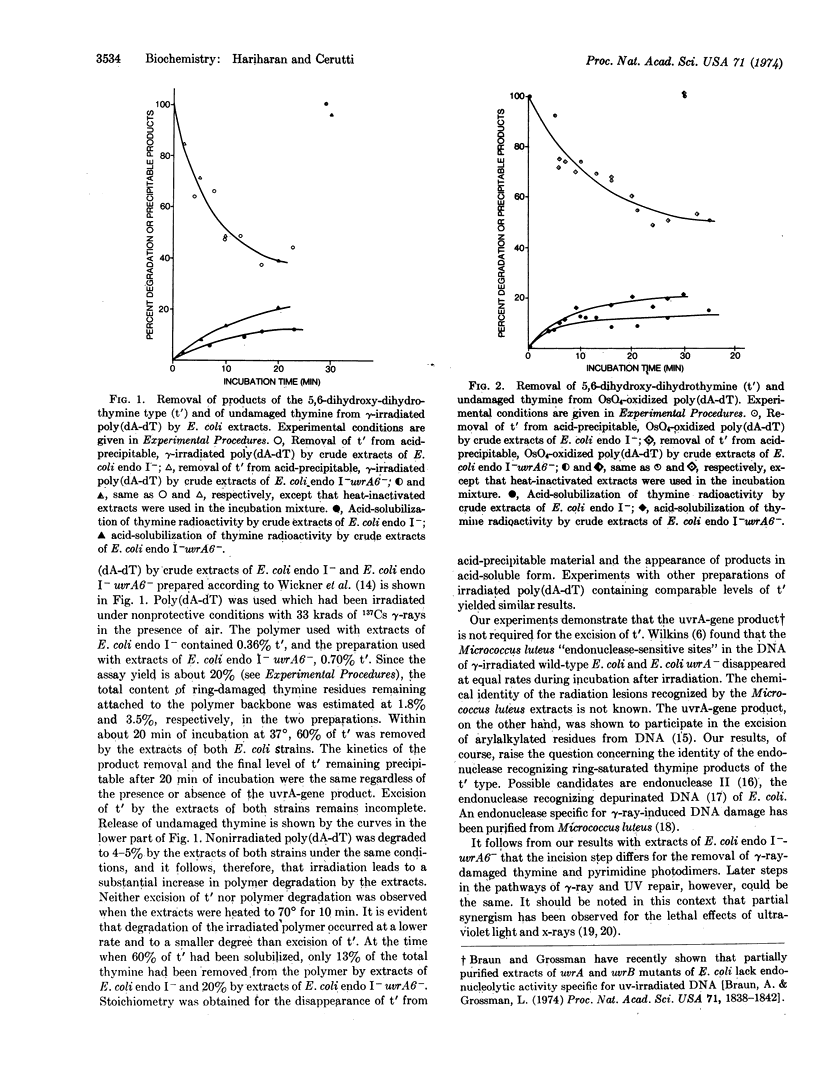
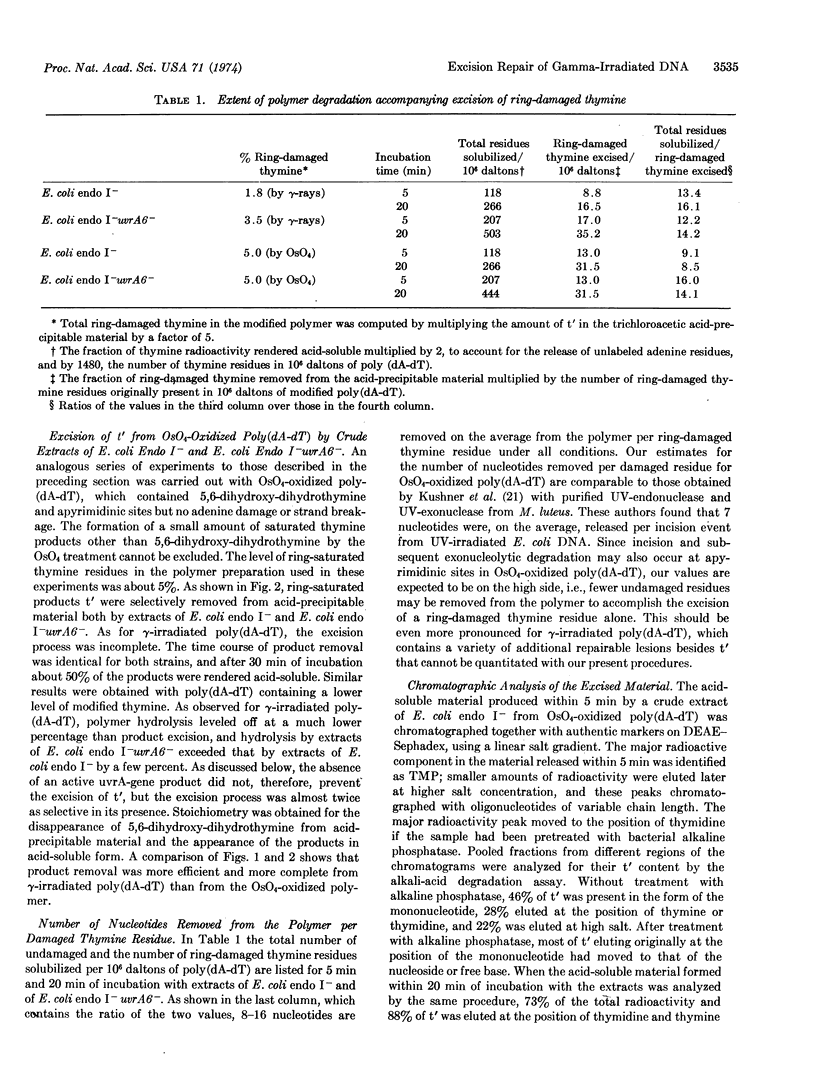
Abstract
Crude extracts of E. coli endo I- and E. coli endo I-uvrA6- possess the ability to remove thymine products of the 5,6-dihydroxy-dihydrothymine type from γ-irradiated or osmium tetroxide-oxidized poly(dA-dT). It is shown that the uvrA-gene product, which is responsible for incision close to photodimers in prereplication ultraviolet repair in E. coli, is not required for, but may aid in, the excision of γ-ray products of the 5,6-dihydroxy-dihydrothymine type. Ring-damaged thymine products are also removed by E. coli extracts from osmium tetroxide-oxidized poly(dA-dT), which contains only 5,6-dihydroxy-dihydrothymine but no strand breakage, indicating that product excision occurs in the absence of radiation-induced breaks. On the average, 8 to 16 nucleotides are removed from the polymer per ring-damaged thymine residue excised by extracts from both strains and for γ-irradiated and osmium tetroxide-oxidized polymer.
Keywords: excision repair, DNA base damage, gamma rays
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beer M., Stern S., Carmalt D., Mohlhenrich K. H. Determination of base sequence in nucleic acids with the electron microscope. V. The thymine-specific reactions of osmium tetroxide with deoxyribonucleic acid and its components. Biochemistry. 1966 Jul;5(7):2283–2288. doi: 10.1021/bi00871a017. [DOI] [PubMed] [Google Scholar]
- Brent T. P. A human endonuclease activity for gamma-irradiated DNA. Biophys J. 1973 Apr;13(4):399–401. doi: 10.1016/S0006-3495(73)85993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton K., Riley W. T. Selective degradation of thymidine and thymine deoxynucleotides. Biochem J. 1966 Jan;98(1):70–77. doi: 10.1042/bj0980070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet J., Teoule R. Peroxydes formés par action du rayonnement gamma sur la thymine en solution aqueuse aérée. Biochim Biophys Acta. 1971 Apr 29;238(1):8–26. [PubMed] [Google Scholar]
- Cerutti P. A. Effects of ionizing radiation on mammalian cells. Naturwissenschaften. 1974 Feb;61(2):51–59. doi: 10.1007/BF00596195. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Xeroderma pigmentosum: a human disease in which an initial stage of DNA repair is defective. Proc Natl Acad Sci U S A. 1969 Jun;63(2):428–435. doi: 10.1073/pnas.63.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., Goldthwait D. A. Endonuclease II of E. coli. I. Isolation and purification. Proc Natl Acad Sci U S A. 1969 Mar;62(3):934–940. doi: 10.1073/pnas.62.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan P. P., Cerutti P. A. Repair of gamma-ray-induced thymine damage in Micrococcus radiodurans. Nat New Biol. 1971 Feb 24;229(8):247–249. doi: 10.1038/newbio229247a0. [DOI] [PubMed] [Google Scholar]
- Hariharan P. V., Cerutti P. A. Formation and repair of gamma-ray induced thymine damage in Micrococcus radiodurans. J Mol Biol. 1972 Apr 28;66(1):65–81. doi: 10.1016/s0022-2836(72)80006-8. [DOI] [PubMed] [Google Scholar]
- Kushner S. R., Kaplan J. C., Ono H., Grossman L. Enzymatic repair of deoxyribonucleic acid. IV. Mechanism of photoproduct excision. Biochemistry. 1971 Aug 31;10(18):3325–3334. doi: 10.1021/bi00794a002. [DOI] [PubMed] [Google Scholar]
- Mattern M. R., Hariharan P. V., Dunlap B. E., Cerutti P. A. DNA-degradation and excision repair in gamma-irradiated Chinese hamster ovary cells. Nat New Biol. 1973 Oct 24;245(147):230–232. doi: 10.1038/newbio245230a0. [DOI] [PubMed] [Google Scholar]
- Okuda A. Inhibition of the UV-ionizing radiation synergism in Escherichia coli B/r by liquid holding between the two irradiations. Photochem Photobiol. 1973 Oct;18(4):335–337. doi: 10.1111/j.1751-1097.1973.tb06429.x. [DOI] [PubMed] [Google Scholar]
- Paterson M. C., Setlow R. B. Endonucleolytic activity from Micrococcus luteus that acts on -ray-induced damage in plasmid DNA of Escherichia coli minicells. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2927–2931. doi: 10.1073/pnas.69.10.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roti J. L., Cerutti P. A. Letter: Gamma-ray induced thymine damage in mammalian cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1974 Apr;25(4):413–417. doi: 10.1080/09553007414550491. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Endonuclease activity toward DNA irradiated in vitro by gamma rays. Nat New Biol. 1973 Feb 7;241(110):170–172. doi: 10.1038/newbio241170a0. [DOI] [PubMed] [Google Scholar]
- Venitt S., Tarmy E. M. The selective excision of arylalkylated products from the DNA of Escherichia coli treated with the carcinogen 7-bromomethylbenz(a)anthracene. Biochim Biophys Acta. 1972 Nov 16;287(1):38–51. doi: 10.1016/0005-2787(72)90328-0. [DOI] [PubMed] [Google Scholar]
- Verly W. G., Paquette Y., Thibodeau L. Nuclease for DNA apurinic sites may be involved in the maintenance of DNA in normal cells. Nat New Biol. 1973 Jul 18;244(133):67–69. doi: 10.1038/newbio244067a0. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Wright M., Wickner S., Hurwitz J. Conversion of phiX174 and fd single-stranded DNA to replicative forms in extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3233–3237. doi: 10.1073/pnas.69.11.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins R. J. Does Escherichia coli possess a DNA excision repair system for gamma-ray damage? Nat New Biol. 1973 Aug 29;244(139):269–271. doi: 10.1038/newbio244269a0. [DOI] [PubMed] [Google Scholar]



