Abstract
Metastatic EGFR-mutant lung cancers are sensitive to the first- and second- generation EGFR tyrosine kinase inhibitors (TKIs), gefitinib, erlotinib, and afatinib, but resistance develops. Acquired resistance (AR) to gefitinib or erlotinib occurs most commonly (>50%) via the emergence of a second-site EGFR mutation, T790M. Two strategies to overcome T790M-mediated resistance are dual inhibition of EGFR with afatinib plus the anti-EGFR antibody, cetuximab (A+C), or mutant-specific EGFR inhibition with AZD9291. A+C and AZD9291 are now also being tested as first-line therapies, but whether these therapies will extend progression-free survival or induce more aggressive forms of resistance in this setting remains unknown. We modeled resistance to multiple generations of anti-EGFR therapies preclinically in order to understand the effects of sequential treatment with anti-EGFR agents on drug resistance and determine the optimal order of treatment. Using a panel of erlotinib/afatinib-resistant cells including a novel patient-derived cell line (VP-2), we found that AZD9291 was more potent than A+C at inhibiting cell growth and EGFR signaling in this setting. 4 of 4 xenograft-derived A+C-resistant cell lines displayed in vitro and in vivo sensitivity to AZD9291, but 4 of 4 AZD9291-resistant cell lines demonstrated cross-resistance to A+C. Addition of cetuximab to AZD9291 did not confer additive benefit in any preclinical disease setting. This work, emphasizing a mechanistic understanding of the effects of therapies on tumor evolution, provides a framework for future clinical trials testing different treatment sequences. This paradigm is applicable to other tumor types in which multiple generations of inhibitors are now available.
Keywords: Lung cancer, EGFR, AZD9291, afatinib, cetuximab
Introduction
Activating mutations in the tyrosine kinase domain of the EGF receptor (EGFR) are found in 10-35% of lung adenocarcinomas, the predominant subtype of non-small cell lung cancer (NSCLC) (1-3). Such mutations, which occur most commonly either as small in-frame deletions in exon 19 (19del) or point mutations in exon 21 (L858R), confer sensitivity to the first- and second-generation EGFR tyrosine kinase inhibitors (TKIs) gefitinib, erlotinib, and afatinib (1-9).
While multiple lines of anti-EGFR therapies have been developed to treat EGFR-mutant tumors, acquired resistance (AR) to these regimens remains a major clinical obstacle. Metastatic EGFR-mutant lung cancers treated with erlotinib or gefitinib in the first-line setting develop into resistant tumors within 9-16 months (10). In over 50% of cases, acquired resistance to erlotinib or gefitinib involves emergence of a second-site EGFR mutation substituting threonine for methionine at position 790 in exon 20 (T790M) (11, 12). Other rarer mechanisms include amplification of the genes encoding the MET and ERBB2 kinases, mutations in BRAF or PIK3CA (10, 13-15), reduced expression of the RAS GTPase neurofibromin (encoded by the gene NF1) (16), and activation of the AXL kinase (17). Histologic changes such as epithelial-tomesenchymal transition (EMT) and development of small-cell lung cancer (SCLC) features have also been detected in a small subset of tumors from patients with AR to first-generation TKIs (17-19).
We previously showed that dual inhibition of EGFR with the second-generation TKI afatinib and the anti-EGFR antibody cetuximab (A+C) induces tumor regression of T790M+ transgenic mouse lung tumors, overcoming a model of primary AR to erlotinib and gefitinib. The addition of cetuximab to afatinib results in simultaneous depletion of phospho- and total EGFR (20). In a subsequent phase Ib clinical trial of A+C, a 29% response rate was observed in patients with AR to gefitinib or erlotinib, regardless of T790M status (21). Thus, a substantial fraction of EGFR-mutant tumors remain dependent on the EGFR signaling axis for survival even after AR to first-generation TKIs. Unfortunately, resistance to A+C has already been observed in patients. For example, activation of mTORC1 signaling may confer resistance to A+C in some tumors (22); however, a complete understanding of the spectrum of resistance mechanisms to A+C is currently lacking.
Third-generation mutant-specific EGFR TKIs, such as AZD9291, CO-1686, and HM61713, have also shown activity in patients with T790M+ AR to gefitinib or erlotinib (23-25). These agents are designed to specifically inhibit mutant EGFR (i.e., 19del-, L858R-, and/or T790M+), sparing the wild-type receptor (26). As these new compounds become widely available for clinical use, patients will be treated with multiple lines of EGFR-targeted therapies with increasing frequency. For example, a patient with EGFR-mutant lung cancer may receive erlotinib or afatinib as first line therapy, with the assumption that when progressive disease develops, the resistant tumor will still be sensitive to other EGFR-targeted therapies such as A+C or AZD9291. If mutant-specific TKIs become available in the first line setting, whether tumor cells with acquired resistance to such inhibitors will be more aggressive or even responsive to subsequent anti-EGFR treatment remains unknown. In summary, the effect of sequential treatment with various anti-EGFR agents on tumor evolution and drug resistance in EGFR-mutant lung cancer remains to be determined. Data demonstrating the optimal sequence of these therapies is needed in order to inform clinical decision-making. Here, we model resistance to anti-EGFR treatments in EGFR mutant lung cancer cell lines in order to further elucidate mechanisms of sensitivity and resistance of tumors to each of the available anti-EGFR therapies. Importantly, previous work from our lab and others has demonstrated that cell line modeling is highly predictive of resistance mechanisms seen in patients, validating use of this preclinical approach (27, 28).
Materials and methods
Cell lines
All cell lines utilized in these studies were authenticated via routine genotyping for known EGFR kinase mutations. Cell lines were kept in continuous culture for no more than 8 weeks and were routinely tested for mycoplasma contamination to ensure accuracy of experimental data. Cells were cultured in RPMI + L-glutamine (Corning) and supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals) and 1% penicillin/streptomycin (Corning) and grown in a humidified incubator with 5% CO2 at 37C. EGFR-mutant cell lines PC-9, HCC827, and H1975 have been maintained in the Pao laboratory since 2004. Isogenic resistant cell lines were derived in the lab as described previously and in this manuscript (27). Briefly, parental cells were cultured with increasing concentrations of TKI starting at the IC30. All resistant cells were maintained in drug, and TKI was refreshed every 72 hours.
Derivation of VP-2 cell line
VP-2 cells were derived from a 70 year-old male never smoker patient who first received 2 cycles of carboplatin/paclitaxel/bevacizumab chemotherapy before starting on erlotinib. He experienced a partial response on erlotinib, with acquired resistance occurring after 18 months. He then received one month of afatinib, with no response, at which time he developed a large pleural effusion which was tapped. Pleural fluid was obtained with informed consent on an IRB-approved protocol (THO-0547). After pelleting the cells and washing 3x in sterile PBS, red blood cells were lysed in ACK buffer (Lonza INC, Allendale, NJ). After lysis, the remaining cell pellet was washed 3x in sterile PBS. The remaining mixture of cells was then distributed into 10cm dishes. Cells were cultured in RPMI supplemented with 10% heat inactivated fetal bovine serum and penicillin-streptomycin as described above. The medium was changed every 1-3 days for approximately 3 months. To verify that the established cell line (named VP-2) harbored EGFR mutations, we performed direct sequencing.
Immunoblotting
Resistant cells were removed from drug selection 72 hours before immunoblotting experiments. Cells were washed on ice with cold PBS and lysed in radioimmunoprecipitation (RIPA) buffer (250mM Tris-HCl pH 7.5, 75mM NaCl, 1% NP-40/IGEPAL, 0.1% sodium dodecyl sulfate) supplemented with complete protease inhibitor cocktail (Roche), 40mM sodium fluoride, 1mM sodium orthovanadate, and 1uM okadaic acid. Lysates were subjected to SDS-PAGE (4-12% gels) followed by immunoblotting with the indicated antibodies and detection by Western Lightning ECL reagent (Perkin-Elmer). The following antibodies were obtained from Cell Signaling Technologies: phospho-ERK (T202/Y204; 1:1000; # 9101), ERK (1:1000, #9102), phospho-AKT (S473; 1:500; #9271), AKT (1:1000; #9272), phospho-S6 (S240/244; 1:1000; #2215), S6 (1:1000; #2217), BIM (1:1000; #2819), HRP-conjugated anti-mouse (1:3000; #7076), and HRP-conjugated anti-rabbit (1:3000; #7074). Phospho-EGFR antibody was obtained from Abcam (Y1068; 1:1000; #EP774Y), EGFR from BD Transduction Laboratories (1:500; #610017), and actin from Sigma-Aldrich (1:3000; #A2066). Phospho-RTK arrays were purchased from R&D Systems (#ARY001B), and assays were run on cells maintained in 10% FBS using the manufacturer’s protocol.
Xenograft studies
Nude mice (nu/nu; Harlan Laboraties) used for in vivo studies were cared for in accordance with guidelines approved by the Memorial Sloan-Kettering Cancer Center (MSKCC) Institutional Animal Care and Use Committee and Research Animal Resource Center (New York, NY). Eight-week-old female mice were injected s.c. with 10 million PC-9/BRc1, PC-9/BRc1/V7, PC-9/BRc1/A+CR6, and PC-9/BRc1/A+CR7 cells. When tumors reached approximately 150mm3, animals were randomized to receive vehicle, the combination of afatinib [25 mg/kg p.o. qd] and cetuximab [50mg/kg i.p. twice per week], or AZD9291 [5mg/kg or 10mg/kg p.o qd], as indicated. Mice were observed daily for signs of morbidity/mortality. Tumors were measured twice weekly using calipers, and volume was calculated using the formula: length x width2 x 0.52. Body weight was also assessed twice weekly. Tumor samples were collected within 8 hours of the last treatment. Portions of each extracted tumor were preserved in 4% paraformaldehyde, flash-frozen in liquid nitrogen, or minced and placed fresh into culture medium for derivation of cell lines.
Soft agar assays
Colony formation of PC-9, PC-9/AZR, PC-9/ERc1, and PC-9/ERc1/AZR cells was assessed using the CytoSelect 96-Well In Vitro Tumor Sensitivity Assay (Soft Agar Colony Formation) kit purchased from Cell BioLabs, Inc. (# CBA-150), according to the manufacturer’s protocol. Briefly 50uL of base agar matrix was dispensed into each well of a 96-well tissue culture plate. 5,000 cells in 75uL of cell suspension agar matrix were dispensed into each well, and 50uL of culture medium was added to each well, containing various drugs as indicated. Fresh medium with drugs was added every 72 hours. After 10 days of incubation, the matrix was solubilized, and MTT reagent was added to each well. The absorbance was measured on a SpectraMax fluorometer at 570nM.
Histology
Xenograft tumors were fixed in 4% paraformaldehyde (PFA) overnight at room temperature, placed in 70% ethanol and sent to Histoserv, Inc. for paraffin embedding and sectioning. 5μm sections were used for hematoxylin and eosin (H&E) staining.
Growth inhibition assays
Short-term (72h) cellular growth inhibition was measured with CellTiter Blue Reagent (Promega, #G8081) according to the manufacturer’s instructions using cells plated in hextuplicate at a density of 3,000 cells per well. Fluorescence was measured on a SpectraMax fluorometer, and growth inhibition was calculated as percentage of vehicle-treated wells. For longer-term cellular growth inhibition assays, 3,000 cells/well were plated in 24-well plates and treated with indicated drug combinations. Media and inhibitors were refreshed every 72 hours, and cells were grown for 10 days or until confluence in untreated wells. Cells were fixed and stained in 20% methanol with .025% crystal violet and washed with water. Dried plates were imaged and staining intensity quantified on the LI-COR Odyssey.
FISH Analysis
Cells were grown in RPMI 1640 with 10% FBS to ~70% confluence, then harvested and fixed in crayon fixative (methanol:acetic acid = 3:1) for FISH analysis. FISH analysis was performed using the EGFR/CEP7 dual-color probe set from Abbott Molecular and following the protocol from Vysis/Abbott Molecular with a few modifications. In brief, the probe targeting EGFR gene was labeled with SpectrumOrange (red), and chromosome 7 centromere probe (CEP7) was labeled with SpectrumGreen (green); nuclei were counterstained with DAPI (blue). FISH signal scoring and capture were performed by Fluorescence microscope (Zeiss) coupled with ISIS FISH Imaging System (Metasystems). Two hundred interphase cells showing optimum hybridization signals were scored.
EGFR cDNA sequencing
Total RNA was extracted from TKI-sensitive and resistant cell lines using the RNeasy mini kit (Qiagen, Germantown, MD). EGFR cDNA was generated via SuperScript III one-step RT-PCR system with platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA). Gene-specific primers (GSPs), EGFR-cDNA-F (5′-CCCCTGACTCCGTCCAGTAT -3′) and EGFR-cDNA-R (5′-TGGCTAGTCGGTGTAAACGT-3′) were used in the system to amplify the entire EGFR cDNA. To sequence through the whole fragment, several other primers were also designed for dideoxynucleotide sequencing, EGFR-cDNA-w1-F (5′- GCAGTGACTTTCTCAGCAACA -3′), EGFR-cDNA-w2-F (5′- GAAATCATACGCGGCAGGAC -3′), EGFR-cDNA-w3-F (5′-TGGAGCCTCTTACACCCAGT -3′), and EGFR-cDNA-w4-F (5′-ATAGTCGCCCAAAGTTCCGT -3′).
Amplicon-based targeted next-generation sequencing
Using Illumina’s online Design Studio, we generated amplicon probes for use with the Illumina MiSeq platform against all exons of selected genes allowing an extension of 25 bases into the introns on either side of each exon. Amplicon probes were designed not to avoid common single-nucleotide polymorphic (SNP) regions, because some somatic SNPs are often included in genomic SNP databases (for example, EGFR c.2369C>T, p.T790M in lung cancers resistant to first-line EGFR therapy is deposited in the dbSNP database, SNP ID: rs121434569) but are still important for biological function.
Next-generation sequencing analysis
Raw paired-end sequencing reads in FASTQ files were evaluated for quality using the FastQC tool v1.10.1 (http://www.bioinformatics.bbsrc.ac.uk/projects/fastqc/) and aligned to the human reference genome (UCSC hg19) using BWA-MEM algorithm v0.7.8 (29) with default parameters. Duplicated reads were removed using Picard MarkDuplicates tool v1.88 (http://picard.sourceforge.net). Next, to improve SNP and indel detection, the aligned reads were improved using the local realignment and base quality score recalibration procedure following the GATK (v2.5-2) Best Practices recommendations (30, 31). The analysis-ready reads from a pair of drug-resistant versus sensitive cell lines were then used to call mutations unique to resistant cell lines using MuTect v1.1.4 (32) with default parameters. All SNVs passed the default filters of MuTect and SNVs flagged for clustered read position filter were included in our final list of MuTect calls. A variant was preserved if <2 reads supported the variant allele in the normal sample, its average base quality was > 30, it was not a strand bias artifact, and its log-odd score calculated by MuTect was > 6.3. We removed known SNPs included in dbSNP build 137 (33). Variant annotation was performed using ANNOVAR tool (v2013-5-9) (34).
Statistical analysis
Quantification of crystal violet assays, soft agar assays, and xenograft data are presented as means ± SD. The Wilcoxon rank sum test was used for (pairwise) group comparisons. All p values are nominal, without adjusting for the study-wise type I error rate. All analyses are conducted using R software version 3.0. unless indicated specifically.
Results
AZD9291 versus A+C in T790M+ cell lines
Clinical data demonstrate efficacy of both AZD9291 and A+C in the T790M+ second-line setting (i.e., AR to erlotinib/gefitinib/afatinib), but the relative potency of these therapies is unknown. Here, we utilized a panel of T790M+ cell lines (Table 1, Fig S1) to compare directly the growth-inhibitory and signaling effects of A+C versus AZD9291 in T790M+ disease. In prior studies, we had derived multiple erlotinib- and afatinib-resistant EGFR mutant cell lines by well-established in vitro dose-escalation protocols (27). Here, we also present data from a novel T790M+ cell line (VP-2) derived directly ex vivo from the pleural fluid of a patient with EGFR-mutant (19del) lung adenocarcinoma and AR to erlotinib (Fig. S2A, Materials and methods). VP-2 cells demonstrated amplification of the EGFR locus by fluorescence in-situ hybridization (FISH), were resistant to growth inhibition by erlotinib and afatinib, and retained sensitivity to AZD9291 (Fig. S2B-D). Consistent with sensitivity to AZD9291, VP-2 cells did not display amplification of MET by array comparative genomic hybridization (aCGH).
Table 1. Drug-sensitive and -resistant cell lines used in this study.
TKI-naïve: PC-9 and HCC827 cells are parental EGFR-mutant cell lines sensitive to all EGFR TKIs. First-line (primary) AR: VP-2 is a novel cell line derived from a pleural effusion of a patient harboring a lung adenocarcinoma with T790M+ acquired resistance to erlotinib and afatinib (see materials and methods for details). PC-9/ERc1 and PC-9/BRc1 are T790M+ clonal cell lines derived from PC-9 cells with in vitro acquired resistance to erlotinib and afatinib (BIBW2992), respectively. HCC827/R1 is a T790M+, erlotinib-resistant cell line derived from HCC827 cells. Parental H1975 cells harbor both the EGFR TKI-sensitizing L858R mutation and the T790M resistance mutation and are erlotinib-resistant in vitro. PC-9/BRc1/V4, -5, and -7 cells were derived from vehicle-treated PC-9/BRc1 xenografts and serve as controls for cells with second-line A+C resistance. PC-9/AZR cells are derived from PC-9 cells with in vitro acquired resistance to AZD9291. Second-line (secondary) AR: PC-9/ERc1/AZR, HCC827/R1/AZR, and H1975/AZR cells were derived from PC-9/ERc1, HCC827/R1, and H1975 cells, respectively, with in vitro acquired resistance to AZD9291. PC-9/BRc1/A+CR3, -5, -6, -7, -8, and -9 cells were derived from six different PC-9/BRc1 xenografts treated with long-term A+C. 19del, EGFR exon19 deletion; L, EGFR L858R point mutation; T, T790M; AR, acquired resistance.
| Cell lines used in this study | |
|---|---|
| TKI resistance status | Name and EGFR genotype |
| TKI-naïve | |
| Parental EGFR-mutant | PC-9 (19del) |
| HCC827 (19del) | |
| First-line (primary) AR | |
| Erlotinib/afatinib-resistant | VP-2 (19del + T) |
| PC-9/ERc1 (19del + T) | |
| PC-9/BRc1 (19del + T) | |
| HCC827/R1 (19del + T) | |
| PC-9/BRc1/V4 (19del + T) | |
| PC-9/BRc1/V5 (19del + T) | |
| PC-9/BRc1/V7 (19del + T) | |
| H1975 (L+T) | |
| AZD9291-resistant | PC-9/AZR (19del) |
| Second-line (secondary) AR | |
| AZD9291-resistant | PC-9/ERc1/AZR (19del + T) |
| HCC827/R1/AZR* (19del) | |
| H1975/AZR (L+T) | |
| A+C-resistant | PC-9/BRc1/A+CR3 |
| PC-9/BRc1/A+CR5** | |
| PC-9/BRc1/A+CR6 | |
| PC-9/BRc1/A+CR7 | |
| PC-9/BRc1/A+CR8 | |
| PC-9/BRc1/A+CR9** | |
HCC827/R1/AZR cells were derived from polyclonal HCC827/R1 (19del+T790M) cells but lost T790M with acquired resistance to AZD9291
PC-9/BRc1/A+CR5 and -9 xenografts were not used as models of A+C resistance because they were derived from xenografts that were either collected while mice were off drug (PC-9/BRc1/A+CR9) or that re-responded and were not resistant to A+C in vivo at the time of collection (PC-9/BRc1/A+CR5).
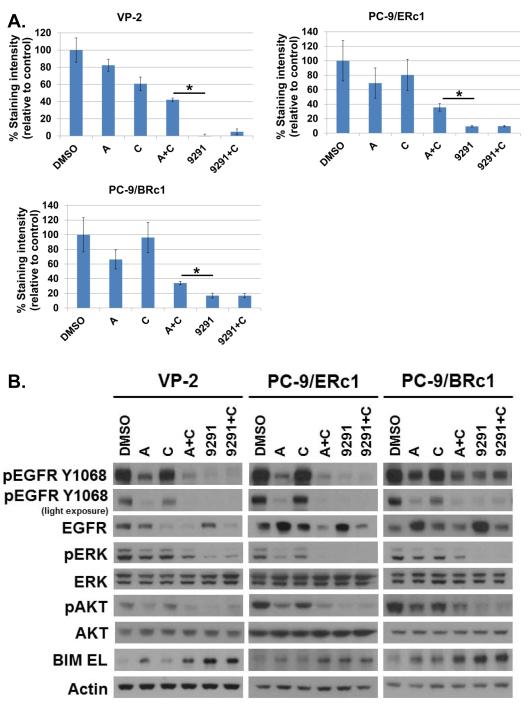
We treated at clinically relevant doses (i.e., at concentrations at or below the Cmax as determined by phase I clinical trial data) T790M+ cell lines VP-2, PC-9/ERc1, PC-9/BRc1, HCC827/R1 (all 19del; T790M), and H1975 (L858R; T790M) with either A+C [50nM; 5ug/mL] or AZD9291 [50nM]. Both A+C and AZD9291 inhibited proliferation of T790M+ cells in long-term (10-day) growth inhibition assays, but AZD9291 induced more growth inhibition than A+C (Fig. 1A, Fig. S2E). Consistent with these findings, analysis of cell lysates showed that both therapies decreased phospho-EGFR and downstream pathways involving phospho-ERK and phospho-AKT at both 6h and 24h, confirming and extending previously published data (Fig. 1B, Fig. S2F) (14, 20, 26). Both A+C and AZD9291 also induced expression of the pro-apoptotic BCL-2 family member BIM, which is required for apoptosis induced by EGFR TKIs in EGFR-mutant cells (Fig. 1B) (35). Collectively, these data confirm the activity of both A+C and AZD9291 in our models of T790M+ acquired resistance to EGFR TKIs and further suggest that AZD9291 may be a more potent inhibitor in this setting.
Figure 1. Afatinib plus cetuximab (A+C) versus AZD9291 in T790M+ cell lines.
A, Quantification of crystal violet staining of VP-2, PC-9/ERc1, and PC-9/BRc1 cells treated in triplicate with DMSO, afatinib (A) [50nM], cetuximab (C) [5ug/mL], afatinib + cetuximab (A+C), AZD9291 (9291) [50nM], or AZD9291 + cetuximab (9291+C) for 10 days. Data are expressed as mean ± standard deviation of triplicate measurements; *p<0.10 for AZD9291 vs. A+C. B, Immunoblotting of cell lysates from VP-2, PC-9/ERc1, and PC-9/BRc1 treated for 24 hours with DMSO, afatinib (A) [50nM], cetuximab (C) [5ug/mL], A+C, AZD9291 (9291) [50nM], or AZD9291 + cetuximab (9291+C) show the effect of each treatment on phospho-EGFR, phospho-ERK, phospho-AKT and BIM EL induction.
It remains unknown whether AZD9291 + cetuximab will have any clinical utility as a combination therapy for EGFR-mutant lung cancer. In order to discern the effects of combining AZD9291 with cetuximab versus treating with AZD9291 alone, we evaluated the signaling and growth inhibitory effects of this combination in the assays described above. While AZD9291 + cetuximab did cause a greater decrease in total EGFR expression, this did not lead to any additive growth-inhibitory or differential downstream signaling effects in our T790M+ cell line models of erlotinib/afatinib resistance, even using various doses of either drug (Fig. 1A-B, Fig. S2G).
Derivation of A+C-resistant cell lines
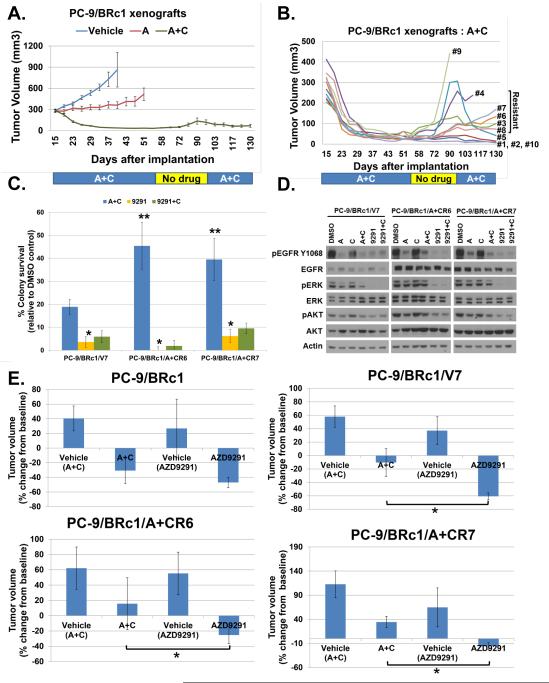
We next sought to test whether AZD9291 could overcome resistance to A+C. Therefore, we derived new A+C-resistant cell lines by subjecting xenografts of PC-9/BRc1 cells (19del; T790M) to long-term treatment with A+C until tumors began to grow in the presence of drug (Fig. 2A-B). Re-derived cell lines from the A+C-treated xenografts showed increased EGFR protein expression (Fig. S3A) and persistent levels of EGFR amplification by fluorescence in situ hybridization (FISH) relative to vehicle-treated controls (Fig. S3B). Phospho-RTK arrays of baseline signaling in two representative A+C-resistant cell lines revealed sustained phospho-EGFR but no increased activation of additional RTKs that would suggest ‘bypass’ signaling as a mechanism of resistance (Fig. S3C). Notably, we also did not see increased phospho-S6 in our A+C-resistant cell lines (Fig. S3A), suggesting that the mechanism of resistance in this model is not due to increased mTOR pathway activity, as previously characterized in separate experiments (22). H&E staining of A+C-resistant tumors did not show features of SCLC morphology, a known mechanism of resistance to first- and second-generation EGFR TKIs (Fig. S3D). A combination of amplicon-based targeted next-generation sequencing (NGS; NCBI SRA Accession ID: SRP049301) and cDNA-based dideoxynucleotide sequencing did not detect any additional mutations in KRAS, PIK3CA, BRAF, or EGFR, ERBB2, ERBB3, or ERBB4. Thus, A+C-resistant lines demonstrated increased EGFR protein without evidence of alternative known resistance mechanisms, suggesting that they might still be dependent on the EGFR signaling axis for survival.
Figure 2. Afatinib plus cetuximab (A+C)-resistant cell lines are sensitive to growth inhibition by AZD9291.
A, To derive A+C-resistant lines, mice bearing PC-9/BRc1 xenografts were treated long-term with either vehicle, afatinib (A) [25 mg/kg p.o], or A+C [25mg/kg p.o.; 50mg/kg i.p.]. Data are expressed as mean ± standard deviation of tumor volumes calculated twice weekly for 10 mice/group. Treatment was withdrawn for days 56-94 to allow tumor re-growth before re-initiation of treatment on day 95. B, Xenograft data of A+C-treated mice from A are plotted as individual tumor curves. Tumors that grew in the presence of drug were deemed resistant (#3, 4, 6, 7 and 8); cell lines were derived from all tumors from which sufficient tissue could be obtained at the point of experiment termination (#3, 5, 6, 7, 8, and 9). C, Quantification of soft agar assays of xenograft-derived A+C-resistant cell lines PC-9/BRc1/A+CR6 and PC-9/BRc1/A+CR7 vs. A+C-sensitive vehicle control cell line PC-9/BRc1/V7 treated for 10 days with either DMSO, A+C [50nM; 5ug/mL], AZD9291 (9291) [50nM], or AZD9291 + cetuximab (9291+C). Data are expressed as mean ± standard deviation of hextuplicate data; *p<0.05 for AZD9291 vs. A+C; **p<0.05 for A+C in PC-9/BRc1/A+CR6 and PC-9/BRc1/A+CR7 vs. PC-9/BRc1/V7. D, Immunoblotting of lysates from PC-9/BRc1/V7, PC-9/BRc1/A+CR6, and PC-9/BRc1/A+CR7 cells treated for 6 hours with DMSO, afatinib (A) [50nM], cetuximab (C) [5ug/mL], A+C, AZD9291 (9291) [50nM], or AZD9291 + cetuximab (9291+C) demonstrate that A+C-resistant cells PC-9/BRc/A+CR6 and PC-9/BRc1/A+CR7 cells are relatively resistant to inhibition of phospho-EGFR, phospho-ERK, and phospho-AKT compared to PC-9/BRc1/V7 cells upon treatment with A+C, but AZD9291 inhibits phospho-EGFR and downstream signaling in both A+C-sensitive and A+C-resistant cell lines. E, Xenograft-derived A+C-resistant PC-9/BRc1/A+CR6 and PC-9/BRc1/A+CR7 cells, xenograft-derived A+C-sensitive PC-9/BRc1/V7 cells, and parental PC-9/BRc1 cells were re-implanted as xenografts in immunodeficient mice and treated for one week with either A+C [25mg/kg p.o.; 50mg/kg i.p.], AZD9291 [10mg/kg p.o.], or respective vehicle controls. Tumor volumes were calculated from twice-weekly caliper measurements, and data are plotted as percentage tumor growth or shrinkage relative to baseline. Data are expressed as mean ± standard deviation of measurements from five mice. *p<0.05 for AZD9291 vs. A+C.
AZD9291 overcomes A+C resistance
Having derived A+C-resistant cell lines, we tested their sensitivity to clinically relevant concentrations of AZD9291 in standard growth inhibition assays. We chose PC-9/BRc1/A+CR6 and PC-9/BRc1/A+CR7 cells for further characterization, because they developed the most resistance to A+C in vivo (Fig. 2B). In soft agar assays, both PC-9/BRc1/A+CR6 and PC-9/BRc1/A+CR7 cells were resistant to growth inhibition by A+C relative to PC-9/BRc1/V7 vehicle control, but colony formation of all cell lines was significantly inhibited in the presence of AZD9291 (Fig. 2C). Consistent with the growth inhibition data, immunoblotting of cell lysates demonstrated less inhibition of phospho-EGFR, phospho- extracellular signal-regulated kinase (phospho-ERK), and phospho-protein kinase B (phospho-PKB/AKT) in A+C-resistant cells than A+C-sensitive PC-9/BRc1/V7 cells upon treatment with A+C; however, AZD9291 diminished EGFR and downstream signaling in both control and A+C-resistant cell lines (Fig. 2D). Thus, AZD9291 appears to overcome resistance to A+C in these cell lines in vitro. As in erlotinib/afatinib resistant cell lines, addition of cetuximab to AZD9291 induced a slightly greater decrease in total EGFR by immunoblotting but no significant change in growth inhibition as compared to AZD9291 alone in A+C-resistant cell lines (Fig. 2C-D).
To confirm our findings in vivo, we re-implanted our original xenograft-derived cell lines subcutaneously into nude mice and measured changes in tumor volume following treatment with AZD9291. Consistent with the in vitro results, parental PC-9/BRc1 and PC-9/BRc1/V7 cells displayed tumor regression following 1 week of treatment with both A+C and AZD9291, but only AZD9291 induced shrinkage of A+C-resistant PC-9/BRc1/A+CR6 and PC-9/BRc1/A+CR7 xenografts (Fig. 2E). Taken together with our in vitro growth inhibition and signaling data, these findings strongly suggest that these preclinical models of A+C resistance maintain reliance on EGFR signaling for survival and that AZD9291 may have clinical efficacy in some settings of A+C resistance.
A+C does not overcome AZD9291 resistance
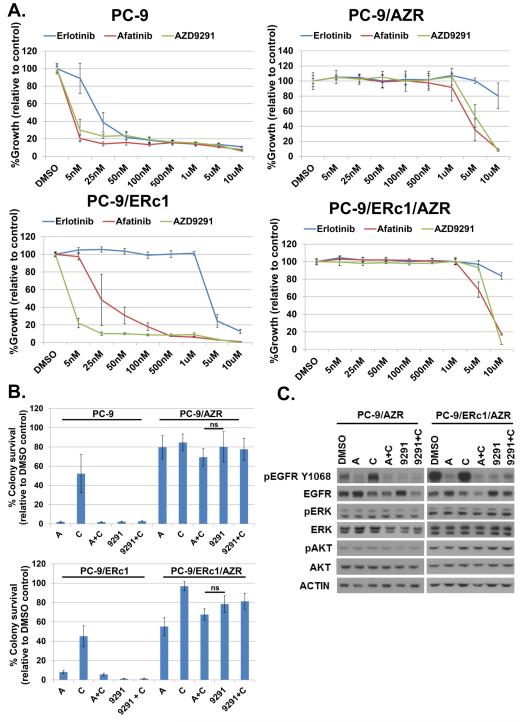
In order to test whether A+C could overcome resistance to AZD9291, we generated four AZD9291-resistant cell lines using well-established TKI dose-escalation protocols (13, 27, 36, 37) (Table 1, Fig. S1). PC-9/AZR cells were derived from TKI-sensitive PC-9 parental cells, mimicking first-line resistance to AZD9291. Three other AZD9291-resistant cell lines (PC-9/ERc1/AZR, HCC827/R1/AZR, H1975/AZR) were derived from T790M+ erlotinib-resistant ‘parental’ cell lines, modeling the clinical setting of second-line resistance resistance to AZD9291. After long-term culture in TKI, AZD9291-resistant PC-9, PC-9/ERc1, HCC827/R1, and H1975 cells acquired the ability to grow in drug concentrations >100 fold (1uM) the initial IC50 (median inhibitory concentration) of the parental cells (Figure 3A and S4A). Resistance was maintained following culture for 16 passages in the absence of AZD9291 (Fig. S4C), suggesting that the phenotype was not reversible as we have observed with erlotinib and afatinib (27). All AZD9291-resistant cells were also cross-resistant to first- and second- generation EGFR TKIs erlotinib and afatinib (Figure 3A and S4A). A combination of amplicon-based targeted NGS (NCBI SRA Accession ID: SRP049329) and cDNA-based dideoxynucleotide sequencing did not detect any acquired mutations in KRAS, PIK3CA, BRAF, or EGFR, ERBB2, ERBB3, or ERBB4 in the setting of AZD9291 resistance; specifically, we did not observe any mutations at Cys797 in EGFR, the site at which AZD9291 makes a covalent bond with EGFR.
Figure 3. AZD9291-resistant cell lines are resistant to growth inhibition by A+C.
A, Cell growth-inhibition assays demonstrate the resistance of PC-9/AZR and PC-9/ERc1/AZR cells to AZD9291, relative to isogenic parental controls PC-9 and PC-9/ERc1, respectively. AZD9291 resistance also confers robust cross-resistance to first- and second-generation EGFR TKIs erlotinib and afatinib. Data are expressed as a percentage of DMSO control and plotted as mean ± standard deviation of hextuplicate data. B, Quantification of soft agar assays of PC-9, PC-9/AZR, PC-9/ERc1, and PC-9/ERc1/AZR cells treated for 10 days with either DMSO, afatinib (A), cetuximab (C), A+C [50nM; 5ug/mL], AZD9291 (9291) [50nM], or AZD9291 + cetuximab (9291+C). Data are expressed as mean ± standard deviation of hextuplicate data; ns= non-significant at p=0.05 for AZD9291 vs. A+C. C, Immunoblotting of lysates from PC-9/AZR and PC-9/ERc1/AZR cells treated for 6 hours with DMSO, afatinib (A) [50nM], cetuximab (C) [5ug/mL], A+C, AZD9291 (9291) [50nM], or AZD9291 + cetuximab (9291+C) demonstrate that AZD9291 can still inhibit phospho-EGFR but not downstream phospho-ERK or phospho-AKT in AZD9291-resistant cells.
In long-term colony formation and growth inhibition assays, all AZD9291-resistant cell lines were resistant to growth inhibition by both AZD9291 and A+C as compared to parental (i.e., AZD9291-sensitive) controls (Fig. 3B and Fig. S4B). Furthermore, immunoblotting of cell lysates from AZD9291-resistant cells treated with AZD9291 for 6 hours showed inhibition of phospho-EGFR but sustained phosphorylation of ERK and AKT (Fig. 3C). The dissociation between phospho-EGFR and phospho-ERK/AKT inhibition in this setting further suggests an EGFR-independent mechanism of resistance to AZD9291. More extensive characterization of these cell lines is ongoing; however, in collaboration with the AstraZeneca project team, we have found that AZD9291-resistant cells often display activation of the MAPK pathway (Eberlein et al, manuscript under review). Consistent with these data, addition of a MEK inhibitor (AZD6244) partially re-sensitized our AZD9291-resistant cells to inhibition of colony formation by AZD9291 (Fig S4D). Addition of cetuximab to AZD9291 diminished total EGFR protein levels in AZD9291-resistant cells but did not increase growth inhibition relative to AZD9291 alone (Fig. 3B-C). Collectively, these studies suggest that resistance to AZD9291 may be mediated by EGFR-independent mechanisms that confer cross-resistance to subsequent anti-EGFR therapies.
Discussion
Patients with metastatic EGFR mutant lung cancer experience dramatic and prolonged benefit from treatment with EGFR TKIs such as gefitinib and erlotinib. Unfortunately, resistance occurs in most cases, with more than half of patients acquiring a second-site T790M mutation. Recently, studies with newer generations of anti-EGFR treatments such as A+C and AZD9291 confirm that some tumors maintain dependence on EGFR signaling even in the setting of disease progression. The optimal sequence of anti-EGFR therapy is now unknown, but preclinical modeling of AR to targeted therapies has proven to be an effective strategy for predicting clinical outcomes. Here, we used existing and new cell line models of resistance to first-, second- and third- generation anti-EGFR therapies in vitro and in vivo in order to determine rationally a treatment sequence that may confer maximal duration of clinical benefit before development of acquired resistance to all available anti-EGFR therapies.
First-line EGFR targeted therapy
Erlotinib, gefitinib, and afatinib are approved for treatment of EGFR-mutant lung tumors in the first-line setting. In addition, preclinical data suggest that AZD9291 could be highly effective in the first-line setting (20, 26, 38), and a trial testing AZD9291 in TKI-naïve patients is underway. However, the question of whether use of AZD9291 in the first-line setting will extend PFS for patients compared to erlotinib, gefitinib, or afatinib alone remains to be determined clinically. Furthermore, it is unclear at this point whether tumors that develop resistance to mutant-specific TKIs in the first-line setting will demonstrate a more aggressive phenotype than erlotinib/gefitinib/afatinib-resistant tumors, or whether they will respond to subsequent anti-EGFR therapies.
Second-line EGFR targeted therapy
Early clinical trial data demonstrate that both A+C and AZD9291 can effectively overcome AR to first- and second-generation EGFR TKIs (21, 23), which we recapitulate in our preclinical models. We further show that while addition of cetuximab to AZD9291 does induce a slightly greater decrease in levels of total EGFR compared to AZD9291 alone, the combination does not provide added growth-inhibitory effect in vitro. This suggests that, at the doses tested, AZD9291 is sufficiently potent as monotherapy to inhibit EGFR T790M signaling below the threshold needed for inhibition of cell growth. However, it is still possible that the combination, through dual targeting of the receptor, could possibly have some increased efficacy in patients and may warrant further investigation clinically in the future.
Third-line EGFR targeted therapy
We show that secondary resistance to A+C may be mediated in some cases by EGFR-dependent mechanisms that can be overcome by AZD9291. Addition of cetuximab to AZD9291 did not appear to confer any added benefit in the setting of A+C resistance. Currently, we do not know how frequently such EGFR dependence will occur. In separate work, we have shown that other models of A+C resistance (derived under different conditions and protocols than the models presented here) are characterized by activation of mTORC1 signaling (22). These data are consistent with the notion that disease progression is due to heterogeneous mechanisms in patients. Ongoing analysis of patients’ tumors with acquired resistance to A+C will shed further light on the proportion of A+C-resistant tumors that retain sensitivity to subsequent anti-EGFR therapies.
In preclinical models of acquired resistance to AZD9291, cells appear to have bypassed EGFR signaling alone for survival. In the presence of drug, they display sustained activation of downstream signaling, despite decreased phospho-EGFR. Consistent with these findings, in contrast to A+C resistance, our models of resistance to AZD9291 displayed cross-resistance to all other anti-EGFR therapies in both the first- and second-line setting. Thus, one potential scenario for cases of T790M+ resistance involves A+C followed by AZD9291 but not vice versa. Confirmation of these findings will require clinical trials testing different treatment sequences.
Previous work by others has implicated increased activation of the mitogen-activated protein kinase 1 (MAPK1)/ERK signaling pathway as a mechanism of acquired resistance to WZ4002, a similar mutant-specific EGFR TKI that never reached clinical development (39). These authors showed that WZ4002 in combination with a MAP-ERK kinase (MEK) inhibitor was sufficient to overcome WZ4002 resistance. Similarly, our ongoing studies of AZD9291 resistance mechanisms implicate MAPK pathway dysregulation (Eberlein et al, manuscript under review), with potential re-sensitization through combined EGFR and MEK inhibition. Clinical testing of such findings will further inform sequential treatment regimens with these therapies.
This study addresses critical and timely questions for patients with EGFR-mutant lung cancer but may also be applicable to oncogene-driven tumor types in general. In vitro preclinical studies in chronic myelogenous leukemia (CML) have shown that the order and concentration of treatment with dasatinib or nilotinib following imatinib resistance has an effect both on the specific resistance mutations that emerge in the target fusion kinase BCR-ABL and on the sensitivity to subsequent targeted therapies (40). Similarly, recent studies indicate that metastatic prostate cancer with acquired resistance to first-generation anti-androgens maintain reliance on androgen receptor signaling for survival (41) and are sensitive to subsequent therapy with second-generation anti-androgens such as enzalutamide. However, enzalutamide resistance can be mediated by ‘bypass’ induction of the glucocorticoid receptor, suggesting that enzalutamide-resistant tumors have escaped dependence on the androgen receptor for survival and that subsequent anti-androgen therapies may be ineffective for such patients (42). Together with our findings in EGFR mutant lung cancer, the studies in CML and prostate cancer highlight the fact that as multiple lines of targeted therapies are utilized with increasing frequency in the clinic, a thorough understanding of the molecular determinants of sensitivity and resistance of tumors to sequential treatment will be critical in order to maximize benefit to patients.
Supplementary Material
Acknowledgements
We would like to thank Dr. Otis Rickman and Charla Walston for their assistance in collection of pleural fluid for establishment of the VP-2 cell line.
Financial support: The authors acknowledge support from the NIH/National Cancer Institute grants WP R01-CA121210 (W. Pao and K. Politi), P01-CA129243 (W. Pao, M. Ladanyi), U54-CA143798 (W. Pao), R00 CA120247 (K. Politi), and R01CA158472 (X. Chen). Additional support was provided by the Joyce Family Foundation (W. Pao), Cornelius Abernathy Craig Chair (W. Pao), the Ingram Professorship Funds (W. Pao), the VICC Cancer Center Core grant (P30-CA68485) (W. Pao), the American Italian Cancer Foundation (V. Pirazzoli), Uniting Against Lung Cancer (K. Politi), and Stand Up to Cancer Innovative Research Grant, a Program of the Entertainment Industry Foundation (SU2C-AACR-IRG0409) (W. Pao). C.B. Meador was supported by Public Health Service Award T32 GM07347 from the National Institute of General Medical Studies for the Vanderbilt Medical-Scientist Training Program and the VICC Melly Family Scholarship. C.M. Lovly was supported by an NIH K12 training grant (K12 CA9060625) and a Damon Runyon Clinical Investigator Award. This work was also supported in part by funding from AstraZeneca.
Footnotes
Conflicts of interest: WP: Consulting: MolecularMD, AstraZeneca, Bristol-Myers Squibb, Symphony Evolution Clovis Oncology, Exelixis, Clarient; Research funding: Enzon, Xcovery, AstraZeneca, Symphogen, Clovis Oncology, Bristol-Myers Squibb; Other: A patent relating to EGFR T790M mutation testing was licensed on behalf of WP, KP and others by MSKCC to MolecularMD. CML has served as a consultant for Pfizer and has been an invited speaker for Abbott and Qiagen. DC is an employee of AstraZeneca.
References
- 1.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 6.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 8.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 9.Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients With Metastatic Lung Adenocarcinoma With EGFR Mutations. J Clin Oncol. 2013;31:3327–34. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 10.Ohashi K, Maruvka YE, Michor F, Pao W. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol. 2013;31:1070–80. doi: 10.1200/JCO.2012.43.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 13.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 14.Takezawa K, Pirazzoli V, Arcila ME, Nebhan CA, Song X, de Stanchina E, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov. 2012;2:922–33. doi: 10.1158/2159-8290.CD-12-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–7. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Bruin EC, Cowell C, Warne PH, Jiang M, Saunders RE, Melnick MA, et al. Reduced NF1 Expression Confers Resistance to EGFR Inhibition in Lung Cancer. Cancer Discov. 2014;4:606–19. doi: 10.1158/2159-8290.CD-13-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44:852–60. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zakowski MF, Ladanyi M, Kris MG, Memorial Sloan-Kettering Cancer Center Lung Cancer OncoGenome G EGFR mutations in small-cell lung cancers in patients who have never smoked. N Engl J Med. 2006;355:213–5. doi: 10.1056/NEJMc053610. [DOI] [PubMed] [Google Scholar]
- 20.Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119:3000–10. doi: 10.1172/JCI38746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janjigian YY, Smit EF, Groen HJ, Horn L, Gettinger S, Camidge DR, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov. 2014;4:1036–45. doi: 10.1158/2159-8290.CD-14-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pirazzoli V, Nebhan C, Song X, Wurtz A, Walther Z, Cai G, et al. Acquired Resistance of EGFR-Mutant Lung Adenocarcinomas to Afatinib plus Cetuximab Is Associated with Activation of mTORC1. Cell Rep. 2014;7:999–1008. doi: 10.1016/j.celrep.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janne PA, Ramalingam SS, Chih-Hsin Yang J, Ahn M-J, Kim D-W, Kim S-W, Planchard D, Ohe Y, Felip E, Watkins C, Cantarini M, Ghiorghiu S, Ranson M. Clinical activity of the mutant-selective EGFR inhibitor AZD9291 in patients (pts) with EGFR inhibitor-resistant non-small cell lung cancer (NSCLC) J Clin Oncol. 2014;32(suppl):5s. abstr 8009^. [Google Scholar]
- 24.Sequist LV, Soria J-C, Gadgeel SM, Wakelee HA, Camidge DR, Varga A, Solomon BJ, Papadimitrakopoulou V, Jaw-Tsai SS, Caunt L, Kaur P, Rolfe L, Allen AR, Goldman JW. First-in-human evaluation of CO-1686, an irreversible, highly selective tyrosine kinase inhibitor of mutations in EGFR (activating and T790M) J Clin Oncol. 2014;32(suppl):5s. abstr 8010^. [Google Scholar]
- 25.Kim D-W, Lee DH, Kang JH, Park K, Han J-Y, Lee J-S, Jang I-J, Kim H-Y, Son J, Kim J-H. Clinical activity and safety of HM61713, an EGFR-mutant selective inhibitor, in advanced non-small cell lung cancer (NSCLC) in patients (pts) with EGFR mutations who had received EGFR tyrosine kinase inhibitors (TKIs) J Clin Oncol. 2014;32(suppl):5s. abstr 8011. [Google Scholar]
- 26.Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–61. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011;3:90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohashi K, Sequist LV, Arcila ME, Moran T, Chmielecki J, Lin YL, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A. 2012;109:E2127–33. doi: 10.1073/pnas.1203530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–95. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, del Angel G, Levy-Moonshine A, et al. Current Protocols in Bioinformatics. John Wiley & Sons, Inc.; 2002. From FastQ Data to High-Confidence Variant Calls: The Genome Analysis Toolkit Best Practices Pipeline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–9. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29:308–11. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong Y, Somwar R, Politi K, Balak M, Chmielecki J, Jiang X, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007;4:e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Godin-Heymann N, Ulkus L, Brannigan BW, McDermott U, Lamb J, Maheswaran S, et al. The T790M “gatekeeper” mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor. Mol Cancer Ther. 2008;7:874–9. doi: 10.1158/1535-7163.MCT-07-2387. [DOI] [PubMed] [Google Scholar]
- 37.Ogino A, Kitao H, Hirano S, Uchida A, Ishiai M, Kozuki T, et al. Emergence of epidermal growth factor receptor T790M mutation during chronic exposure to gefitinib in a non small cell lung cancer cell line. Cancer Res. 2007;67:7807–14. doi: 10.1158/0008-5472.CAN-07-0681. [DOI] [PubMed] [Google Scholar]
- 38.Ramalingam S, Ohe Y, Nogami N, Yang JC-H, Eberlein C, Ashton S, Mellor M, Spitzler P, Meador CB, Ichihara E, Cross D, Pao W, Ballard P, Hughes G, Cantarini M, Frewer P, Ghiorghiu S, Janne PA. Pre-clinical and Clinical Evaluation of AZD9291, a Mutation-specific Inhibitor, in treatment-naïve EGFR Mutated NSCLC. ESMO. 2014:454P. Poster. [Google Scholar]
- 39.Ercan D, Xu C, Yanagita M, Monast CS, Pratilas CA, Montero J, et al. Reactivation of ERK signaling causes resistance to EGFR kinase inhibitors. Cancer Discov. 2012;2:934–47. doi: 10.1158/2159-8290.CD-12-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauer RC, Sanger J, Peschel C, Duyster J, von Bubnoff N. Sequential inhibitor therapy in CML: in vitro simulation elucidates the pattern of resistance mutations after second- and third-line treatment. Clin Cancer Res. 2013;19:2962–72. doi: 10.1158/1078-0432.CCR-13-0052. [DOI] [PubMed] [Google Scholar]
- 41.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 42.Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–22. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.