Abstract
Background
This study evaluated the risk factors associated with racial disparities in female breast cancer mortality for African-American and Hispanic women at the census tract level in Texas from 1995 to 2005.
Methods
Data on female breast cancer cases were obtained from the Texas Cancer Registry. Socioeconomic and demographic data were collected from Census 2000. Network distance and driving times to mammography facilities were estimated using Geographic Information System techniques. Demographic, poverty and spatial accessibility factors were constructed using principal component analysis. Logistic regression models were developed to predict the census tracts with significant racial disparities in breast cancer mortality based on racial disparities in late-stage diagnosis and structured factors from the principal component analysis.
Results
Late-stage diagnosis, poverty factors, and demographic factors were found to be significant predictors of a census tract showing significant racial disparities in breast cancer mortality. Census tracts with higher poverty status were more likely to display significant racial disparities in breast cancer mortality for both African Americans (odds ratio [OR], 2.43; 95% confidence interval [CI], 1.95–3.04) and Hispanics (OR, 5.30; 95% CI, 4.26–6.59). Spatial accessibility was not a consistent predictor of racial disparities in breast cancer mortality for African-American and Hispanic women.
Conclusion
Physical access to mammography facilities does not necessarily reflect a greater utilization of mammogram screening, possibly owing to financial constraints. Therefore, a metric measuring access to health care facilities is needed to capture all aspects of access to preventive care. Despite easier physical access to mammography facilities in metropolitan areas, great resources and efforts should also be devoted to these areas where racial disparities in breast cancer mortality are often found.
Introduction and Background
Substantial and widening breast cancer disparities among diverse groups currently exist in the United States (Feuer et al., 1993; Sturgeon, Schairer, Grauman, Ghormli, & Devesa, 2004). In particular, striking disparities with respect to stage of diagnosis and mortality have been observed among different racial and sociodemographic groups (Chu et al., 1996; Smigal et al., 2006). Racial disparities in breast cancer mortality have widened since the 1980s largely owing to a lower decline in mortality within minority groups relative to non-Hispanic Whites (Menashe, Anderson, Jatoi, & Rosenberg, 2009). Thus, identifying the factors responsible for racial disparities in breast cancer mortality is imperative in reducing the gap among ethnic groups. Socioeconomic status (SES) is a critical factor with a paradoxical influence on breast cancer incidence and mortality: Incidence rates are higher within the more affluent and educated groups, although survival rates are higher and mortality rates are lower among these groups (Clarke et al., 2002; Hsu, Glaser, & West, 1997; Singh, 2003).
Financial and physical accessibility to health care is a major concern for health planners and health policymakers in addressing health inequity. Poor access to health services results in diagnoses at more advanced disease stages, which typically lead to more expensive treatments (Haynes & Smedley, 1999). This study adopted Geographic Information System technology to compute network distance and driving time based on road infrastructure, a more accurate and realistic measure of separation between a person’s residence and the location of a health care facility compared with Euclidean distance (McLafferty & Wang, 2009).
Most epidemiologic studies pool together all cases from different geographic regions according to certain characteristics among racial groups. This technique can result in incorrect assessment of racial disparities owing to marked discrepancies in SES, access to screening facilities, and quality of cancer treatment across regions (Coughlin, Thompson, Hall, Logan, & Uhler, 2002; Grann et al., 2006). Few studies have focused on geographical disparities in female breast cancer mortality while taking into account racial groups and relevant risk factors, especially access to mammography facilities by network distance and travel time.
The authors have recently examined how racial disparities in late-stage diagnosis are correlated with racial disparities in mortality for breast cancer (Tian, Wilson, & Zhan, 2011). The present study builds on the baseline established in the previous work both methodologically and etiologically. In particular, it drills down within specific risk factors to determine which one contributes the most to racial disparities in female breast cancer mortality at the census tract level and considers the additional factor of how spatial accessibility to mammography facilities impacts racial disparities. From a methodological and technique perspective, it extends earlier work by taking advantage of Geographic Information System techniques to 1) quantify and map racial disparities in late-stage diagnosis and mortality for breast cancer between African-American/Hispanic women and non-Hispanic Whites at the census tract level in Texas, and 2) measure spatial accessibility to mammography facilities and explore how this factor and others (racial disparities in late-stage diagnosis, demographic factors, and poverty status) impact the existence of significant racial disparities in breast cancer mortality.
Data and Methods
Data
Breast cancer incidence and mortality data from 1995 to 2005 were obtained from the Texas Cancer Registry and the Center of Health Statistics, Texas Department of State Health Services. The Surveillance, Epidemiology, and End Results Summary Stage 1997 was used for the data in 1995 through 1999, whereas the Surveillance, Epidemiology, and End Results Summary Stage 2000 was used to categorize stages of breast cancer cases diagnosed in 2000 through 2003. In this study, regional (coded as 2–5) and distant (coded as 7) cancer cases were defined as late stage. Mortality cases were extracted from death certificates with breast cancer as primary cause of death. For all age groups and races, a total of 44,515 and 26,910 cases were reported for late-stage and breast cancer mortality, respectively. However, 12% of the mortality cases were not successfully mapped because some records had incomplete address information. With respect to breast cancer mortality, the percentage of cases being disqualified was similar across the three racial groups. Differences in age distribution across census tracts in Texas were taken into account by age adjusting the rates of late-stage diagnosis and mortality in non-Hispanic Whites, African Americans, and Hispanics using the 2000 U.S. standard million population.
Demographic and socioeconomic data was retrieved from the 2000 Census Summary File 3. In this study, demographic variables include the percentage of African-American women, percentage of Hispanic women, percentage of minority women, and population density for each census tract. SES indicators contain the measurement of rural/urban residence, educational attainment, unemployment rate, median household income, and poverty level.
The mammography facilities data were obtained from the Texas Mammography Accreditation Program within the Texas Department of State Health Services. There were 605 mammography facilities in operation in 2000 with the Federal Drug Administration’s approval. Only 5% of mammography facilities were added between 1995 and 2000 in Texas; therefore, it is reasonable to estimate the spatial accessibility to mammography facilities using 2000 data. The mammography facilities were geocoded using a JavaScript program to address-match each physical address into latitude and longitude coordinates based on Google Maps Application Programming Interface. Network distance and travel time from census tract centroid to the closest mammography facilities were estimated using the Network Analyst Extension in ArcInfo 9.3 to quantify spatial access to health care. The average network distance and travel time to the five closest mammography facilities, as suggested by Tarlov, Zenk, Campbell, Warnecke, and Block (2009), were selected as the other metrics in measuring spatial accessibility to mammography facilities to represent the choices available to a patient in selecting preferred facilities. Moreover, mammography density was calculated as the number of mammography facilities within a 30-mile buffer from each census tract centroid per 1,000 females for all ages. A radius of 30 miles has been suggested by previous authors as a “reasonable driving distance” (Rahman, Price, Dignan, Lindquist, & Jordan, 2009).
Methods
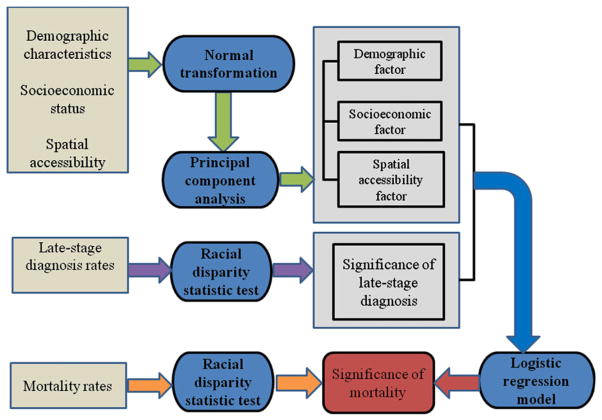
The logical flow of our methodology is shown in Figure 1. Below, we elaborate in detail on each component and the linkages between. Racial disparities were quantified on an absolute scale using age-adjusted rate difference between the target groups (African American/Hispanics) and the reference group (non-Hispanic Whites):
| (1) |
where r̄(ui) is the population-weighted average of age-adjusted rates, n1(ui) and r1(ui) represent the population size and cancer age-adjusted incidence/mortality rates of the reference group in region ui, and n2(ui) and r2(ui) are the same quantities for the target group. A positive RD(ui) indicates that breast cancer incidence/mortality within the target groups is elevated relative to the reference group, whereas a negative RD(ui) suggests the opposite. The null hypothesis of equality of rates between groups was tested to determine which census tracts were experiencing significant racial disparities in breast cancer rates between African-American/Hispanic and non-Hispanic White females. The disparity analysis was performed in STIS 1.8, developed by BioMedware (AvRuskin et al., 2004).
Figure 1.
Flow chart of the methods used in the study.
To address issues of multicolinearity in regression, the original variables were replaced by uncorrelated linear combinations of these variables created using principal component analysis in SPSS 16.0 (Harman, 1976; SPSS, Inc., Chicago, IL). Two variables (percentage of population unemployed, median household income) had skewness and kurtosis values above 3 and were normalized using the procedure described in Goovaerts and Jacquez (2004).
A logistic regression model (Zar, 1974) was constructed using STIS 1.8 to predict the likelihood for a census tract to display significant racial disparities in breast cancer mortality based on the predictors derived from principal component analysis. The probability of a significant racial disparity, pui, was modeled as:
| (2) |
In this function, βj is the coefficient of the variable xj in the regression, β0 is the intercept, and e is the natural logarithm. A log likelihood chi-square test was used to test the overall significance of the logistic model. Wald’s chi-square test was used to test the statistical significance for each predictor individually.
Results
Table 1 lists summary statistics for sociodemographic variables and spatial-accessibility for all 4,388 census tracts in Texas. The average percentages of African-American and Hispanic women were 12% and 31%, respectively, in 2000. On average, 20.32% of the population was living in rural areas in Texas. The average percentage of females with less than a college education is 52.22% and 7.11% of the population were unemployed. The mean population percentage living under the poverty line was 16.22% among all census tracts in Texas. The median household income was $41,184 for all census tracts on average.
Table 1.
Descriptive Statistics of Census Variables for Demographic, Socioeconomic, and Spatial Accessibility Factors for 4,388 Census Tracts in Texas
| Variable | Description | Mean | Standard Deviation | Skewness | Kurtosis | |
|---|---|---|---|---|---|---|
| Percentage of African-American women | Total number of African-American women by total females | 12.00 | 19.00 | 2.58 | 6.71 | |
| Demographic factor | Percentage of Hispanic women | Total number of Hispanic women by total females | 31.00 | 28.00 | 1.04 | −0.14 |
| Percentage of minority women | Total population of African-American and Hispanic women by the total females | 43.00 | 30.00 | 0.45 | −1.16 | |
| Population density | Total population by the area (km2) | 1,079 | 1,219 | 3.17 | 30.01 | |
| Percentage of rural population | Total population living in rural areas by the total population for each census tract | 20.32 | 36.00 | 1.52 | 0.56 | |
| Socioeconomic factor | Percentage of population with less than a college education | Total females with less than a college education by the total female population for each census tract | 52.22 | 20.43 | −0.30 | −0.66 |
| Percentage of population under unemployment | Population under unemployment by the total population in labor | 7.11 | 6.11 | 3.77 | 33.05 | |
| Percentage of population under the poverty line | Population living federal poverty line by the total population for each census tract | 16.22 | 12.13 | 1.25 | 2.04 | |
| Median household income | Median household income in 1999 | 41,184 | 21,489 | 2.16 | 8.15 | |
| Density of mammography facilities | Number of mammography facilities per 1000 females within a 30-mile buffer of each census tract centroid | 69.45 | 1,480.71 | 64.08 | 4,189.24 | |
| Spatial accessibility | Travel distance to the closest facility | Travel distance from centroid of each census tract to the closest facility on transportation network (miles) | 7.29 | 12.22 | 6.54 | 71.09 |
| Average travel distance to the five closest facilities | Average of travel distance from centroid of each census tract to the five closest facilities on transportation network (miles) | 13.18 | 16.15 | 3.68 | 25.00 | |
| Travel time to the closest facility | Travel time from centroid of each census tract to the closest facility on the transportation network (minutes) | 11.97 | 17.47 | 5.63 | 56.84 | |
| Average travel time to the five closest facilities | Average travel time from centroid of each census tract to the five closest facilities on transportation network (minutes) | 19.82 | 22.82 | 3.39 | 21.26 |
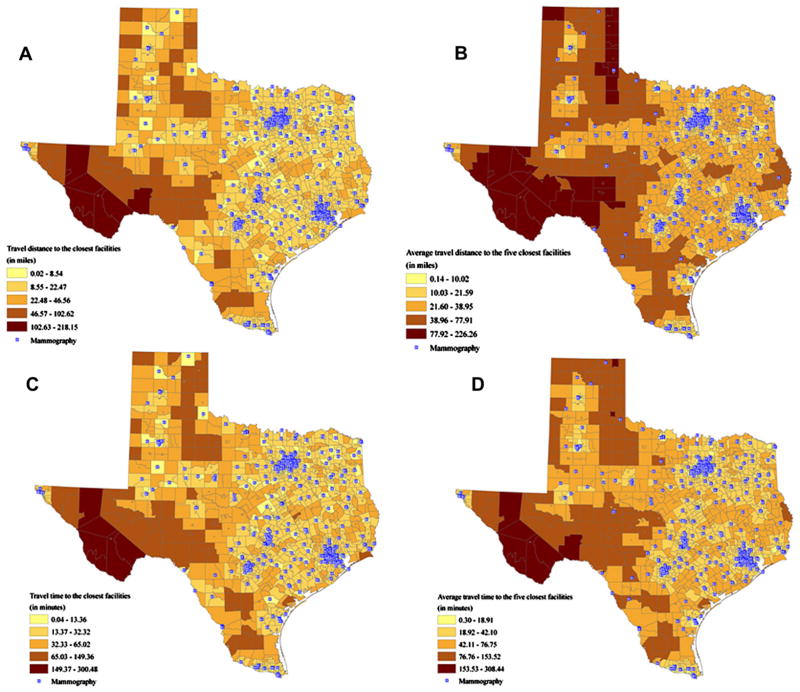
Network travel distance from each tract centroid to the closest mammography facility was approximately 7.29 miles on average, whereas averaged network distance to the five closest mammography facilities had a mean of 13.18 miles (Figure 2A, B). The average travel time from a census tract centroid to the closest mammography facility was 11.97 minutes and when averaged over the five closest facilities, the time was 19.82 minutes (Figure 2C, D). The Southwestern region of Texas required much longer travel times and distances than metropolitan areas, such as Houston, Dallas, Austin, and San Antonio (Figure 2).
Figure 2.
Measures of spatial accessibility to mammography facilities. (A) Travel distance to the closest facility. (B) Average travel distance to the five closest facilities. (C) Travel time to the closest facility. (D) Average travel time to the five closest facilities.
Test of Racial Disparities in Breast Cancer Late-Stage Diagnosis and Mortality
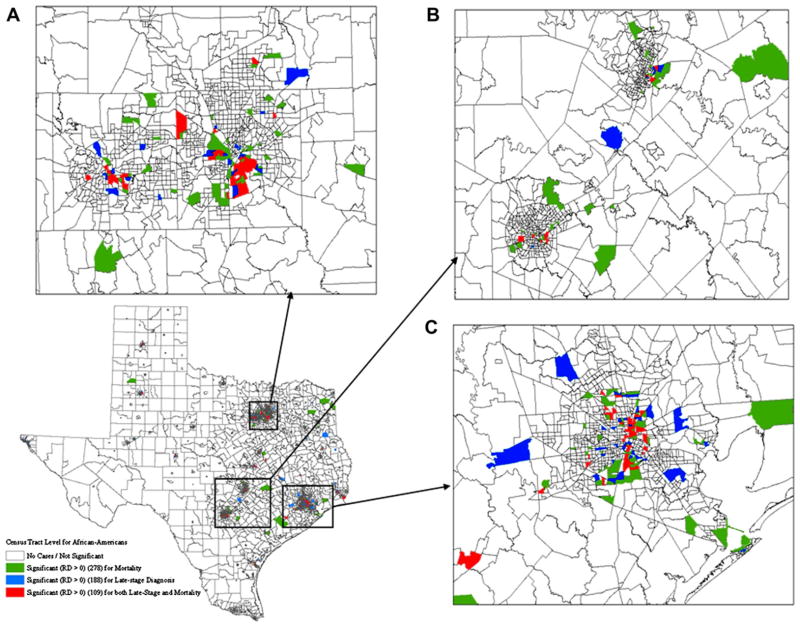
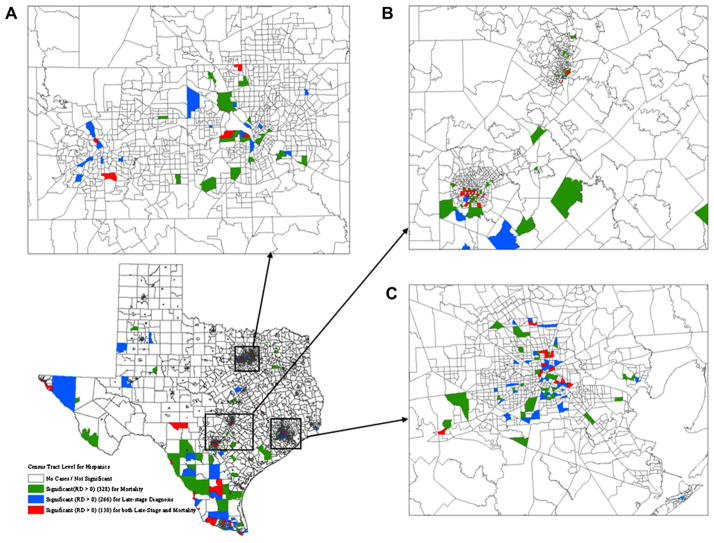
For both African-American and Hispanic females, significant disparities were observed in fewer census tracts for late-stage diagnosis relative to mortality and results for the two types of racial disparities did not overlap spatially (Figures 3 and 4). For African Americans, significant racial disparities in late-stage diagnosis, mortality, or both were observed in 188, 278, and 109 census tracts, respectively (Figure 3). With respect to Hispanic women, 130 census tracts were tested significant for racial disparities in both late-stage diagnosis and breast cancer mortality, which have 266 and 328 census tracts found significant, respectively (Figure 4). Both African-American and Hispanic women had a great number of census tracts experiencing significant racial disparities in the metropolitan areas of Dallas, Austin–San Antonio, and Houston (Figures 3A–C and 4A–C). Beside the metropolitan areas, these tracts with higher mortality rates for Hispanic women were found along the Southwest border of Texas as well owing to the high concentration of the Hispanic population.
Figure 3.
Geographic distributions of census tracts with significant racial disparities in breast cancer late-stage diagnosis and mortality for African-American women. (A) Magnified section of Dallas. (B) Magnified section of Austin-San Antonio. (C) Magnified section of Houston.
Figure 4.
Geographic distributions of census tracts with significant racial disparities in breast cancer late-stage diagnosis and mortality for Hispanic women. (A) Magnified section of Dallas. (B) Magnified section of Austin–San Antonio. (C) Magnified section of Houston.
Factor Analysis
The three principal components with eigenvalues above 1 are listed in Table 2. They explained 75% of the total variance in the dataset. The rotated component matrix in Table 3 showed that the first component mainly represented seven variables: Mammography, population densities, percentage of rural population, travel distance, travel time to the closest facilities, average travel distance, and time to the five closest facilities. Signs of factor loadings indicate that the density of mammography facilities within a 30-mile buffer had an inverse relationship with driving distance and travel time. The first component can be interpreted as a composite measurement of spatial accessibility to mammography facilities.
Table 2.
Percentage of Variance Explained by the Principal Components Before and after Varimax Rotation
| Component | Initial Eigenvalues
|
Rotation Sums of Squared Loadings
|
||||
|---|---|---|---|---|---|---|
| Total | % of Variance | Cumulative % | Total | % of Variance | Cumulative | |
| 1 | 5.281 | 37.723 | 37.723 | 5.136 | 36.686 | 36.686 |
| 2 | 4.106 | 29.329 | 67.052 | 4.190 | 29.926 | 66.612 |
| 3 | 1.147 | 8.190 | 75.242 | 1.208 | 8.630 | 75.242 |
| 4 | 0.874 | 6.240 | 81.482 | |||
| 5 | 0.660 | 4.717 | 86.199 | |||
| 6 | 0.484 | 3.461 | 89.659 | |||
| 7 | 0.395 | 2.822 | 92.482 | |||
| 8 | 0.341 | 2.438 | 94.919 | |||
| 9 | 0.278 | 1.987 | 96.906 | |||
| 10 | 0.196 | 1.402 | 98.307 | |||
| 11 | 0.131 | 0.937 | 99.244 | |||
| 12 | 0.084 | 0.600 | 99.844 | |||
| 13 | 0.018 | 0.126 | 99.970 | |||
| 14 | 0.004 | 0.030 | 100.000 | |||
Table 3.
Factor Loadings Obtained after Varimax Rotation of the First Three Principal Components
| Component
|
|||
|---|---|---|---|
| 1 | 2 | 3 | |
| Percentage of minority | −0.244 | 0.843 | 0.140 |
| Percentage of Hispanic | −0.242 | 0.721 | −0.419 |
| Percentage of population less than a college education | 0.166 | 0.854 | −0.045 |
| Percentage of population living under the poverty line | 0.008 | 0.906 | 0.022 |
| Median household income | −0.101 | −0.857 | −0.062 |
| Percentage of population with unemployment | −0.046 | 0.777 | 0.125 |
| Mammography density | −0.640 | −0.107 | 0.351 |
| Population density | −0.837 | 0.141 | 0.052 |
| Percentage of rural population | 0.745 | −0.105 | −0.046 |
| Travel distance to the closest facilities | 0.881 | −0.010 | 0.020 |
| Average travel distance to the five closest facilities | 0.925 | 0.060 | −0.061 |
| Travel time to the closest facilities | 0.882 | −0.036 | 0.014 |
| Average travel time to the five closest facilities | 0.933 | 0.023 | −0.064 |
| Percentage of African American | −0.180 | 0.152 | 0.924 |
The poverty factor contained six variables with high loadings: Percentage of minority, percentage of Hispanics, percentage of population with less than a college education, percentage of population living under poverty line, median household income, and percentage of population that was unemployed. Median household income was negatively correlated with that factor (−0.857), whereas all other variables had positive correlations, including unemployment rate and poverty level. The third factor consisted of a single variable: Percentage of African Americans with a high correlation coefficient of 0.924. Among all other variables, the percentage of Hispanics had the second highest factor loading, yet much lower (−0.419).
Logistic Regression Analysis for African-American Women
Table 4 summarizes the logistic regression results for African-American women with the estimated parameters, significance level of each individual independent variable, odds ratio (OR) and 95% confidence interval (CI). All the independent variables were highly significant in the logistic model with p-values of less than .01. The regression model with adjusted R2 of 0.47 indicated that a census tract with significant racial disparities in late-stage diagnosis was 4.08 (95% CI, 2.61–6.39) times more likely to display significant racial disparities in mortality. Spatial accessibility factor had an OR of 0.62 (95% CI, 0.50–0.76), which indicates that a census tract with shorter driving distance and time to mammography facilities was more likely to display significant racial disparities. This result can be explained by the fact that most census tracts with significant racial disparities were found in metropolitan areas, where cluster of mammography facilities are often located, leading to greater ease and convenience of mammogram examination. Poverty factor, with a significant OR of 2.43 (95% CI, 1.95–3.04), suggested that census tracts with lower SES were more likely to experience significant racial disparities in breast cancer mortality for African-American women relative to their non-Hispanic White counterparts. Percentage of African Americans (a demographic factor) also played an important role in determining the possibility that a census tract tested significant for racial disparities in breast cancer mortality (OR, 3.45; 95% CI, 2.89–4.13).
Table 4.
Results of Logistic Regression Models for African-American and Hispanic women*
| Independent Variables | Parameter Estimate | Standard Error | p-Value | Odds Ratio (95% CI) |
|---|---|---|---|---|
| African-American women | ||||
| Significance of racial disparities in late-stage diagnosis | 1.41 | 0.13 | <.01 | 4.08 (2.61–6.39) |
| Spatial accessibility | −0.48 | 0.23 | <.01 | 0.62 (0.50–0.76) |
| Poverty factor | 0.89 | 0.11 | <.01 | 2.43 (1.95–3.04) |
| Demographic factor | 1.24 | 0.11 | <.01 | 3.45 (2.89–4.13) |
| Hispanic women | ||||
| Significance of racial disparities in late-stage diagnosis | 1.49 | 0.20 | <.01 | 4.42 (3.01–6.51) |
| Spatial accessibility | −0.08 | 0.07 | .28 | 0.92 (0.80–1.07) |
| Poverty factor | 1.67 | 0.11 | <.01 | 5.30 (4.26–6.59) |
| Demographic factor | −0.31 | 0.07 | <.01 | 0.73 (0.63–0.84) |
The presence of significant racial disparities in breast cancer mortality was the dependent variable.
Logistic Regression Analysis for Hispanic Women
The logistic regression model had adjusted R2 of 0.46 for Hispanic women, which means that about 46% of the variance in the presence of significant racial disparities in breast cancer mortality could be partly explained by the set of predictors. The logistic regression results are summarized in Table 4, with significance level and odds ratio for each predictor. The regression model for Hispanic women indicated that a census tract with significant racial disparities in late-stage diagnosis was 4.42 (95% CI, 3.01–6.51) times more likely to be associated with significant racial disparities for mortality. Unlike African-American women, spatial accessibility to mammography facilities was not a significant predictor in the logistic model with a p-value of .28.
Compared with African-American women, Hispanic women had much higher odds ratio for poverty factor. For Hispanic women, a census tract with higher poverty had 5.3 (95% CI, 4.26–6.59) times greater likelihood for having significant racial disparities of breast cancer mortality. Interestingly, the percentage of African Americans as the demographic factor was a significant predictor in the logistic regression model for Hispanic women. Its odds ratio was 0.73 (95% CI, 0.63–0.84). This indicated that a census tract with a smaller percentage of African Americans had higher likelihood to display significantly higher breast cancer mortality for Hispanic females compared with non-Hispanic Whites. This result could be attributable to the higher concentrations of Hispanic populations in these tracts because the percentage of African Americans was negatively associated with the percentage of Hispanics.
Discussion
In this study, we assessed the association between significant racial disparities in breast cancer mortality and a set of primary predictors, including the significance of racial disparities in late-stage diagnosis, demographic, poverty, and spatial accessibility factors. Census tracts with significant racial disparities in breast cancer mortality were found within the metropolitan areas of Houston, Dallas, and Austin–San Antonio. For Hispanic women, the Southwestern border of Texas also revealed significant racial disparities in breast cancer mortality. There are five key points that should be noted below.
First, our findings are consistent with previous studies that showed a strong connection between racial disparities in breast cancer mortality and late-stage diagnosis (Lannin et al., 1998; Li, Malone, & Daling, 2003). If a census tract exhibited significant racial disparities in late-stage diagnosis, this tract was more likely to display significant racial disparities in breast cancer mortality as well. Minority groups (African Americans and Hispanics) are frequently reported to have a higher likelihood of being diagnosed at a later stage (Boyer-Chammard, Taylor, & Anton-Culver, 1999). In addition, cancer treatment differences could explain the lower survival and higher mortality rates after diagnosis among minority groups (Chevarley & White, 1997; Joslyn & West, 2000). For example, Li and colleagues (2003) concluded that African Americans and Mexican-Americans had a 1.4- to 3.6-fold greater likelihood of being diagnosed with stage IV breast cancer and were less likely to undergo surgical treatment required by the 2000 National Comprehensive Cancer Network Standards.
Hispanic women in counties along the U.S.-Mexico border receive mammography screening less often than their counterparts who live in non-border counties (Coughlin, Uhler, Richards, & Wilson, 2003), because they do not have the adequate access to these screening facilities, as reported in our study. Lack of mammography screening could lead to the excessive late-stage diagnosis and consequently higher mortality rates for Hispanic women in contrast with the reference group of non-Hispanic Whites, especially in the Southwest region of Texas. Factors impacting stage diagnosis and survival rates of breast cancer are different, which could provide some explanation as to why racial disparities in breast cancer late-stage diagnosis and mortality did not fully overlap geographically.
Second, the results of this study agree with the argument that SES plays a significant role in racial disparities of breast cancer (Bradley, Given, & Roberts, 2002; Singh, 2003). This study found that a census tract with high poverty status was about five times more likely to experience significant racial disparities in breast cancer mortality. In geographic regions with lower SES, African-American and Hispanic women, who had less spatial access to health care resources, were more vulnerable than their White counterparts. Minority women may not benefit from current medical advancement and intervention programs to the same extent as non-Hispanic Whites because of lack of health insurance and financial support as well as quality of health care (Chu, Tarone, & Brawley, 1999; Fiscella, Franks, Gold, & Clancy, 2000). Consequently, SES has a profound implication on public health through multifaceted pathways ranging from affordability of health insurance, to knowledge of health issues, to perceptions of early detection owing to cultural beliefs, and to nutrition and life-style behaviors (Baldwin, Taplin, Friedman, & Moe, 2004; Goodman, 1999; Lannin et al., 1998).
Next, this study further substantiated the well-known fact that SES was an essential driving force for the deterioration of health outcomes experienced by the minority and disadvantaged groups owing to cost concerns. For instance, even within the same racial groups of Hispanic women, high SES individuals were reported to have higher utilization of mammography facilities (Stein, Fox, & Murata, 1991). Population-based SES measured at the aggregate level can provide information not only on individual health, but also on the contextual effects of community characteristics on individual health. Contextual SES at the ecological level were highly associated with individual SES, which eventually shaped individual health by influencing their behaviors, health care access and community support (Robert, 1998; Wen, Browning, & Cagney, 2003).
Fourth, these results suggest that spatial accessibility to mammography facilities is a significant predictor for African-American women in determining if a census tract has significant racial disparities in breast cancer mortality. In this study, access to mammography facilities was used as a surrogate measure for access to health care for cancer treatment (Wang, McLafferty, Escamilla, & Luo, 2008). As expected, the travel distances and driving times were shorter within urban areas because of the concentration of health care facilities and physicians, leading to more convenient geographical access to health care facilities (Jordan, Roderick, Martin, & Barnett, 2004). Although most African-American women in Texas reside in metropolitan areas providing better physical access to mammography facilities, African Americans tend to be diagnosed at later stages and have lower survival and higher mortality rates. Thus, better physical access to mammography facilities does not necessarily reflect a greater utilization of mammograms, which may be because of financial constraints (Rahman et al., 2009).
This study contributes additional evidence to the literature with regard to the contentious association between geographical obstruction and health care utilization (Arcury et al., 2005; Athas, Adams-Cameron, Hunt, Amir-Fazli, & Key, 2000; Nattinger, Kneusel, Hoffmann, & Gilligan, 2001). For Hispanic women, spatial accessibility was not a significant covariate in the regression model because census tracts with significant racial disparities in breast cancer mortality were largely found within metropolitan areas, which have shorter driving distances and times to mammography facilities, as well as on Southwest border, which conversely took longer driving times and distances as a result of inadequate mammography services provided in these remote areas. Correspondingly, the effect of spatial accessibility on racial disparities in breast cancer mortality was not as evident, perhaps because unlike African-American, Hispanic women reside in both rural and urban areas, which have markedly different patterns of access to mammography facilities (McLafferty & Wang, 2009).
Moreover, previous studies have shown no consensus on the impact of spatial accessibility to mammography facilities. Tarlov and colleagues (2009) concluded that the mean network distance between mammography facilities and patients’ residence in Chicago had no significant association with the stage of diagnosis. However, another study found that longer driving distance to the closest mammography facility was a significant risk factor for predicting advanced stage diagnosis in breast cancer for Hispanic and White women in Los Angeles County (Gumpertz, Pickle, Miller, & Bell, 2006). Goovaerts (2010) reached a similar conclusion for White women in three Michigan counties. These inconsistent results may be partially explained by utilizing different metrics to measure spatial accessibility. Thus, the impact of spatial accessibility to mammography facilities deserves further investigation.
Last, the percentage of the African-American population within each census tract had an opposite influence on the presence of significant racial disparities in breast cancer mortality for African-American and Hispanic women. If a census tract had more African-American women, this census tract was more likely to show significant racial disparities in breast cancer mortality for African Americans and vice versa for Hispanics. A close examination of geographic distributions for these two groups highlighted the fact that African-American and Hispanic women had different preferences for their residence locations in Texas. This result may have implication for strategic intervention programs.
This study has a few limitations that should be noted. Primary care physicians and health care facilities were not included in the spatial accessibility analysis owing to a lack of data availability at the census tract level. The reference population was only available in 2000 Census to estimate late-stage diagnosis and breast cancer mortality rates over the period from 1995 to 2005, which led us to quantify spatial accessibility using only the mammography facilities in operation in 2000. On the other hand, it was reasonable to use the 2000 Census population because it was a midpoint from 1995 to 2005. Another limitation of the study is that breast cancer late-stage and mortality cases may represent different populations owing to the long breast cancer survival. This limitation is, however, mitigated by the fact that we are looking at racial disparities, and hence comparing differences between populations. In addition, the maps of driving distances and times to the mammography facilities reveal the existence of an “edge effect” along the border of Texas with other states and effect with Mexico, because only mammography facilities within the state of Texas were considered. For example, in the northeast corner of Texas along the border with Arkansas, longer average driving distances and travel times could possibly be slightly reduced by considering facilities from nearby states, because women living along the border may have the tendency to seek mammograms in adjacent regions. However, it still takes longer travel distance to screening facilities because there are no major cities along the Southwestern region, except for those cities that border Mexico.
Another limitation of this study was to assume that the population lives in the centroid of each census tract, potentially leading to inaccurate estimates of spatial access, in particular for large census tracts. However, given the lack of detailed data on mammography facility utilization, this study has improved the quantification of spatial accessibility by using a transportation network. Although the road network and number of mammography facilities did not change significantly over the period under study, the estimation of travel distance between mammography facilities and census tract centroids may be subject to measurement errors.
Conclusion
This study explored a range of factors that could control the presence of significant racial disparities in breast cancer mortality at the census tract level in a spatial context. We found that significance of racial disparities in late-stage diagnosis, poverty status, and the percentage of African Americans in a tract are significant predictors of whether a census tract has significant racial disparities in breast cancer mortality for both African-American and Hispanic women. The inconsistent results found between African-American and Hispanic women with respect to spatial accessibility of mammography facilities call for further investigation on how access to preventative care impacts cancer risks. Physical access to mammography facilities does not necessarily reflect the actual utilization of mammogram because it ignores financial constraints. Therefore, a metric measuring access to health care facilities is warranted to capture all aspects of access to preventive care.
This study revealed that urban areas were equipped with adequate screening facilities, but African-American and Hispanic women still had a higher frequency of late-stage diagnosis and breast cancer mortality, which underpins the importance of other known and unknown factors, especially adequate health insurance and financial means for accessing health care. Along the Southwest border region of Texas, racial disparities in breast cancer could be minimized by enhancing geographical access to health clinics while considering the socioeconomic challenges that Hispanic women are confronted with. Despite easier physical access to mammography facilities in metropolitan areas, great resources and efforts should be directed to the metropolitan areas as well, where racial disparities in breast cancer mortality were often found to be most concentrated.
Acknowledgments
This article is based on one chapter of Nancy Tian’s dissertation completed at Texas State University-San Marcos under F. Benjamin Zhan’s supervision. Benjamin Zhan’s work was in part supported by a Chang Jiang Scholars Award and Wuhan University. Pierre Goovaerts’ research was supported in part by grants R44-CA132347-02 and R43-CA135814-01 from the National Cancer Institute (NCI). The authors thank the Texas Department of State Health Services and the Texas Cancer Registry for providing the data used in the research.
References
- Arcury TA, Gesler WM, Preisser JS, Sherman J, Spencer J, Perin J. The effects of geography and spatial behavior on health care utilization among the residents of a rural region. Health Services Research. 2005;40:135–156. doi: 10.1111/j.1475-6773.2005.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athas WF, Adams-Cameron M, Hunt WC, Amir-Fazli A, Key CR. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. JNCI Journal of the National Cancer Institute. 2000;92:269. doi: 10.1093/jnci/92.3.269. [DOI] [PubMed] [Google Scholar]
- AvRuskin GA, Jacquez GM, Meliker JR, Slotnick MJ, Kaufmann AM, Nriagu JO. Visualization and exploratory analysis of epidemiologic data using a novel space time information system. International Journal of Health Geographics. 2004;3:26. doi: 10.1186/1476-072X-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin LM, Taplin SH, Friedman H, Moe R. Access to multi-disciplinary cancer care. Cancer. 2004;100:701–709. doi: 10.1002/cncr.20030. [DOI] [PubMed] [Google Scholar]
- Boyer-Chammard A, Taylor TH, Anton-Culver H. Survival differences in breast cancer among racial/ethnic groups: a population-based study. Cancer Detection and Prevention. 1999;23:463. doi: 10.1046/j.1525-1500.1999.99049.x. [DOI] [PubMed] [Google Scholar]
- Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. JNCI Journal of the National Cancer Institute. 2002;94:490. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- Chevarley F, White E. Recent trends in breast cancer mortality among white and black US women. American Journal of Public Health. 1997;87:775. doi: 10.2105/ajph.87.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu KC, Tarone RE, Brawley OW. Breast cancer trends of black women compared with white women. Archives of Family Medicine. 1999;8:521. doi: 10.1001/archfami.8.6.521. [DOI] [PubMed] [Google Scholar]
- Chu KC, Tarone RE, Kessler LG, Ries LAG, Hankey BF, Miller BA, et al. Recent trends in US breast cancer incidence, survival, and mortality rates. JNCI Cancer Spectrum. 1996;88:1571. doi: 10.1093/jnci/88.21.1571. [DOI] [PubMed] [Google Scholar]
- Clarke CA, Glaser SL, West DW, Ereman RR, Erdmann CA, Barlow JM, et al. Breast cancer incidence and mortality trends in an affluent population: Marin County, California, USA, 1990–1999. Breast Cancer Research. 2002;4:R13. doi: 10.1186/bcr458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin SS, Thompson TD, Hall HI, Logan P, Uhler RJ. Breast and cervical carcinoma screening practices among women in rural and nonrural areas of the United States, 1998–1999. Cancer. 2002;94:2801–2812. doi: 10.1002/cncr.10577. [DOI] [PubMed] [Google Scholar]
- Coughlin SS, Uhler RJ, Richards T, Wilson KM. Breast and cervical cancer screening practices among Hispanic and non-Hispanic women residing near the United States-Mexico border, 1999–2000. Family & Community Health. 2003;26:130. doi: 10.1097/00003727-200304000-00006. [DOI] [PubMed] [Google Scholar]
- Feuer EJ, Wun LM, Boring CC, Flanders WD, Timmel MJ, Tong T. The lifetime risk of developing breast cancer. JNCI Journal of the National Cancer Institute. 1993;85:892. doi: 10.1093/jnci/85.11.892. [DOI] [PubMed] [Google Scholar]
- Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. JAMA. 2000;283:2579. doi: 10.1001/jama.283.19.2579. [DOI] [PubMed] [Google Scholar]
- Goodman E. The role of socioeconomic status gradients in explaining differences in US adolescents’ health. American Journal of Public Health. 1999;89:1522. doi: 10.2105/ajph.89.10.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goovaerts P. Visualizing and testing the impact of place on late-stage breast cancer incidence: A non-parametric geostatistical approach. Health & Place. 2010;16:321–330. doi: 10.1016/j.healthplace.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goovaerts P, Jacquez GM. Accounting for regional background and population size in the detection of spatial clusters and outliers using geo-statistical filtering and spatial neutral models: the case of lung cancer in Long Island, New York. International Journal of Health Geographics. 2004;3:14. doi: 10.1186/1476-072X-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grann V, Troxel AB, Zojwalla N, Hershman D, Glied SA, Jacobson JS. Regional and racial disparities in breast cancer-specific mortality. Social Science & Medicine. 2006;62:337–347. doi: 10.1016/j.socscimed.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Gumpertz ML, Pickle LW, Miller BA, Bell BS. Geographic patterns of advanced breast cancer in Los Angeles: associations with biological and sociodemographic factors (United States) Cancer Causes and Control. 2006;17:325–339. doi: 10.1007/s10552-005-0513-1. [DOI] [PubMed] [Google Scholar]
- Harman HH. Modern factor analysis. Chicago: University of Chicago Press; 1976. [Google Scholar]
- Haynes MA, Smedley BD. The unequal burden of cancer: an assessment of NIH research and programs for ethnic minorities and the medically underserved. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- Hsu JL, Glaser SL, West DW. Racial/ethnic differences in breast cancer survival among San Francisco Bay Area women. Journal of the National Cancer Institute. 1997;89:1311. doi: 10.1093/jnci/89.17.1311. [DOI] [PubMed] [Google Scholar]
- Jordan H, Roderick P, Martin D, Barnett S. Distance, rurality and the need for care: access to health services in South West England. International Journal of Health Geographics. 2004;3:21. doi: 10.1186/1476-072X-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslyn SA, West MM. Racial differences in breast carcinoma survival. Cancer. 2000;88:114–123. doi: 10.1002/(sici)1097-0142(20000101)88:1<114::aid-cncr16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, Edwards MS. Inflence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA. 1998;279:1801. doi: 10.1001/jama.279.22.1801. [DOI] [PubMed] [Google Scholar]
- Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Archives of Internal Medicine. 2003;163:49. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- McLafferty S, Wang F. Rural reversal? Cancer. 2009;115:2755–2764. doi: 10.1002/cncr.24306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menashe I, Anderson WF, Jatoi I, Rosenberg PS. Underlying causes of the black-white racial disparity in breast cancer mortality: a population-based analysis. JNCI Journal of the National Cancer Institute. 2009;101:993. doi: 10.1093/jnci/djp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattinger AB, Kneusel RT, Hoffmann RG, Gilligan MA. Relationship of distance from a radiotherapy facility and initial breast cancer treatment. Journal of the National Cancer Institute. 2001;93:1344. doi: 10.1093/jnci/93.17.1344. [DOI] [PubMed] [Google Scholar]
- Rahman S, Price JH, Dignan M, Lindquist PS, Jordan TR. Access to mammography facilities and detection of breast cancer by screening mammography: AGIS approach. International Journal of Cancer Prevention. 2009;2:403. [PMC free article] [PubMed] [Google Scholar]
- Robert SA. Community-level socioeconomic status effects on adult health. Journal of Health and Social Behavior. 1998;39:18–37. [PubMed] [Google Scholar]
- Singh GK. Area socioeconomic variations in US cancer incidence, mortality, stage, treatment, and survival, 1975–1999. Washington; DC: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2003. [Google Scholar]
- Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL, et al. Trends in breast cancer by race and ethnicity. CA: A Cancer Journal for Clinicians. 2006;56:168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- Stein JA, Fox SA, Murata PJ. The influence of ethnicity, socioeconomic status, and psychological barriers on use of mammography. Journal of Health and Social Behavior. 1991;32:101–113. [PubMed] [Google Scholar]
- Sturgeon SR, Schairer C, Grauman D, Ghormli LE, Devesa S. Trends in breast cancer mortality rates by region of the United States, 1950–1999. Cancer Causes and Control. 2004;15:987–995. doi: 10.1007/s10552-004-1092-2. [DOI] [PubMed] [Google Scholar]
- Tarlov E, Zenk SN, Campbell RT, Warnecke RB, Block R. Characteristics of mammography facility locations and stage of breast cancer at diagnosis in Chicago. Journal of Urban Health. 2009;86:196–213. doi: 10.1007/s11524-008-9320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N, Wilson JG, Zhan FB. Spatial association of racial/ethnic disparities between late-stage diagnosis and mortality for female breast cancer: where to intervene? International Journal of Health Geographics. 2011;10:24. doi: 10.1186/1476-072X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, McLafferty S, Escamilla V, Luo L. Late-stage breast cancer diagnosis and health care access in Illinois*. The Professional Geographer. 2008;60:54–69. doi: 10.1080/00330120701724087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen M, Browning CR, Cagney KA. Poverty, affluence, and income inequality: neighborhood economic structure and its implications for health. Social Science & Medicine. 2003;57:843–860. doi: 10.1016/s0277-9536(02)00457-4. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. Vol. 620. Englewood Cliffs, NJ: Prentice-Hall; 1974. [Google Scholar]