receptors, the mean values reported herein are likely to underestimate the developmental changes within this subpopulation, i.e., the developmental changes occurring within the μ receptor expressing subpopulation are likely to be quite profound. The extent to which κ- or δ-opioid receptor expressing astrocyte subpopulations overlap or interact with μ receptor expressing subpopulations is uncertain and warrants further study.
Opioids increase intracellular Ca2+ in astrocytes
The cellular mechanisms underlying Ca2+ mobilization and/or maintenance of steady state levels are diverse 16. These include: alterations in Ca2+ influx through voltage- or ligand-gated Ca2+ channels 16, Ca2+ extrusion from the cell (e.g., via Ca2+-ATPase or the Na+/Ca2+ exchanger within the plasma membrane), Ca2+ binding proteins, or changes in the release or sequestration of Ca2+ from internal storage sites 5,42. Ca2+ increases in astrocytes can result from increased extracellular K+, mechanical deformation, the stimulation of specific cell surface receptors, or by intercellular signaling through gap junctions 10,18,30,47,48,52. Ca2+ increases can be mediated by influx through L or T type Ca2+ channels 52, or by IP3, Ca2+, or eicosonoid-induced (ryanodine-sensitive) release from intracellular stores 8. Intercellular Ca2+ signaling among astrocytes has been proposed as a mechanism of long-distance communication between glia, and may be fundamentally important in normal nervous system function or following injury 18,30,52.
There is an absolute requirement for Ca2+ for entry into and exit from mitosis 41. Cells deprived of Ca2+ cannot advance from the G1/S phase of the cell division cycle 35,41. Ca2+-dependent protein kinases are central in regulating astrocyte proliferation and differentiation 32. Therefore, it was not surprising that manipulating [Ca2+]i affected DNA synthesis in astroglia. There was an optimal range of [Ca2+]i which favored DNA synthesis in astroglia and opioids inhibited DNA synthesis within this range. However, at abnormally high or low Ca2+ concentrations, respectively, the effect of morphine on DNA synthesis was negated or no longer inhibitory. For example, morphine had no additive effect on DNA synthesis in the presence of calcium ionophore. High [Ca2+]i levels resulting from ionophore treatment potentially saturated Ca2+ calmodulin-dependent effectors regulating cell replication and/or were cytotoxic through indirect mechanisms. ParadoxicalIy, at very low [Ca2+]o, morphine increased the rate of DNA synthesis. Under these conditions, morphine might have compensated for decreased [Ca2+]o by mobilizing Ca2+ from intracellular stores. Thus, our results suggest that, depending on the starting concentration, relative increases in [Ca2+]i can increase or decrease the rate of DNA synthesis in astrocytes. Similar observations have been noted in other neural cell types. For example, at optimal concentrations Ca2+ permits plasticity and survival, but at extreme low or high concentrations, respectively, Ca2+ promotes stasis or death 29,33,34.
Opioid-dependent declines in astroglial proliferation are likely to be mediated through release of Ca2+ from intracellular stores. In the presence of very low [Ca2+]o, opioids can increase [Ca2+]i and can affect DNA synthesis. Conversely, if IP3-sensitive Ca2+ release or Ca2+-dependent Ca2+ release are prevented, opioids no longer have any differential effect on DNA synthesis compared to non-opioid treated astrocytes. Similarly, other investigators report that extracellular Ca2+ is not necessary to evoke the anti-proliferative effects of excitatory amino acids such as glutamate in cultured astrocytes 38. In these studies, proliferation is inhibited through increased IP3 turnover without stimulating 45Ca2+ influx 38. Since glutamate substantially increases [Ca2+]i in astroglia 18,30,52, it is likely that Ca2+ release from internal stores accompanies changes in inositol phospholipid hydrolysis in the above studies. Moreover, although opioids are reported to cause increases in astrocyte [Ca2+]i by opening L-type Ca2+ channels 4,15, continuous nifedipine treatment (even at 10 μM concentrations) does not prevent morphine-induced reductions in BrdU incorporation or alterations in cell size and shape. Apparently, the influx of Ca2+ through L-type Ca2+ channels is not a mechanism by which opioids affect astrocyte proliferation or differentiation.
Our findings suggest that the release of Ca2+ from IP3-dependent stores is a requirement for DNA synthesis, as well as the maintenance of cellular morphology, in astrocytes. This is consistent with findings that thapsigargin-induced depletion of IP3-sensitive intracellular Ca2+ stores causes cell cycle arrest 35,41, and agrees with findings that opioids affect DNA synthesis by affecting IP3 turnover in fetal brain cell aggregates 2–4. Moreover, [Ca2+]i mobilization is associated with altered IP3 turnover and coincides with altered DNA synthesis by C6 glioma cells 4. Thapsigargin depletes IP3-sensitive Ca2+ stores by inhibiting endoplasmic reticulum Ca2+-ATPases (SERCAs) and by causing leakage of Ca2+ from these sites 59. Whether IP3-induced increases in [Ca2+]i mediate opioid-dependent reductions in DNA synthesis cannot be addressed in the present study based on BrdU incorporation studies alone, since prolonged thapsigargin treatment (48 h) caused a near cessation in DNA synthesis and dramatic alterations in morphology--irrespective of morphine treatment. However, the ability of thapsigargin to attenuate opioid-dependent rises in [Ca2+]i shows that opioids can release Ca2+ through an IP3-dependent mechanism, but do not provide a causal link between the opioid-dependent inhibition of IP3-induced Ca2+ release and alterations in astroglial growth or differentiation.
Importantly, the ability of dantrolene to block the effects of morphine on BrdU incorporation and cellular morphology provides novel insight to the mechanism by which opioids inhibit astrocyte development. Dantrolene blocks Ca2+-dependent Ca2+ release from internal stores 39. Normally, Ca2+-dependent Ca2+ release is thought to be important in amplifying intracellular Ca2+ signals in astroglia 8, and/or may potentiate IP3-dependent Ca2+ release 17. Our findings suggest that opioids affect cell growth and differentiation by amplifying intracellular Ca2+ signals. Opioids may potentiate Ca2+ signals evoked by opioid or non-opioid stimuli. Lastly, dantrolene does not block Ca2+ signaling through gap junctions 8, and therefore does not block morphine’s action by preventing intercellular signaling among astrocytes. Conversely, intercellular signaling may permit opioid-induced Ca2+ signals to be propagated to astrocytes that lack opioid receptors.
During development, astroglia can affect the proliferation, migration, differentiation and survival of neurons and neuronal progenitors 7. In the adult central nervous system, astrocytes function to preserve the ionic and metabolic extracellular milieu, are involved in intercellular signaling, and provide key neural responses to injury 47,48. It has been proposed that endogenous opioids 56, as well as opiate drugs 23,55, affect neural development by inhibiting the genesis of astrocytes. Presumably, a component of the neurobehavioral defects seen in the offspring of opiate-dependent mothers results from the impact of opiate drugs on astroglia 23,55,57. Thus, findings that opioids can affect astrocyte growth and differentiation through changes in Ca2+ homeostasis suggest a basic mechanism by which opioids modulate nervous system development.
Acknowledgments
We wish to thank Dr. Theo Schoenmakers (Univ. Nijmegen, The Netherlands) for kindly providing a copy of the Chelator (version 1.00) software used in initial calibrations of our fura-2 system. We also thank Ms. Carol Turbek and Ms. Rong Zhou for technical assistance. This work was supported by grant DA 06204 and a Major Equipment Grant from NIDA, and by the University of Kentucky Medical Center Vice Chancellor’s Office to KFH.
References
- 1.Arvidsson U, Riedl M, Chakrabarti S, Lee J-H, Nakano AH, Dado RJ, Loh HH, Law P-Y, Wessendorf MW, Elde R. Distribution and targeting of a μ-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barg J, Belcheva MM, Coscia CJ. Evidence for the implication of phosphoinositol signal transduction in μ-opioid inhibition of DNA synthesis. J Neurochem. 1992;59:1145–1152. doi: 10.1111/j.1471-4159.1992.tb08357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barg J, Belcheva MM, Rowinski J, Coscia CJ. kappa-opioid agonist modulation of [3H]thymidine incorporation into DNA: Evidence for the involvement of pertussis toxin-sensitive G protein-coupled phosphoinositide turnover. J Neurochem. 1993;60:1505–1511. doi: 10.1111/j.1471-4159.1993.tb03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barg J, Belcheva MM, Zimlichman R, Levy R, Saya D, Mchale RJ, Johnson FE, Coscia CJ, Vogel Z. Opioids inhibit endothelin-mediated DNA synthesis, phosphoinositide turnover, and Ca2+ mobilization in rat C6 glioma cells. J Neurosci. 1994;14:5858–5864. doi: 10.1523/JNEUROSCI.14-10-05858.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 6.Boersma CJC, Van Leeuwen FW, O’Brien WG, Law GJ, Mason WT, Bicknell RJ. Dynorphin 1-17 delays the vasopressin induced mobilization of intracellular calcium in cultured astrocytes from the rat neural lobe. J Neuroendocrinol. 1993;5:583–590. doi: 10.1111/j.1365-2826.1993.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 7.Cameron RS, Rakic P. Glial Cell Lineage in the Cerebral Cortex: A Review and Synthesis. Glia. 1991;4:124–137. doi: 10.1002/glia.440040204. [DOI] [PubMed] [Google Scholar]
- 8.Charles AC, Dirksen ER, Merrill JE, Sanderson MJ. Mechanisms of intercellular calcium signaling in glial cells studied with dantrolene and thapsigargin. Glia. 1993;7:134–145. doi: 10.1002/glia.440070203. [DOI] [PubMed] [Google Scholar]
- 9.Cheng B, McMahon DG, Mattson MP. Modulation of calcium current, intracellular calcium levels and cell survival by glucose deprivation and growth factors in hippocampal neurons. Brain Res. 1993;607:275–285. doi: 10.1016/0006-8993(93)91517-v. [DOI] [PubMed] [Google Scholar]
- 10.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 11.Crain SM, Shen KF. Opioids can evoke direct receptor-mediated excitatory effects on sensory neurons. Trends Pharmacol Sci. 1990;11:77–81. doi: 10.1016/0165-6147(90)90322-y. [DOI] [PubMed] [Google Scholar]
- 12.Duggan AW, North RA. Electrophysiology of opioids. Pharmacol Rev. 1984;35:219–281. [PubMed] [Google Scholar]
- 13.Eriksson PS, Hansson E, Rönnbäck L. Mu and delta opiate receptors in neuronal and astroglial primary cultures from various regions of the brain-coupling with adenylate cyclase, localisation on the same neurones and association with dopamine (D1) receptor adenylate cyclase. Neuropharmacology. 1991;30:1233–1239. doi: 10.1016/0028-3908(91)90170-g. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson PS, Hansson E, Rönnbäck L. δ and κ opiate receptors in primary astroglial cultures. Part II: Receptor sets in cultures from various brain regions and interactions with β-receptor activated cyclic AMP. Neurochem Res. 1992;17:545–551. doi: 10.1007/BF00968781. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson PS, Nilsson M, Wagberg M, Hansson E, Rönnbäck L. Kappa-opioid receptors on astrocytes stimulate L-type Ca2+ channels. Neurosci. 1993;54:401–407. doi: 10.1016/0306-4522(93)90261-d. [DOI] [PubMed] [Google Scholar]
- 16.Fasolato C, Innocenti B, Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol Sci. 1994;15:77–83. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 17.Finch EA, Turner TJ, Goldin SM. Calcium as a coagonist of inositol 1,4,5-trisphosphate-inducd calcium release. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- 18.Finkbeiner SM. Glial calcium. Glia. 1993;9:83–104. doi: 10.1002/glia.440090202. [DOI] [PubMed] [Google Scholar]
- 19.Gosztonyi G, Schmidt V, Nickel R, Rothschild MA, Camacho S, Siegel G, Zill E, Pauli G, Schneider V. Neuropathologic analysis of postmortal brain samples of HIV-seropositive and -seronegative i.v. drug addicts. Forensic Sci Int. 1993;62:101–105. doi: 10.1016/0379-0738(93)90052-c. [DOI] [PubMed] [Google Scholar]
- 20.Grynkiewicz G, Poenie M, Tsien RY. A new generation of calcium indicators with greatly improved fluorescence properties. J Biol Chem. 1985;2603440:3440–3450. [PubMed] [Google Scholar]
- 21.Gurwell JA, Duncan MJ, Hauser KF. Role of κ opioid receptors in type 1 astrocyte growth. Soc Neurosci Abstr. 1993;19 [Google Scholar]
- 22.Hauser KF, Osborne JG, Stiene-Martin A, Melner MH. Cellular localization of proenkephalin mRNA and enkephalin peptide products in cultured astrocytes. Brain Res. 1990;522:347–353. doi: 10.1016/0006-8993(90)91482-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauser KF, Stiene-Martin A. Characterization of opioid-dependent glial development in dissociated and organotypic cultures of mouse central nervous system: Critical periods and target specificity. Dev Brain Res. 1991;62:245–255. doi: 10.1016/0165-3806(91)90172-f. [DOI] [PubMed] [Google Scholar]
- 24.Hauser KF, Stiene-Martin A. Opiates and the regulation of nervous system development: Evidence from in vitro studies. In: Hammer RP Jr, editor. Neurobiology of Opiates. CRC Press; Boca Raton, Florida: 1993. pp. 23–61. [Google Scholar]
- 25.Jin W, Lee NM, Loh HH, Thayer SA. Opioid-induced inhibition of voltage-gated calcium channels parallels expression of omega-conotoxin-sensitive channel subtype during differentiation of NG108-15 cells. Brain Res. 1993;607:17–22. doi: 10.1016/0006-8993(93)91484-a. [DOI] [PubMed] [Google Scholar]
- 26.Jin W, Lee NM, Loh HH, Thayer SA. Opioids mobilize calcium from inositol 1,4,5-trisphosphate-sensitive stores in NG108-15 cells. J Neurosci. 1994;14:1920–1929. doi: 10.1523/JNEUROSCI.14-04-01920.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson SM, Fleming WW. Mechanisms of Cellular Adaptive Sensitivity Changes: Applications to Opioid Tolerance and Dependence. Pharmacol Rev. 1989;41:435–487. [PubMed] [Google Scholar]
- 28.Kaneko S, Yuasa J, Takahashi H, Satoh M. Functional expression of Ca2+-mobilizing opioid receptors in Xenopus oocytes injected with rat brain mRNA. Mol Brain Res. 1994;22:69–75. doi: 10.1016/0169-328x(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 29.Kater SB, Mattson MP, Cohan C, Connor J. Calcium regulation of the neuronal growth cone. Trends Neurosci. 1988;11:315–321. doi: 10.1016/0166-2236(88)90094-x. [DOI] [PubMed] [Google Scholar]
- 30.Kim WT, Rioult MG, Cornell-Bell AH. Glutamate-induced calcium signaling in astrocytes. Glia. 1994;11:173–184. doi: 10.1002/glia.440110211. [DOI] [PubMed] [Google Scholar]
- 31.Maderspach K, Solomonia R. Glial and neuronal opioid receptors: apparent positive cooperativity observed in intact cultured cells. Brain Res. 1988;441:41–47. doi: 10.1016/0006-8993(88)91381-9. [DOI] [PubMed] [Google Scholar]
- 32.Mangoura D, Sogos V, Pelletiere C, Dawson G. Differential regulation of phospholipase C and D by phorbol esters and the physiological activators carbachol and glutamate in astrocytes from chicken embryo cerebrum and cerebellum. Dev Brain Res. 1995;87:12–21. doi: 10.1016/0165-3806(95)00047-h. [DOI] [PubMed] [Google Scholar]
- 33.Mattson MP. Calcium as sculptor and destroyer of neural circuitry. Exp Gerontol. 1992;27:29–49. doi: 10.1016/0531-5565(92)90027-w. [DOI] [PubMed] [Google Scholar]
- 34.Mattson MP, Barger SW. Roles for calcium signaling in structural plasticity and pathology in the hippocampal system. Hippocampus. 1993;3(Spec No):73–87. [PubMed] [Google Scholar]
- 35.Means AR, Rasmussen CD. Calcium, calmodulin and cell proliferation. Cell Calcium. 1988;9:313–319. doi: 10.1016/0143-4160(88)90012-7. [DOI] [PubMed] [Google Scholar]
- 36.Melner MH, Low KG, Allen RG, Nielson CP, Young SL, Saneto RP. The regulation of proenkephalin expression in a distinct population of glial cells. EMBO J. 1990;9:791–796. doi: 10.1002/j.1460-2075.1990.tb08175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Millan MJ. Kappa-opioid receptors and analgesia. Trends Pharmacol Sci. 1990;11:70–76. doi: 10.1016/0165-6147(90)90321-x. [DOI] [PubMed] [Google Scholar]
- 38.Nicoletti F, Magrì G, Ingrao F, Bruno V, Catania MV, Dell’Albani P, Condorelli DF, Avola R. Excitatory amino acids stimulate inositol phospholipid hydrolysis and reduce proliferation in cultured astrocytes. J Neurochem. 1990;54:771–777. doi: 10.1111/j.1471-4159.1990.tb02317.x. [DOI] [PubMed] [Google Scholar]
- 39.Ohta T, Ito S, Ohga A. Inhibitory action of dantrolene on Ca-induced Ca2+ release from sarcoplasmic reticulum in guinea pig skeletal muscle. Eur J Pharmacol. 1990;178:11–19. doi: 10.1016/0014-2999(90)94788-y. [DOI] [PubMed] [Google Scholar]
- 40.Prather PL, McGinn TM, Claude PA, Liu-Chen LY, Loh HH, Law PY. Properties of a kappa-opioid receptor expressed in CHO cells: Interaction with multiple G-proteins is not specific for any individual Ga subunit and is similar to that of other opioid receptors. Mol Brain Res. 1995;29:336–346. doi: 10.1016/0169-328x(94)00264-f. [DOI] [PubMed] [Google Scholar]
- 41.Reddy GP. Cell cycle: regulatory events in G1-->S transition of mammalian cells. J Cell Biochem. 1994;54:379–386. doi: 10.1002/jcb.240540404. [DOI] [PubMed] [Google Scholar]
- 42.Richter C, Kass GE. Oxidative stress in mitochondria: its relationship to cellular Ca2+ homeostasis, cell death, proliferation, and differentiation. Chem Biol Interact. 1991;77:1–23. doi: 10.1016/0009-2797(91)90002-o. [DOI] [PubMed] [Google Scholar]
- 43.Ruzicka BB, Fox CA, Thompson RC, Akil H, Watson SJ. Opioid receptor mRNA expression in primary cultures of glial cells derived from different rat brain regions. Regul Pept. 1994;54:251–252. [Google Scholar]
- 44.Ruzicka BB, Thompson RC, Watson SJ, Akil H. The regulation of astroglial proenkephalin and opioid receptor mRNA expression by interleukin-1β. Soc Neurosci Abstr. 1995;21:1358. [Google Scholar]
- 45.Schmahl W, Funk R, Miaskowski U, Plendl J. Long-lasting effects of naltrexone, an opioid receptor antagonist, on cell proliferation in developing rat forebrain. Brain Res. 1989;486:297–300. doi: 10.1016/0006-8993(89)90515-5. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz JP, Nishiyama N, Wilson D, Taniwaki T. Receptor-mediated regulation of neuropeptide gene expression in astrocytes. Glia. 1994;11:185–190. doi: 10.1002/glia.440110212. [DOI] [PubMed] [Google Scholar]
- 47.Shao Y, McCarthy KD. Regulation of astroglial responsiveness to neuroligands in primary culture. Neuroscience. 1993;55:991–1001. doi: 10.1016/0306-4522(93)90313-5. [DOI] [PubMed] [Google Scholar]
- 48.Shao Y, McCarthy KD. Plasticity of astrocytes. Glia. 1994;11:147–155. doi: 10.1002/glia.440110209. [DOI] [PubMed] [Google Scholar]
- 49.Shinoda H, Marini AM, Cosi C, Schwartz JP. Brain region and gene specificity of neuropeptides gene expression in cultured astrocytes. Science. 1989;245:415–417. doi: 10.1126/science.2569236. [DOI] [PubMed] [Google Scholar]
- 50.Shinoda H, Marini AM, Schwartz JP. Developmental expression of the proenkephalin and prosomatostatin genes in cultured cortical and cerebellar astrocytes. Dev Brain Res. 1992;67:205–210. doi: 10.1016/0165-3806(92)90220-q. [DOI] [PubMed] [Google Scholar]
- 51.Slotkin T. Perinatal exposure to methadone: how do early biochemical alterations cause neurofunctional disturbances? Prog Brain Res. 1988;73:265–279. doi: 10.1016/S0079-6123(08)60509-9. [DOI] [PubMed] [Google Scholar]
- 52.Sontheimer H. Voltage-dependent ion channels in glial cells. Glia. 1994;11:156–172. doi: 10.1002/glia.440110210. [DOI] [PubMed] [Google Scholar]
- 53.Spruce BA, Curtis R, Wilkin GP, Glover DM. A neuropeptide precursor in cerebellum: Proenkephalin exists in subpopulations of both neurons and astrocytes. EMBO J. 1990;9:1787–1795. doi: 10.1002/j.1460-2075.1990.tb08303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stiene-Martin A, Elde RP, Hauser KF. Regional and temporal differences in μ, δ, and kappa opioid receptor expression by murine type 1 astrocytes from the cerebral cortex, hippocampus, cerebellum, and striatum in vitro. Soc Neurosci Abstr. 1995;21:1360. [Google Scholar]
- 55.Stiene-Martin A, Gurwell JA, Hauser KF. Morphine alters astrocyte growth in primary cultures of mouse glial cells: Evidence for a direct effect of opiates on neural maturation. Dev Brain Res. 1991;60:1–7. doi: 10.1016/0165-3806(91)90149-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stiene-Martin A, Hauser KF. Opioid-dependent growth of glial cultures: Suppression of astrocyte DNA synthesis by Met-enkephalin. Life Sci. 1990;46:91–98. doi: 10.1016/0024-3205(90)90041-o. [DOI] [PubMed] [Google Scholar]
- 57.Stiene-Martin A, Mattson MP, Hauser KF. Opiates selectively increase intracellular calcium in developing type 1 astrocytes: Role of calcium in morphine-dependent morphologic differentiation. Dev Brain Res. 1993;76:189–196. doi: 10.1016/0165-3806(93)90207-q. [DOI] [PubMed] [Google Scholar]
- 58.Tang T, Kiang JG, Cote T, Cox BM. Antisense oligonucleotide to Gαi2 protein a-subunit sequence inhibits an opioid-induced increase in intracellular free calcium in ND8-47 neuroblastoma x DRG hybrid cells. FASEB J. 1995;9:A97. [PubMed] [Google Scholar]
- 59.Thastrup O, Cullen PJ, Drobek BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci (USA) 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thorlin T, Eriksson PS, Nilsson M, Hansson E, Rönnbäck L. Co-localized opioid and glutamate receptors on astroglial cells - Possible regulators of synaptic transmission. Soc Neurosci Abstr. 1994;20:1731. [Google Scholar]
- 61.Vaysse PJ-J, Zukin RS, Fields KL, Kessler JA. Characterization of opioid receptors in cultured neurons. J Neurochem. 1990;55:624–631. doi: 10.1111/j.1471-4159.1990.tb04179.x. [DOI] [PubMed] [Google Scholar]
- 62.Vogel Z, Nah S-Y, Saya D, Levy R, Attali B, Barg J. Signal transduction of opiate receptors in spinal cord cells. J Toxicol Toxin Rev. 1994;13:115–123. [Google Scholar]
- 63.Wang L, Gintzler AR. Bimodal opioid regulation of cyclic AMP formation: Implications for positive and negative coupling of opiate receptors to adenylyl cyclase. J Neurochem. 1994;63:1726–1730. doi: 10.1046/j.1471-4159.1994.63051726.x. [DOI] [PubMed] [Google Scholar]
- 64.Zagon IS, McLaughlin PJ. Identification of opioid peptides regulating proliferation of neurons and glia in the developing nervous system. Brain Res. 1991;542:318–323. doi: 10.1016/0006-8993(91)91585-o. [DOI] [PubMed] [Google Scholar]