Abstract
Neutral cues, after being reliably paired with noxious events, prompt defensive engagement and amplified sensory responses. To examine the neurophysiology underlying these adaptive changes, we quantified the contrast-response function of visual cortical population activity during differential aversive conditioning. Steady-state visual evoked potentials (ssVEPs) were recorded while participants discriminated the orientation of rapidly flickering grating stimuli. During each trial, luminance contrast of the gratings was slowly increased and then decreased. Right-tilted gratings (CS+) were paired with loud white noise but left-tilted gratings (CS−) were not. The contrast-following waveform envelope of ssVEPs showed selective amplification of the CS+ only during the high-contrast stage of the viewing epoch. Findings support the notion that motivational relevance, learned in a time frame of minutes, affects vision through a response gain mechanism.
Human observers are able to flexibly select relevant sensory information (e.g., about features, objects, or spatial locations pertinent to behavioral states or goals) at the cost of other information. This process of selection is required because perceptual systems have limited capacity, a property that is particularly constraining in the context of complex environments with potential sources of reward or danger. Accordingly, theoretical and empirical work has addressed the question to what extent sensory cues representing threat or reward attain preferential access and are processed in a facilitated fashion. This research has generally converged to demonstrate that the sensory representation of motivationally relevant (appetitive or aversive) stimuli is amplified, often leading to heightened neurophysiological responses and greater behavioral accuracy (e.g., M. M. Bradley et al., 2003; Keil et al., 2003). In the visual system, threat-related stimuli (e.g., angry faces and attack scenes) or fear-conditioned stimuli are processed in a facilitated fashion in visual search (e.g., Koster, Crombez, Van Damme, Verschuere, & De Houwer, 2004), spatial attention (e.g., Bocanegra & Zeelenberg, 2011), and contrast perception paradigms (e.g., Phelps, Ling, & Carrasco, 2006). This threat advantage is evidenced by faster and more efficient detection (e.g., Fox et al., 2000; Öhman, Flykt, & Esteves, 2001), more pronounced hemodynamic activity in the extended visual cortices (e.g., M. M. Bradley et al., 2003; Sabatinelli, Bradley, Fitzsimmons, & Lang, 2005), as well as electrocortical facilitation (e.g., Ito, Larsen, Smith, & Cacioppo, 1998; Junghöfer, Bradley, Elbert, & Lang, 2001) in widespread cortical areas.
Differential fear conditioning is a mechanism through which one neutral stimulus (conditioned stimulus: CS+) efficiently acquires motivational relevance by its co-occurrence with an aversive stimulus (unconditioned stimulus: US), whereas another neutral stimulus (the CS−) is never paired with the US. After few contingent pairings, the CS+ alone typically elicits measurable defensive responses, as evidenced by verbal, behavioral, and physiological measures (Miskovic & Keil, 2012). A plethora of electrophysiological (e.g., Kluge et al., 2011) and fMRI studies (Armony & Dolan, 2002; Morris, Öhman, & Dolan, 1999) in human observers and experimental animals have documented differential engagement of widespread brain areas in response to aversively conditioned stimuli, including the amygdala, thalamus, insula, as well as frontal and sensory cortices. Associative fear conditioning has been related to learned response amplification of CS+ features in sensory cortex, again suggesting sensory prioritization of motivationally (or behaviorally) relevant events (here, the CS+) compared to neutral events. In human vision, responses to both grating stimuli (Stolarova, Keil, & Moratti, 2006) and face-shape conjunctions (Damaraju, Huang, Barrett, & Pessoa, 2009) are amplified in lower-tier visual cortices, after being reliably paired with a US. It has been argued that CS+ specific sensory amplification may reflect re-entrant bias signals originating in anterior brain structures sensitive to threat (M. M. Bradley et al., 2003; Miskovic & Keil, 2013). Although initially a slow process, such a re-entry based mechanism may result in local re-tuning of early sensory neurons if massive pairing is maintained over extended time periods (Keil, 2004; Stolarova et al., 2006). This notion is supported by findings with scalp-recorded brain potentials demonstrating differential amplitude enhancement for the CS+ in visual cortex as early as 60–100 ms after onset of a CS+, but only after hundreds of trials of differential fear conditioning (Stolarova et al., 2006). In the same vein, extensive aversive conditioning gradually increased the amplitude and synchrony of early evoked oscillations of early occipital cortical regions (Keil, Stolarova, Moratti, & Ray, 2007). Thus, changes in network connectivity among visual neurons may underlie the evolution of heightened sensitivity to features signaling learned threats and/or rewards (Miskovic & Keil, 2012). Similar to human visual cortex, unit activity in rodent auditory cortex during tone/shock conditioning manifested altered tuning and heightened phase-locked gamma oscillations (i.e., enhanced coordination of neurons encoding the CS+) at tonotopic sites sensitive to the shock-paired tone frequency (Headley & Weinberger, 2013). What is currently not known, however, is the neurophysiological mechanism mediating the heightened cortical representation of CS+ related features in the human visual system during the initial stages of fear learning. In vision research, questions regarding the mechanism of changes in visual discrimination are often addressed by measuring an observer’s or a neuron’s response to stimuli presented at varying levels of achromatic contrast. Occurring at the earliest levels of the cortical visual hierarchy (see Carrasco, 2011), contrast sensitivity may characterize not only the spatiotemporal properties of the neurons in the retinocortical pathways (Hicks, Lee, & Vidyasagar, 1983), but also the strength of the initial perceptual signal towards primary visual cortex (Boynton, Demb, Glover, & Heeger, 1999). Although the nature of contrast perception may largely reflect bottom-up feature processing (Laretzaki, Plainis, Argyropoulos, Pallikaris, & Bitsios, 2010), it is worth noting that contrast information is influenced by a vast array of selection criteria, such as directed attention or motivational relevance. For instance, a threat cue may enhance contrast sensitivity when low-contrast stimuli are presented in the attended visual field (Phelps et al., 2006). Single-cell electrophysiology studies have also suggested that neuronal activity in the primary visual cortex is modulated by attention-related feedback signals (Vidyasagar & Pigarev, 2007).
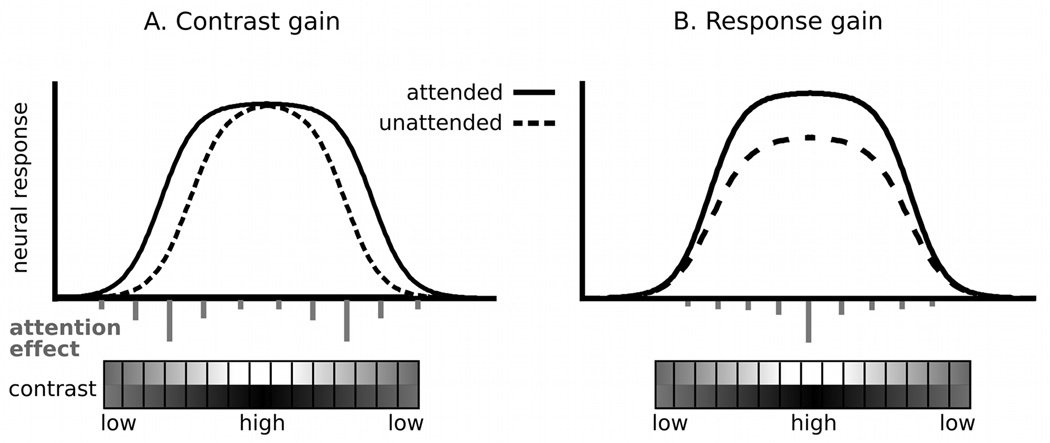
At least two different mechanisms have been identified in the extant literature as to how perceptual sensitivity can be modulated by stimulus relevance: the contrast gain and response gain mechanisms, which are most extensively described and examined in studies of visual selective attention (e.g., Ling & Carrasco, 2006; Liu, Pestilli, & Carrasco, 2005; Reynolds & Heeger, 2009). According to the contrast gain hypothesis (Figure 1A), task relevance results in top-down modulation that increases visual neuronal responses to intermediate-contrast stimuli (e.g., Carrasco, Ling, & Read, 2004; Martínez-Trujillo & Treue, 2002; Reynolds, Pasternak, & Desimone, 2000) by lowering the threshold at which responses occur (i.e., an increase in sensitivity), prior to saturation of the neural contrast-response function (CRF; see Kim, Grabowecky, Paller, Muthu, & Suzuki, 2007). Conversely, the response gain of sensory neurons (Figure 1B) is characterized by a multiplicative scaling of the neural responses across the entire dynamic range, maximally benefitting the perception of high luminance contrast of an attended stimulus (e.g., Morrone, Denti, & Spinelli, 2004). In addition, attention may increase the perception of both the intermediate and high contrast range of a stimulus, referred to as an additive (or mixed) gain mechanism (e.g., Buracas & Boynton, 2007; Huang & Dobkins, 2005), 2005). Thus, changes in the contrast-response function can be readily quantified and related to neurophysiological mechanisms that underlie sensory amplification. The present report uses this strategy to characterize the mechanism by which the sensory response to a fear-conditioned stimulus is heightened.
Figure 1.
The contrast and response gain models of visual attention. The relative degrees of the shift in the CRF mediated by attentional effects at different stimulus contrasts are depicted as the length of vertical gray lines.
Recent electrophysiological work has begun to address how prioritization of motivationally relevant stimuli may be accompanied by changes in perceptual sensitivity of visual neurons on a macroscopic level. For instance, after viewing scenes varying in emotional content, the contrast-modulation of visual evoked potentials supported the additive gain model (Song & Keil, 2013). Extending these findings, the present study examines how the neurophysiological contrast-response function is modulated when a stimulus acquires fear-relevance through differential fear conditioning. Employing the steady state visual evoked potential (ssVEP; Regan, 1989), we measure the continuous cortical engagement of visual areas low in the visual hierarchy in response to a contrast-varying Gabor patch stream, the orientation of which predicts CS status (i.e., CS+ versus CS−). The ssVEP is an oscillatory response of neuronal populations in visual cortex, elicited by a stimulus modulated at a fixed frequency (Müller, Teder-Sälejärvi, & Hillyard, 1998). Here, we record ssVEPs as the luminance contrast of the driving CS gratings is gradually increased for the first half and decreased for the second half of each trial, resulting in a waxing-waning pattern of stimulus contrast. This enables us to measure the electrocortical contrast-response function as the envelope of the time-varying ssVEP across each trial. If the visual cortical facilitation of fear-associated features is accompanied by a diminished discrimination threshold (in line with the contrast gain model), the ssVEP amplitude of the CS+ is expected to be greater than the CS− specifically during time segments with intermediate contrast. Alternatively, if the strength of sensory representation of fear-associated features in visual cortex is enhanced by a multiplicative gain factor across all levels of contrast (supportive of the response gain model), then amplitude differences between the CS+ and CS− should be greater during time segments with higher compared to low or intermediate contrast.
Method
Participants
Among 27 undergraduate students of the University of Florida recruited for psychology credit, 21 students (13 females; age M =19.05 years, SD =1.32) participated in the present study. All participants were screened for a personal and family history of photic epilepsy. Experimental procedures were approved by the institutional review board of the University of Florida, and participants gave written informed consent. Based on this analysis, six participants did not show reliable entrainment by the visual stimulus and were excluded from the analysis.
Stimuli
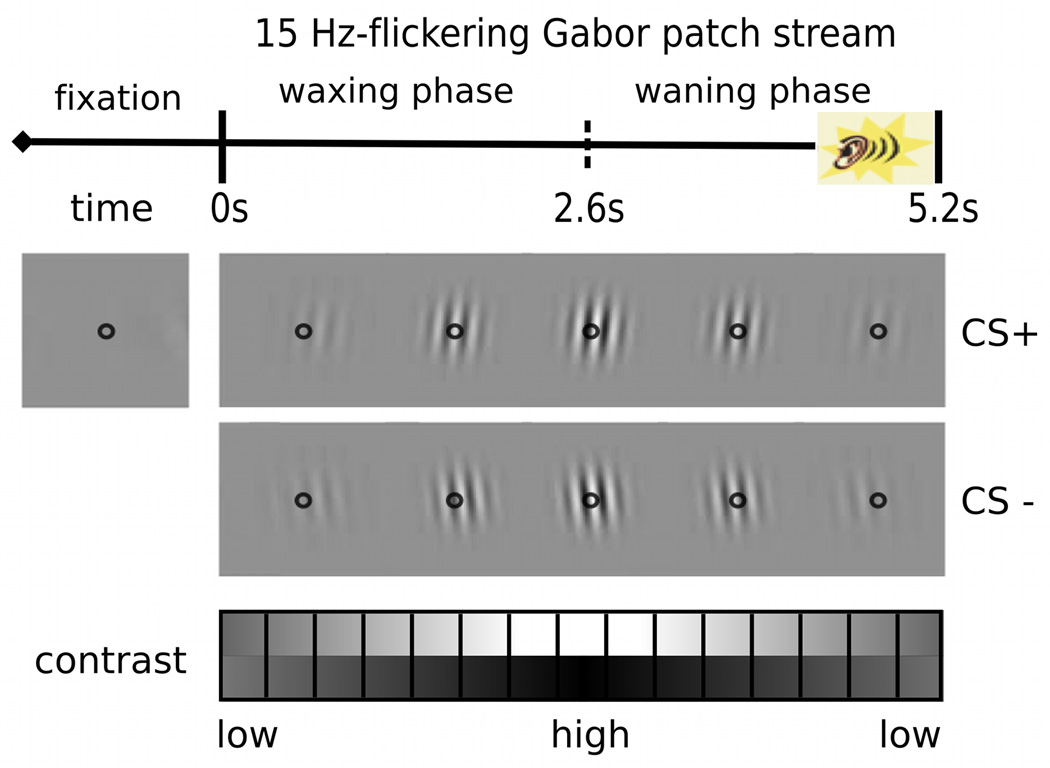
All visual stimuli were generated and presented using MATLAB (R2007b; Mathworks, Inc., Natick, MA, USA) and the Psychophysics Toolbox (Brainard, 1997; Pelli, 1997). They were displayed centrally with a fixation point (a small black circle) on a uniform gray background on a 23-inch LED (Samsung LS23A950) monitor with a vertical refresh rate of 120 Hz. The CS was a continuous stream of slightly tilted (5° clockwise or counterclockwise) sinusoidal grating multiplied with a Gaussian envelope (i.e., Gabor patches, 4.52 degrees) with a spatial frequency of 2 cycles per degree. The gratings were turned on and off at a rate of 7.5 Hz for a duration of 5 seconds per trial. Thus, brightness at a given pixel of the Gabor patch changed 15 times per second, resulting in a 15 Hz modulation rate. Over the course of one trial, luminance contrast of the patches gradually increased and decreased over time (maximum Michelson luminance contrast 0.392), resulting in a waxing-waning pattern of stimulus contrast on a trial basis. The auditory US was a 90-dB sound pressure level (SPL) white noise played through speakers placed behind the participant.
During the acquisition phase of the experiment, the CS+ stimulus was a right-tilted Gabor patch stream consistently presented together with the US during the last second of each CS+ trial, whereas the visual CS− stimuli was a left-tilted Gabor patch stream, always presented without US. Differential delay (Pavlovian) fear conditioning as employed here is a standard procedure used in animals and humans and is known to differ in many ways from other forms of associative learning (e.g., trace conditioning). It involves a significant overlap between the CS+ and the US, which co-terminate. In addition to different neurophysiological mechanisms underlying other conditioning paradigms (e.g., Bangasser, Waxler, Santollo, & Shors, 2006; Miskovic & Keil, 2013), delay conditioning is appropriate for the present design as it requires fewer trials than other forms of associative learning (see Beylin et al., 2001; Pavlov, 1927). In our implementation, the probability of pairing of CS+ and US was 100% for the first 4 CS+ trials and then decreased to 40% contingency across individuals. This was done to enable comparisons of paired versus unpaired CS+ trials, with the latter not including the observers’ response to the US, including its associated artifacts. In terms of the 16 CS+ during the intermittent conditioning, 55% of the first half CS+ stimuli and 45% of the second half CS+ stimuli were paired with the US. Each type of CSs was evaluated by the participants at the end of the experiment regarding average hedonic valence and emotional arousal, using the Self-Assessment Manikin (SAM; Bradley & Lang, 1994) 9-point scale.
Design and Procedure
After having given informed consent, participants viewed the display at a distance of 57 cm and were instructed to fixate at the center of the screen, marked by a small black circle, throughout the experiment. The experimental design involved a two-alternative forced-choice (2AFC) orientation discrimination task during the presentation of each flickering stream of tilted Gabor patches. Each session started with four practice trials to demonstrate the procedure and make sure that all individuals understood the task correctly. In total, there were 120 trials, organized into three blocks: habituation, acquisition, and extinction. During habituation, participants were shown 40 CS alone trials where they would not receive any US. For the following 40 conditioning trials, participants were instructed about the presence of the US, but they were not told that a specific stimulus or a feature of the CS (i.e., right-tilt) predicted the US. Finally, 40 extinction trials were presented without any explicit instruction regarding the absence of US for the visual CS. The sequence of stimulus presentation during an acquisition block is illustrated schematically in Figure 2. After the display of a central fixation point (1∼4 s), the target stream of flickering Gabor patches appeared at the center of the screen for a duration of 5200 ms. Participants were asked to press either the left or the right mouse button as soon as they identified the orientation of the Gabor patch, clockwise or counterclockwise, respectively. Their awareness of the contingency between the CS+ and the US was measured at the end of the experimental session after each participant completed the self-assessment manikin, indicating hedonic valence (pleasure) and emotional arousal for both CSs. Participants were also asked to report if they were able to predict the presence of the US in each trial and - if this was the case - whether or not their ratings were related to the learned contingency.
Figure 2.
Schematic illustration of one trial. Inter-trial intervals (fixation) varied randomly between 1000∼4000ms. The unconditioned stimulus (US; 90dB white noise) was played at the last second of the conditioned trials. In each trial, participants were asked to indicate whether the Gabor patch was tilted to the right or to the left.
EEG Recordings and Data Analysis
The Electroencephalogram (EEG) was continuously recorded from 257 electrodes using a HydroCel Electrical Geodesics (EGI) dense-array system. Data were digitized at a rate of 250 Hz, using the vertex sensor (Cz) as the recording reference. All electrode impedances were kept below 50 kΩ as recommended for the EGI high input impedance amplifier (Ferree, Luu, Russell, & Tucker, 2001). Offline EEG data were pre-processed using the EMEGS (ElectroMagnetoEncephalograph) toolbox for MATLAB (Peyk, DeCesarei, & Junghöfer, 2011). A low-pass filter of 40 Hz was applied, and epochs of 400 ms pre- and 5200 ms post-onset of the flickering Gabor patch stream were extracted. Using the procedure of artifact rejection proposed by Junghöfer et al. (1997), trials with artifacts were identified and excluded based on the distribution of statistical parameters of EEG epochs (absolute value, standard deviation, maximum of the temporal differential) extracted across time points, for each channel: Histograms of theses parameters were generated for each channel and statistical index. Individual channels that were more than 5 standard deviations above the median of channels throughout all trials for one or more statistical indices were globally interpolated on the level of continuous data, using spherical spline interpolation from the full channel set. The outlying trials for each channel were excluded by a combination of statistical thresholds (2 standard deviations above the median was used as a starting criterion) and visual inspection. This leads to a variable exclusion of trials by channel, which is manually adjusted by the user in a final step (see Junghöfer et al. 1997). The number of channels excluded in individual trials ranged from 7 to 62 across individuals and trials (M = 22.86, SD = 10.92). It was ensured that the interpolated channels were not located in the same region of the scalp across trials, by excluding trials in which clusters of bad electrodes did not allow representation of scalp voltages resulting from forward calculation (Peyk et al., 2011). The number of channels excluded overall ranged from 2 to 4, (M= 3.62, SD=0.67). For interpolation and all subsequent analyses, data were arithmetically transformed to the average reference. After artifact rejection, the average number of retained trials across individuals was 100.91 ± 16.56 of 120 total trials. For each condition, an average (± SD) of 35.76 ± 2.68 trials in the habituation block, 32.67 ± 2.77 trials in the acquisition block, and 32.48 ± 2.84 trials in the extinction block were retained.
Steady-state VEP Analysis
Artifact free epochs of the voltage data were averaged for six conditions: habituation CS+ (17.90 ± 2.74 trials), habituation CS− (17.86 ± 2.61 trials), acquisition CS+ (15.57 ± 2.79 trials), acquisition CS− (17.10 ± 2.74 trials), extinction CS+ (16.48 ± 2.56 trials), and extinction CS− (16.00 ± 3.11 trials). At each of the resulting 256 source locations, the time-varying ssVEP amplitude at the stimulation frequency of 15-Hz was extracted by means of complex modulation (Regan, 1989). More trials of the CS− than the CS+ condition were retained in the acquisition block (paired t(20) = −2.46, p = .023)1, but no significant differences in the number of trials were observed for the habituation (paired t(20) = .15, p = .89) and the extinction block (paired t(20) = 1.16, p = .26).
Statistical Analysis
Behavioral data and SAM ratings
Behavioral accuracy and median reaction times (RTs) of each individual were averaged for each CS type of the three experiment blocks. Effects of conditions on task performance were examined by means of the repeated-measures ANOVA having two within-subject factors of CS type and experiment block. Due to the highly skewed distribution of percentage error in the orientation discrimination task, Fisher’s Z transformation was applied for the error data before performing ANOVA. Self-reported hedonic valence and emotional arousal of CS+ were compared to CS− in each block using repeated-measures ANOVA having two within-subject factors: block and CS type. Post-hoc analyses were conducted on both behavioral data and SAM ratings using paired samples t-tests. A significance level of .05 was used for all statistical analyses.
EEG data
First, the reliable presence of phase-locked ssVEP signals evoked by the flickering Gabor patch stream in each observer and each condition was examined using the circular T-square statistic, developed by Victor and Mast (1991). To eliminate stimulus onset ERPs and the very small ssVEP signal during very low contrast periods, we used the time window of 600 to 4800ms after Gabor onset in calculating this index. The T-square statistic, a measure of phase and power consistency across segments, was computed (for each channel and participant as well as for each condition average) based on Fourier spectra for non-overlapping data segments (using the FFT command in MATLAB, without windowing or normalization), containing three cycles of the signal (=200 ms), resulting in (4800-600)/200 = 21 sub-segments. The resulting T-square value is Chi-square distributed and a p-value can be assigned for each sensor. We required significant T-square values for the majority of occipital sensors (Oz and its 16 nearest neighbors) for a subject to be included. Based on this criterion, six participants did not show reliable entrainment by the visual stimulus and were excluded from the analysis.
To compare CS+ and CS− conditions at high temporal resolution, the 15 Hz-ssVEP complex demodulated time-varying amplitudes at each electrode and time point were analyzed by means of permutation-controlled t-tests and chi-square goodness-of-fit tests. A statistical comparison between CS+ and CS− trials in each block was chosen in keeping with earlier work suggesting that safety learning is not associated with heightened visual responding (a threat-related cortical response bias; e.g., Miskovic & Keil, 2013). Furthermore, direct comparisons between the CS+ and CS− trials in each block avoid contamination with temporal order effects (e.g., habituation) that may occur in conditioning paradigms, where the temporal order of habituation, conditioning/acquisition, and extinction cannot be counterbalanced or manipulated. Applying a significance level of .05 (two-tailed), significance thresholds were first determined for the experiment blocks (habituation, acquisition, and extinction) by calculating 1000 permutation data sets (e.g., Groppe, Urbach, & Kutas, 2011) containing all electrodes and time points of the original data, but with CS+ and CS− conditions randomly shuffled across subjects. Then, t-tests comparing the permutated data sets were calculated and the maximum t-value for each topography entered the test distribution, the 0.025 and 0.975 tails of which served as critical values.
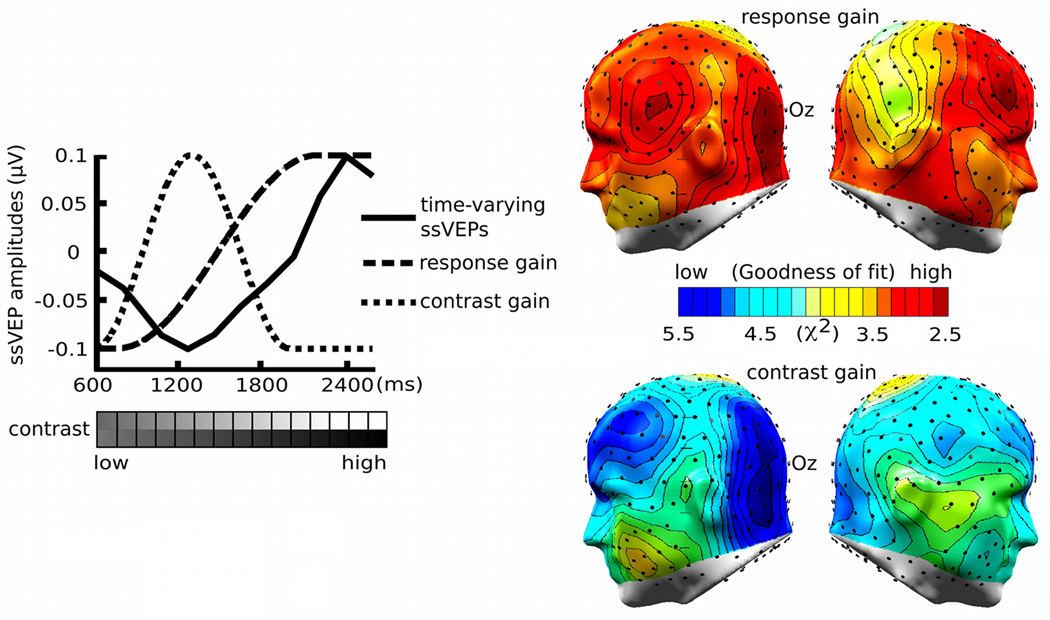
In order to determine the goodness-of-fit of the two models of contrast-response functions (contrast gain versus response gain), we computed the chi-square statistic for the actual and expected values of normalized amplitude differences between the CS+ and CS− during acquisition vis-à-vis each model. This measure of model fit was chosen because (1) the chisquare index is simple to calculate and does not require selection for additional parameters, and (2) it is often used in electrophysiology in the context of other models such as dipole fits or to quantify the appropriateness of distributed source models (Ranken, Stephen, & George, 2004). Models and data were considered only for the waxing-contrast phase (see figure 4), because this part of the data was unaffected by the US presentation in the acquisition block. Model-predicted values were normalized by the maximum value and observed amplitude differences were normalized using a range-correction method at each electrode. A chi-square value at each electrode location was then computed for the fit between the model and the data, for each electrode location and participant. The chi-square statistic of each model was spatially mapped on cortical areas of the brain, thereby visualizing the contrast-dependent conditioning effects.
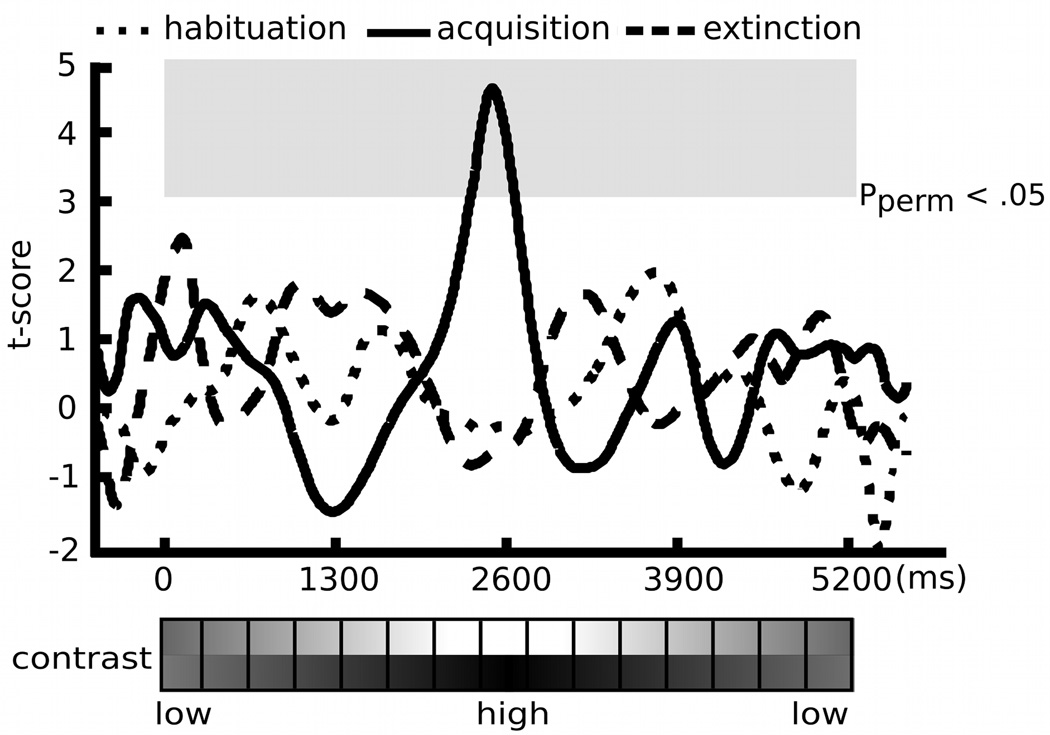
Figure 4.
Permutation-controlled t-tests on time-varying ssVEP amplitude differences between the CS+ and the CS− condition in each experimental block (habituation, acquisition, and extinction). Significance thresholds of the amplitude difference were determined for each block separately, at each time point and each scalp location (e.g., sensor 125). Applying a significance level of .05 (two-tailed), the conditioning-induced amplitude difference was statistically significant only during the acquisition block, depicted as the gray square.
Results
Behavioral Data and SAM Ratings
Table 1 displays the RTs and percentage error rates in the different conditions. All participants performed the orientation discrimination task with high accuracy. The average error rate across the six conditions was 9.17%. The repeated-measures ANOVA on the error data using Fisher’s Z transformation revealed no main effect of CS type (CS+ and CS−) or block (habituation, acquisition, and extinction), and no interaction effect of CS type by block. There was a main effect of CS type on RTs (F(1,20) = 6.32, p = .021), suggesting that participants were slower in identifying the orientation of left-tilted Gabor patches (CS−), as compared with right-tilted patches (CS+). Self-report of emotional arousal for each stimulus type showed the effects expected during fear conditioning. A 3 × 2 repeated-measures ANOVA revealed a significant block by CS type interaction (F(2,40) = 37.58, p < .001), and main effects of block and CS type (F(2,40) = 22.21, p < .001; F(1,20) = 26.16, p < .001). Post-hoc analysis using the paired t-tests indicated that the CS+ stimuli were rated as more arousing than CS− stimuli only during the acquisition block (t(20) = 7.01, p < .001). Both CS types were rated as relatively neutral (M=4.8, SD=1.28) in terms of hedonic valence (pleasure), showing no significant effects of block, CS type, or block by CS type interaction (all p > .1). Means (standard deviations) hedonic valence and emotional arousal ratings for the CS+ and CS− are summarized in Table 2.
Table 1.
Means (Standard deviations) of error rates and response times in an orientation discrimination task.
| Habituation | Acquisition | Extinction | ||||
|---|---|---|---|---|---|---|
| CS+ | CS− | CS+ | CS− | CS+ | CS− | |
| Error rates | 0.07 (0.08) |
0.11 (0.13) |
0.06 (0.07) |
0.10 (0.14) |
0.11 (0.15) |
0.13 (0.20) |
| Response times (ms) |
1117.98 (341.07) |
1203.68 (425.07) |
1088.50 (281.24) |
1091.80 (274.24) |
1062.13 (328.88) |
1061.78 (293.67) |
Table 2.
Means (Standard deviations) of self-reported hedonic valence and arousal ratings (SAM) of the CS+ and the CS− stimulus stream.
| Habituation | Acquisition | Extinction | ||||
|---|---|---|---|---|---|---|
| CS+ | CS− | CS+ | CS− | CS+ | CS− | |
| Valence | 5.1 (0.8) | 5.0 (0.9) | 4.5 (1.7) | 5.1 (1.1) | 4.5 (1.5) | 4.6 (1.7) |
| Arousal | 2.4 (1.8) | 2.8 (2.0) | 5.6. (1.8) | 2.5 (1.8) | 2.6 (1.9) | 2.2 (1.8) |
EEG Data
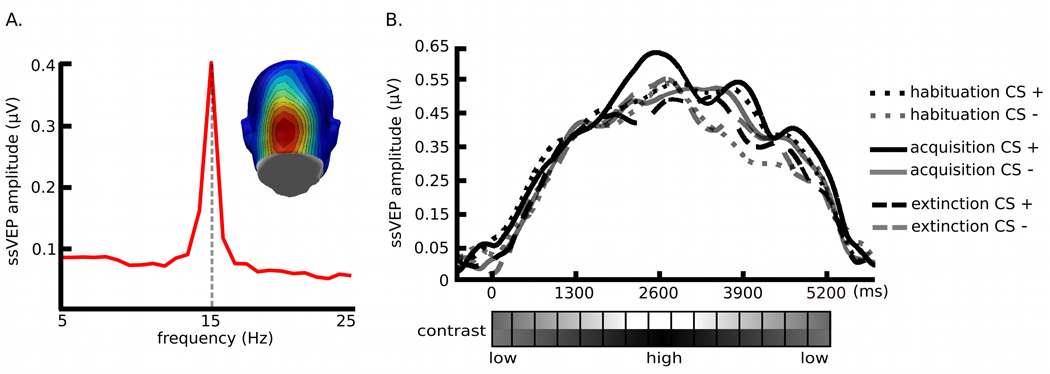
The target Gabor patch stream reliably evoked steady-state responses in visual cortex, as shown in the complex-demodulated ssVEP envelope, which follows the contour of time-varying stimulus contrast (Figure 3). In addition, the Gabor-evoked ssVEP amplitude waveform was differentially modulated by the experimental manipulations of CS types and blocks. Permutation tests focused on the hypothesis as to whether conditioning effects on the ssVEP amplitude can be characterized by the contrast, response, or additive gain model of attention. The permutation-corrected t-waveforms for CS types within each experimental block are shown in figure 4, suggesting that the time-varying amplitude of the CS+ stream, as compared with the CS− stream, was selectively enhanced during the high-contrast segment of the acquisition block (pperm< .05)2. Amplitude difference between the CS+ and the CS− was neither significant for contrast levels of Gabor patches which preceded the US nor during which the US was presented. In addition, the permutation analysis on the habituation vs. the acquisition block for each CS type (P > .05; i.e., the t-score at each contrast level was below the critical t-value of 2.94, 2.97, and 2.91 in the habituation, acquisition, and extinction block, respectively) suggests that there were no temporal order effects across different contrast levels. The chi-square goodness-of-fit tests on amplitude differences between the CS+ and CS− stream showed that the response gain model was a better fit to the conditioning effects on neural mass population activity in lower-tier visual areas (minimum χ2 (8, N=21) = 2.28), compared to the contrast gain model (minimum χ2 (8, N=21) = 3.15; see Figure 5).
Figure 3.
(A) The location of the time-averaged ssVEP over the visual cortex is demonstrated by the topography. (B) Time course of 15-Hz ssVEP amplitude in frequency domain obtained by complex demodulation averaged across 21 participants for a subset of posterior electrode locations and Oz. The reference point of the ssVEP data is the onset of each Gabor patch stream, depicted as ‘0 ms’. The envelope of ssVEP amplitudes follows different stimulus contrasts of the Gabor patch stream. The black dotted line is for habituation CS+, the gray dotted line is for habituation CS−, the black solid line is for acquisition CS+, the gray solid line is for acquisition CS−, the black dashed line is for extinction CS+, and the gray dashed line is for extinction CS−.
Figure 5.
Normalized time-varying ssVEP amplitudes during the waxing-contrast phase of the acquisition block and the chi-square Goodness-of-fit tests of the response and contrast gain models. The solid line is for the range-corrected time-varying amplitude difference between the CS+ and the CS− condition, the dashed line is for the simulated response gain model, and the dotted line is for the simulated contrast gain model. The chi-square goodness-of-fit tests on conditioning-induced amplitude differences showed that the response gain model was a better fit to the conditioning effects on neural mass population activity in the slightly lateralized visual cortex (posterior sensor clusters around Oz), compared to the contrast gain model. The chi-square values over the left and right hemispheres are demonstrated by the topographies, which depict the lowest chi-square value as dark red and the highest as dark blue.
Discussion
The present study examined the question to what extent a fear-conditioned stimulus influences the sensitivity of neural population activity in visual cortex to continuous changes in contrast. In line with previous findings, early visual cortical activity was amplified by a stimulus that acquired motivational relevance (i.e., motivated attention; Lang, Bradley, & Cuthbert, 1997) through association with an aversive event. To characterize the nature of this facilitation, we measured the effects of differential fear conditioning on the neurophysiological contrast-response function for the population activity of visual neurons. Within a trial, Gabor patches continuously changed contrast following an inverted U-shaped function. During acquisition, the CS+ patches (right-tilted) were paired with a loud noise US during the last second of the CS+ trial, when orientation was readily visible, but contrast was well below the maximum. Although stimulus contrast per se did not predict the presence of an aversively arousing US, visual cortical responses toward the CS+ stream were enhanced selectively for the portion of the trial characterized by high contrast, as compared with the CS− stream (left-tilted patches). Moreover, number of US-paired CS+ and unpaired CS+ trials did not differ in the first versus second half of the acquisition block (t(20) = −1.747, p = .096), suggesting that the amplitude difference of CS+ and CS− at high contrast viewing epochs was not affected by the temporal distribution of USs across trials. By contrast, no evidence was found supporting a contrast gain mechanism that heightens contrast sensitivity by selective amplification for intermediate contrast. Importantly, no amplitude difference in response to left-tilted versus right-tilted patches was found during the habituation block, suggesting no initial bias in the continuous electrocortical responses to a particular orientation. We also observed that the discrimination response – which was emitted approximately 1000 ms before the ssVEP difference occurred (see Figure 4) – for the CS+ and CS− did not differ during the acquisition block. Thus, an interpretation of ssVEPs being differentially affected by motor responses occurring during the waxing phase of each trial is not supported by the behavioral data. Based on the permutation t-tests and the chi-square goodness of fit tests of the hypothetical gain models, we conclude that time-varying ssVEPs show selective visual cortical facilitation in response to threat-related stimuli, characterized by a response gain, i.e., multiplicative scaling of neural responses (see Figure 4–Figure 5).
Determining the nature of the contrast-response function during fear conditioning may have the potential to shed light on psychophysical and neurophysiological mechanisms (e.g., neurotransmitter systems and neural networks) mediating fear learning in the human visual system. Previous human psychophysical work has studied the threshold and magnitude of contrast-dependent visual processing in the context of selective attention tasks (e.g., Ling & Carrasco, 2006). In this literature, paying selective attention to a task-relevant stimulus is thought to induce the contrast gain, thus facilitating perception of an attended stimulus by scaling the sensory input (i.e., lowering the contrast threshold for the input signal), which allows for greater sensory sensitivity. Alternatively, changes in response gain are often taken to imply that attention facilitates a stimulus-dependent process, which multiplicatively amplifies neuronal response magnitude (i.e., scaling the output signal). The present study supports a response gain mechanism for features associated with motivational relevance. Specifically, the data suggest that the magnitude of neural population activity in visual cortex is multiplicatively amplified for stimuli with threat-related orientations. The finding of a multiplicative response amplification of lower-tier visual activity is consistent with our previous study using task-irrelevant emotional scenes followed by contrast-varying target gratings (Song & Keil, 2013). In this earlier study, the ssVEP data suggested that the benefits of affective engagement for subsequent target processing followed the additive pattern of a contrast and a response gain (i.e., a hybrid model, see Huang & Dobkins, 2005). Thus, in both cases, changing the affective state of the observer strongly modulated visual cortical responses at the peak of the contrast function.
These findings allow the formulation of more specific hypotheses for future work, based on psychophysics studies on gain modulation. For instance, signal enhancement of sensory cortex in response to a target following emotional stimuli may operate via processes similar to those postulated for exogenous spatial attention (e.g., Ling & Carrasco, 2006). Changes in neural mass activity for CS features may entail a discriminative amplification mechanism of lower-tier visual areas, in which the association of a certain feature of the target with threat leads to increased neural firing rate (i.e., a change in slope and asymptote of the contrast-response curve) as its contrast increases. In a hypothetical account, once the strength of signal carrying information about a threat-associated feature exceeds (i.e., orientation) an observer’s discrimination threshold, the bottom-up representation of other threat-predicting stimulus features (i.e., contrast) may further be enhanced. Without a change in the discrimination threshold or sensitivity, response amplification of motivationally-relevant stimuli can occur in conjunction with re-entrant bias signals from arousal-sensitive brain regions to visual cortex (see Lang & Bradley, 2010). Moreover, recent discussions of contrast and response gain models have drawn on the normalization model of attention (Reynolds & Heeger, 2009) and have argued that the size of the target vis-à-vis the field of view may in itself bias the system towards contrast or response gain, when attending to specific features. Thus, the fact that the stimulus array in the present study was larger (4.52 degrees) than in our previous work (2.01 degrees) may have contributed to the clear pattern of response gain emerging here.
In addition to psychophysical mechanisms, the evidence of response gain in visual cortex has been associated with specific neurophysiological mechanisms. For instance, work in rodents and non-human primates has suggested that neural response gain is related to cholinergic (acetylcholine; ACh) and GABAergic neuromodulation: Neocortical cholinergic projections within primary and secondary sensory cortices are attributed to facilitate bottom-up stimulus processing (e.g., increasing signal-to-noise ratio or response selectivity; Bentley, Vuilleumier, Thiel, Driver, & Dolan, 2003) by permitting lower-tier visual neurons to be responsive to an attentional signal (e.g., Deco & Thiele, 2011). In associative fear learning, activation or blockade of muscarinic and nicotinic ACh receptor sites (Tinsley, Quinn, & Fanselow, 2004) was shown to facilitate or inhibit the expression of fear responses to CS+ stimuli (Thiel, Friston, & Dolan, 2002; Weinberger, 1998). Thus, contrast-dependent neural gain in visual cortex may emerge via response amplification mediated by cholinergic projections. Consistent with this notion, recent work has suggested that response gain emerges as a result of cholinergic modulation in the rodent and primate V1 (Soma, Shimegi, Osaki, & Sato, 2012; Soma, Shimegi, Suematsu, & Sato, 2013). According to the authors, ACh maintains sensory gain at a certain level for normal visual function and improves an animal’s ability to detect and discriminate visual stimuli during selective attention. Anatomical and neurophysiological studies on cholinergic afferents into sensory areas have demonstrated that the activation of motivational circuits in the brain engages ACh signaling and facilitates information gathering and learning, especially in the context of associative fear conditioning. ACh fibers primarily originate in the nucleus basalis of the basal forebrain, which is proposed to relay “evaluative processing within regions such as the amygdala to selection and learning mechanisms in the thalamus and cortical regions such as the prefrontal and sensory cortex” (Bentley et al., 2003, p. 59). Especially when modulated by the alertness of an animal, the contrast response of thalamic signal to visual cortex follows the pattern of response gain (Cano, Bezdudnaya, Swadlow, & Alonso, 2006; Disney, Aoki, & Hawken, 2007). This line of evidence is consistent with a hypothetical role of the thalamus and the amygdala in establishing “an associative fear trace in sensory cortex” (Miskovic & Keil, 2012, p. 1237).
Another source of neuromodulation during fear learning may depend on the GABAergic system. Previous studies have demonstrated that interactions between excitatory and inhibitory circuits involved in visual processing may critically depend on GABAergic interneurons (Ascoli et al., 2008), especially in regard to neuronal responsiveness, sensitivity, and selectivity for stimulus properties (Katzner, Busse, & Carandini, 2011). Using local administration of the selective GABAA antagonist gabazine, Katzner and colleagues (2011) found that GABA inhibition of cat V1 controlled the sensitivity of visual neurons by scaling their output (response) gain, without affecting their input (or contrast) gain. Particularly in regard to fear conditioning, the disruptive effects of GABA within the amygdala (especially the basolateral amygdala) on acquisition and consolidation of fear memories have been widely demonstrated (Makkar, Zhang, & Cranney, 2010). Considering the re-entrant projections from the amygdala to the visual cortex (Sabatinelli, Lang, Bradley, Costa, & Keil, 2009), GABA inhibition throughout the feedback neural circuitry that may mediate a complex array of ‘survive-defending’ behaviors (Lang & Bradley, 2013) may be crucial for enhanced discrimination of threat-associated features.
There are limitations of the present study that should be taken into consideration. The present research design specifically highlighted affective learning and experience as factors that modulate visual processing, which may complicate direct comparisons with gain models based on selective attention tasks. To the extent that the ssVEP is a measure of neural mass activity, it is not clear if the response gain observed here emerges when neuronal populations act to suppress non-relevant stimulus features, or when only amplifying overall visual cortical engagement in the context of a threat cue. Thus, further studies may need to clarify the competitive or facilitative nature of fear-induced response amplification by utilizing spatial- or feature-based attention paradigms, and psychophysics studies. In addition, an early vs. late trial analysis during acquisition could not be performed due to the relatively small number of trials in each condition. Here, the number of trials was kept close to the minimum possible to achieve compliance and avoid fatigue as well as loss of EEG quality over time. Future studies may use approaches specifically targeting contrast and response gain changes over the course of the experiment.
In conclusion, the present work demonstrates that learned contingencies between a neutral stimulus and an aversive event modulate the contrast-response function of visual neurons on a macroscopic level. Reliably paired with aversive auditory stimuli, visual threat cues evoked increased ssVEP amplitudes as their contrast increased, whereas safety cues did not. This pattern is consistent with the neurophysiological mechanism of response gain, in which task-driven attention multiplicatively increases stimulus-dependent responses, without altering contrast sensitivity. Consistent with a cholinergic and GABAergic modulation of response gain in the animal model, these learning-dependent changes may be the starting point of a cascade of neural and behavioral processes aiming to continuously adapt and optimize responses to a changing environment.
Acknowledgments
This research was supported by National Institute of Mental Health Grants R01 MH084932-02, R01 MH097320, the Ministerio de Ciencia e Innovación Grant I + D + i PSI2009-07066 awarded to A.K. and an American Psychological Association Dissertation Award by Science Directorate to I.S..
Footnotes
When repeating the analyses with equal trial counts for CS+ and CS− (by eliminating CS-trials from the analysis), the permutation t-test results (described below) of amplitude difference between the CS+ and CS− during conditioning remained stable: A slightly greater t-value of 4.65 was observed for the high-contrast CS+ amplitude enhancement. This suggests that the present results were not driven by signal-to-noise effects.
To test this effect using a more traditional analytical strategy, the normalized amplitudes of CS+ and CS− stimuli at three contrast viewing epochs (i.e., low, intermediate, and high) in the acquisition block were averaged across 16 nearest neighbors of site Oz. The repeated-measures ANOVA (CS type x contrast) showed a main effect of contrast (F(2,40) = 4.40, p = .019) and a trend for an interaction of CS type by contrast (F(2,40) = 3.16 , p = .053). A post-hoc analysis using the paired sample t-tests suggested that the difference amplitude of CS+ and CS− stimuli during acquisition was significant at high contrast viewing epochs (t(20) = 3.32, p = .003), but not at low (t(20) = −.098, p> .1) or intermediate contrast viewing epochs (t(20) = .972, p> .1).
References
- Armony JL, Dolan RJ. Modulation of spatial attention by fear-conditioned stimuli: an event-related fMRI study. Neuropsychologia. 2002;40(7):817–826. doi: 10.1016/s0028-3932(01)00178-6. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Group PIN. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9(7):557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Waxler DE, Santollo J, Shors TJ. Trace conditioning and the hippocampus: the importance of contiguity. J Neurosci. 2006;26(34):8702–8706. doi: 10.1523/JNEUROSCI.1742-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Effects of attention and emotion on repetition priming and their modulation by cholinergic enhancement. J Neurophysiol. 2003;90(2):1171–1181. doi: 10.1152/jn.00776.2002. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty? Neurobiol Learn Mem. 2001;76(3):447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Bocanegra BR, Zeelenberg R. Emotion-induced trade-offs in spatiotemporal vision. J Exp Psychol Gen. 2011;140(2):272–282. doi: 10.1037/a0023188. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Demb JB, Glover GH, Heeger DJ. Neuronal basis of contrast discrimination. Vision Res. 1999;39(2):257–269. doi: 10.1016/s0042-6989(98)00113-8. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The Self-Assessment Manikin and the semantic differential. Journal of Behavior Therapy & Experimental Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behav Neurosci. 2003;117(2):369–380. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Buracas GT, Boynton GM. The effect of spatial attention on contrast response functions in human visual cortex. J Neurosci. 2007;27(1):93–97. doi: 10.1523/JNEUROSCI.3162-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano M, Bezdudnaya T, Swadlow HA, Alonso JM. Brain state and contrast sensitivity in the awake visual thalamus. Nat Neurosci. 2006;9(10):1240–1242. doi: 10.1038/nn1760. [DOI] [PubMed] [Google Scholar]
- Carrasco M. Visual attention: the past 25 years. Vision Res. 2011;51(13):1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S. Attention alters appearance. Nat Neurosci. 2004;7(3):308–313. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E, Huang YM, Barrett LF, Pessoa L. Affective learning enhances activity and functional connectivity in early visual cortex. Neuropsychologia. 2009;47(12):2480–2487. doi: 10.1016/j.neuropsychologia.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Thiele A. Cholinergic control of cortical network interactions enables feedback-mediated attentional modulation. Eur J Neurosci. 2011;34(1):146–157. doi: 10.1111/j.1460-9568.2011.07749.x. [DOI] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ. Gain modulation by nicotine in macaque v1. Neuron. 2007;56(4):701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clin Neurophysiol. 2001;112(3):536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Fox E, Lester V, Russo R, Bowles RJ, Pichler A, Dutton K. Facial Expressions of Emotion: Are Angry Faces Detected More Efficiently? Cogn Emot. 2000;14(1):61–92. doi: 10.1080/026999300378996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe DM, Urbach TP, Kutas M. Mass univariate analysis of event-related brain potentials/fields II: Simulation studies. Psychophysiology. 2011;48(12):1726–1737. doi: 10.1111/j.1469-8986.2011.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley DB, Weinberger NM. Fear conditioning enhances γ oscillations and their entrainment of neurons representing the conditioned stimulus. J Neurosci. 2013;33(13):5705–5717. doi: 10.1523/JNEUROSCI.4915-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks TP, Lee BB, Vidyasagar TR. The responses of cells in macaque lateral geniculate nucleus to sinusoidal gratings. J Physiol. 1983;337:183–200. doi: 10.1113/jphysiol.1983.sp014619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Dobkins KR. Attentional effects on contrast discrimination in humans: evidence for both contrast gain and response gain. Vision Res. 2005;45(9):1201–1212. doi: 10.1016/j.visres.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Ito TA, Larsen JT, Smith NK, Cacioppo JT. Negative information weighs more heavily on the brain: the negativity bias in evaluative categorizations. J Pers Soc Psychol. 1998;75(4):887–900. doi: 10.1037//0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Bradley MM, Elbert TR, Lang PJ. Fleeting images: a new look at early emotion discrimination. Psychophysiology. 2001;38(2):175–178. [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Leiderer P, Berg P, Rockstroh B. Mapping EEG-potentials on the surface of the brain: a strategy for uncovering cortical sources. Brain Topogr. 1997;9(3):203–217. doi: 10.1007/BF01190389. [DOI] [PubMed] [Google Scholar]
- Katzner S, Busse L, Carandini M. GABAA inhibition controls response gain in visual cortex. J Neurosci. 2011;31(16):5931–5941. doi: 10.1523/JNEUROSCI.5753-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A. The role of human prefrontal cortex in motivated perception and behavior: a macroscopic perspective. In: Otani S, editor. Prefrontal Cortex: from synaptic plasticity to cognition. New York: Kluwer; 2004. pp. 245–267. [Google Scholar]
- Keil A, Gruber T, Müller MM, Moratti S, Stolarova M, Bradley MM, Lang PJ. Early modulation of visual perception by emotional arousal: evidence from steady-state visual evoked brain potentials. Cogn Affect Behav Neurosci. 2003;3(3):195–206. doi: 10.3758/cabn.3.3.195. [DOI] [PubMed] [Google Scholar]
- Keil A, Stolarova M, Moratti S, Ray WJ. Adaptation in human visual cortex as a mechanism for rapid discrimination of aversive stimuli. Neuroimage. 2007;36(2):472–479. doi: 10.1016/j.neuroimage.2007.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Grabowecky M, Paller KA, Muthu K, Suzuki S. Attention induces synchronization-based response gain in steady-state visual evoked potentials. Nat Neurosci. 2007;10(1):117–125. doi: 10.1038/nn1821. [DOI] [PubMed] [Google Scholar]
- Kluge C, Bauer M, Leff AP, Heinze HJ, Dolan RJ, Driver J. Plasticity of human auditory-evoked fields induced by shock conditioning and contingency reversal. Proc Natl Acad Sci U S A. 2011;108(30):12545–12550. doi: 10.1073/pnas.1016124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster EH, Crombez G, Van Damme S, Verschuere B, De Houwer J. Does imminent threat capture and hold attention? Emotion. 2004;4(3):312–317. doi: 10.1037/1528-3542.4.3.312. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Biol Psychol. 2010;84(3):437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. Appetitive and Defensive Motivation: Goal-Directed or Goal-Determined? Emot Rev. 2013;5(3):230–234. doi: 10.1177/1754073913477511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert MM. Motivated attention: Affect, activation and action. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and Orienting: Sensory and Motivational Processes. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1997. pp. 97–135. [Google Scholar]
- Laretzaki G, Plainis S, Argyropoulos S, Pallikaris IG, Bitsios P. Threat and anxiety affect visual contrast perception. J Psychopharmacol. 2010;24(5):667–675. doi: 10.1177/0269881108098823. [DOI] [PubMed] [Google Scholar]
- Ling S, Carrasco M. Sustained and transient covert attention enhance the signal via different contrast response functions. Vision Res. 2006;46(8-9):1210–1220. doi: 10.1016/j.visres.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Pestilli F, Carrasco M. Transient attention enhances perceptual performance and FMRI response in human visual cortex. Neuron. 2005;45(3):469–477. doi: 10.1016/j.neuron.2004.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar SR, Zhang SQ, Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology. 2010;35(8):1625–1652. doi: 10.1038/npp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Trujillo J, Treue S. Attentional modulation strength in cortical area MT depends on stimulus contrast. Neuron. 2002;35(2):365–370. doi: 10.1016/s0896-6273(02)00778-x. [DOI] [PubMed] [Google Scholar]
- Miskovic V, Keil A. Acquired fears reflected in cortical sensory processing: a review of electrophysiological studies of human classical conditioning. Psychophysiology. 2012;49(9):1230–1241. doi: 10.1111/j.1469-8986.2012.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V, Keil A. Perceiving Threat In the Face of Safety: Excitation and Inhibition of Conditioned Fear in Human Visual Cortex. Journal of Neuroscience. 2013;33(1):72–78. doi: 10.1523/JNEUROSCI.3692-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci U S A. 1999;96(4):1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone MC, Denti V, Spinelli D. Different attentional resources modulate the gain mechanisms for color and luminance contrast. Vision Res. 2004;44(12):1389–1401. doi: 10.1016/j.visres.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Müller MM, Teder-Sälejärvi W, Hillyard SA. The time course of cortical facilitation during cued shifts of spatial attention. Nat Neurosci. 1998;1(7):631–634. doi: 10.1038/2865. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. J Exp Psychol Gen. 2001;130(3):466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. Oxford, UK: Oxford University Press; 1927. [Google Scholar]
- Pelli DG. The Video Toolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Peyk P, DeCesarei A, Junghöfer M. Electro Magneto Encephalograhy Software: overview and integration with other EEG/MEG toolboxes. Computational Intelligence and Neuroscience. 2011;2011 doi: 10.1155/2011/861705. Article ID 861705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychol Sci. 2006;17(4):292–299. doi: 10.1111/j.1467-9280.2006.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranken DM, Stephen JM, George JS. MUSIC seeded multi-dipole MEG modeling using the Constrained Start Spatio-Temporal modeling procedure. Neurol Clin Neurophysiol. 2004;2004:80. [PubMed] [Google Scholar]
- Regan D. Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine. New York: Elsevier; 1989. [Google Scholar]
- Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61(2):168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26(3):703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24(4):1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Bradley MM, Costa VD, Keil A. The timing of emotional discrimination in human amygdala and ventral visual cortex. J Neurosci. 2009;29(47):14864–14868. doi: 10.1523/JNEUROSCI.3278-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma S, Shimegi S, Osaki H, Sato H. Cholinergic modulation of response gain in the primary visual cortex of the macaque. J Neurophysiol. 2012;107(1):283–291. doi: 10.1152/jn.00330.2011. [DOI] [PubMed] [Google Scholar]
- Soma S, Shimegi S, Suematsu N, Sato H. Cholinergic modulation of response gain in the rat primary visual cortex. Sci Rep. 2013;3:1138. doi: 10.1038/srep01138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Keil A. Affective engagement and subsequent visual processing: effects of contrast and spatial frequency. Emotion. 2013;13(4):748–757. doi: 10.1037/a0031553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolarova M, Keil A, Moratti S. Modulation of the C1 visual event-related component by conditioned stimuli: evidence for sensory plasticity in early affective perception. Cereb Cortex. 2006;16(6):876–887. doi: 10.1093/cercor/bhj031. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Friston KJ, Dolan RJ. Cholinergic modulation of experience-dependent plasticity in human auditory cortex. Neuron. 2002;35(3):567–574. doi: 10.1016/s0896-6273(02)00801-2. [DOI] [PubMed] [Google Scholar]
- Tinsley MR, Quinn JJ, Fanselow MS. The role of muscarinic and nicotinic cholinergic neurotransmission in aversive conditioning: comparing pavlovian fear conditioning and inhibitory avoidance. Learn Mem. 2004;11(1):35–42. doi: 10.1101/lm.70204. [DOI] [PubMed] [Google Scholar]
- Victor JD, Mast J. A new statistic for steady-state evoked potentials. Electroencephalogr Clin Neurophysiol. 1991;78(5):378–388. doi: 10.1016/0013-4694(91)90099-p. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR, Pigarev IN. Modulation of neuronal responses in macaque primary visual cortex in a memory task. Eur J Neurosci. 2007;25(8):2547–2557. doi: 10.1111/j.1460-9568.2007.05483.x. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Physiological memory in primary auditory cortex: characteristics and mechanisms. Neurobiol Learn Mem. 1998;70(1–2):226–251. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]