Abstract
Background
Patients undergoing hemodialysis (HD) often develop cerebral disease complications. Furthermore, cerebral regional saturation of oxygen (rSO2) was previously reported to be significantly lower in HD patients than in healthy subjects. We aimed to identify the factors affecting the cerebral rSO2 in HD patients.
Methods
Fifty-four HD patients (38 men and 16 women; mean age, 67.7 ± 1.2 years, HD duration, 6.5 ± 1.9 years) were recruited. Cerebral rSO2 was monitored at the forehead before HD using an INVOS 5100C (Covidien Japan, Tokyo, Japan).
Results
The rSO2 levels were significantly lower in HD patients compared with healthy controls (49.5 ± 1.7% vs. 68.9 ± 1.6%, p <0.001). Multiple regression analysis showed that cerebral rSO2 independently associated with pH (standardized coefficient: -0.35), HD duration (standardized coefficient: -0.33), and serum albumin concentration (standardized coefficient: 0.28). Furthermore, the rSO2 was significantly lower in HD patients with diabetes mellitus (DM), compared with patients without DM (46.8 ± 1.7% vs. 52.1 ± 1.8%, p <0.05).
Conclusions
In HD patients, cerebral rSO2 was affected by multiple factors, including pH, HD duration, and serum albumin concentration. Furthermore, this is the first report describing significantly lower levels of rSO2 in HD patients with DM than in those without DM.
Introduction
Central nervous system (CNS) dysfunction, such as uremic encephalopathy, cognitive impairment, and dementia, is a frequent complication of patients undergoing hemodialysis (HD). [1] Cerebrovascular accident (CVA) was described as the fourth leading cause of death in HD patients according to the annual report of the Japanese Society for Dialysis Therapy in 2011. [2] Magnetic resonance imaging (MRI) is a useful tool for detecting morphological changes in the brain and therefore evaluating CVA; in addition, silent cerebral infarction detected by MRI has been found to associate with the severity of cognitive impairment in HD patients. [3] However, imaging methods like MRI and computed tomography can only provide information about organic lesions in the brain, and cannot evaluate the functional status such as cerebral blood flow and cerebral oxygenation. Recently, near-infrared spectroscopy (NIRS) has been used as a tool to measure the regional saturation of oxygen (rSO2), a marker of tissue oxygenation, at the frontal cerebral cortex in a variety of clinical situations, and has shown the change of critical balance between arterial oxygen delivery and cerebral oxygen consumption. [4–7] Cerebral rSO2 was reported to be significantly lower in HD patients than in healthy controls. [1,8] Few reports, however, have examined the relationship between cerebral oxygenation in HD patients and clinical parameters. Therefore, in this study, we aimed to elucidate the clinical factors influencing cerebral rSO2 in HD patients.
Methods
In this study, we included HD patients who met the following criteria: (1) patients with end-stage renal disease receiving intermittent HD and (2) patients with unimpaired consciousness. The exclusion criteria were: (1) coexisting disease including chronic obstructive pulmonary disease, apparent neurological disorder, and chronic hypotension (defined as systolic blood pressure <100 mmHg), and (2) history of cerebrovascular disease and dementia. Not all patients enrolled in this study underwent imaging examinations such as computed tomography and MRI for detecting cerebral ischemia, carotid artery stenosis or aortic stenosis. Therefore, we cannot completely exclude the existence of ischemic conditions in each patient. However, we excluded HD patients with apparent neurological disorder, history of cerebrovascular disease, and dementia; therefore, it could be considered that cerebral ischemia, carotid artery stenosis or aortic stenosis had no clinical effect in the HD patients enrolled in our study. Fifty four HD patients were recruited (38 men and 16 women; mean age, 67.7 ± 1.2 years, HD duration, 6.5 ± 1.9 years). The causes of chronic renal failure were diabetes mellitus (DM, 27 patients), chronic glomerulonephritis (14 patients), nephrosclerosis (4 patients), polycystic kidney disease (4 patients), and other (5 patients). Each patient received maintenance HD 2 or 3 times a week, and the duration of the HD sessions was 3 or 4 h. The patients’ general characteristics are summarized in Table 1. All participants signed informed consent to participate in this study. This study was approved by the Institutional Review Board of Saitama Medical Center, Jichi Medical University, Japan (No. RIN13-39), and Nishikawa Town Hospital, Japan (No. 1/4/2013), and conforms to the provisions of the Declaration of Helsinki (as revised in Tokyo in 2004). In addition, 28 healthy volunteers (18 men and 10 women, mean age, 43.4 ± 3.6 years) were recruited as the control group.
Table 1. Patients Characteristics and the correlation between cerebral rSO2 and clinical parameters.
| mean ± SE | p value | r | |
|---|---|---|---|
| Total number pf patients (male/female) | 54 (38/16) | ||
| Age (years) | 67.7 ± 1.2 | NS | |
| Disease | |||
| diabetes mellitus | 27 | ||
| chronic glomerulonephritis | 14 | ||
| nephrosclerosis | 4 | ||
| polycystic kidney disease | 4 | ||
| others | 5 | ||
| HD duration (years) | 6.6 ± 0.9 | <0.01 | -0.35 |
| Weight gain (%) | 3.4 ± 0.2 | NS | |
| Systolic blood pressure (mmHg) | 139.7 ± 2.8 | NS | |
| Diastolic blood pressure (mmHg) | 73.2 ± 1.8 | NS | |
| pH | 7.38 ± 0.0 | <0.01 | -0.42 |
| pCO2 (mmHg) | 37.6 ± 0.6 | NS | |
| pO2 (mmHg) | 81.9 ± 2.2 | NS | |
| HCO3- (mEq/L) | 21.6 ± 0.3 | NS | |
| Sat O2 (%) | 94.8 ± 0.6 | NS | |
| Hb (g/dL) | 9.9 ± 0.2 | <0.01 | 0.44 |
| Arterial O2 content (mL/dL) | 12.8 ± 0.2 | <0.01 | 0.40 |
| ESAs dose (U/week) | 5375 ± 510 | NS | |
| BUN (mg/dL) | 52.1 ± 2.2 | <0.05 | 0.29 |
| Cr (mg/dL) | 8.5 ± 0.3 | NS | |
| Na (mEq/L) | 137.1 ± 0.5 | <0.01 | 0.45 |
| K (mEq/L) | 4.5 ± 0.1 | <0.05 | 0.28 |
| Ca (mg/dL) | 8.9 ± 0.1 | <0.05 | -0.28 |
| P (mg/dL) | 4.7 ± 0.2 | <0.01 | 0.35 |
| Total protein (g/dL) | 6.2 ± 0.1 | NS | |
| Serum albumin (g/dL) | 3.3 ± 0.1 | <0.01 | 0.41 |
| Serum osmolarity (mosm/kg-H2O) | 301.5 ± 1.4 | <0.05 | -0.33 |
| Plasma glucose (mg/dL) | 156.6 ± 8.5 | <0.01 | 0.36 |
| HbA1c (%) | 5.9 ± 0.2 | NS | |
| C-reactive protein (mg/dL) | 2.2 ± 0.6 | <0.01 | -0.36 |
Monitoring of cerebral oxygenation and clinical laboratory measurement
Cerebral rSO2 was monitored at the forehead with an INVOS 5100C saturation monitor (Covidien Japan, Tokyo, Japan), which utilizes NIRS technology. This instrument uses a light-emitting diode, which transmits near-infrared light at 2 wavelengths (735 and 810 nm), and 2 silicon photodiodes which act as light detectors; results are read as a single numerical value that represents the rSO2 [9,10]. All data obtained by this instrument were immediately and automatically stored in sequence. Interobserver variance for this instrument, that is, reproducibility of the rSO2 measurement, is acceptable as previously reported. Therefore, rSO2 is considered reliable when estimating the actual cerebral oxygenation. [11]
Prior to HD, the recruited patients rested in the supine position for at least 10 min in order to reduce the influence of postural change. An rSO2 measurement sensor was attached to the patient’s forehead for measurement in the resting state. Thereafter, rSO2 was measured for 5 min before HD, and we evaluated the mean rSO2 for 5 min, as a marker of cerebral oxygenation, in each patient. Blood samples were obtained from each patient under room air. It was previously reported that samples obtained from the radial artery or those from an arterial line at the arteriovenous fistula presented similar values when evaluating the parameters of oxygen status, including pH, oxygen pressure (pO2: mmHg), and oxygen saturation (SpO2: %). [12] Therefore, prior to HD we obtained all blood samples, including blood gas analysis, from the arterial site of arteriovenous fistulae in each patient.
Arterial O2 content (CaO2) and serum osmolality (sOsm) were calculated using the following equations:
[13]
[14] where Hb represents the hemoglobin concentration (g/dL). Na represents the serum sodium concentration (mEq/L), PG represents the plasma glucose level (mg/dL), and BUN represents the blood urea nitrogen concentration (mg/dL).
Erythropoiesis-stimulating agents (ESAs) were administered for the treatment of renal anemia, and calculations of the optimum ESA dose (U/week) were based on a method reported previously, [15] where a ratio of 1:200 was used to convert the dose for long-acting ESAs, including darbepoetin-α and continuous erythropoietin receptor activator, into a short-acting recombinant human erythropoietin equivalent dose for each patient. [15] The rSO2 in healthy controls was measured for at least 5 min in the supine position in a manner similar to that in HD patients.
Analysis
Data were expressed as mean ± standard error (SE). The Student’s t-test for non-paired values was used for comparing 2 groups, and Mann–Whitney U test was used for comparison of nonparametric variables between 2 groups. Correlations between 2 groups were evaluated by Pearson’s correlation coefficient and linear regression analysis. Multiple regression analysis was performed using parameters that showed a significant correlation with cerebral rSO2. A difference of p <0.05 was considered significant.
Results
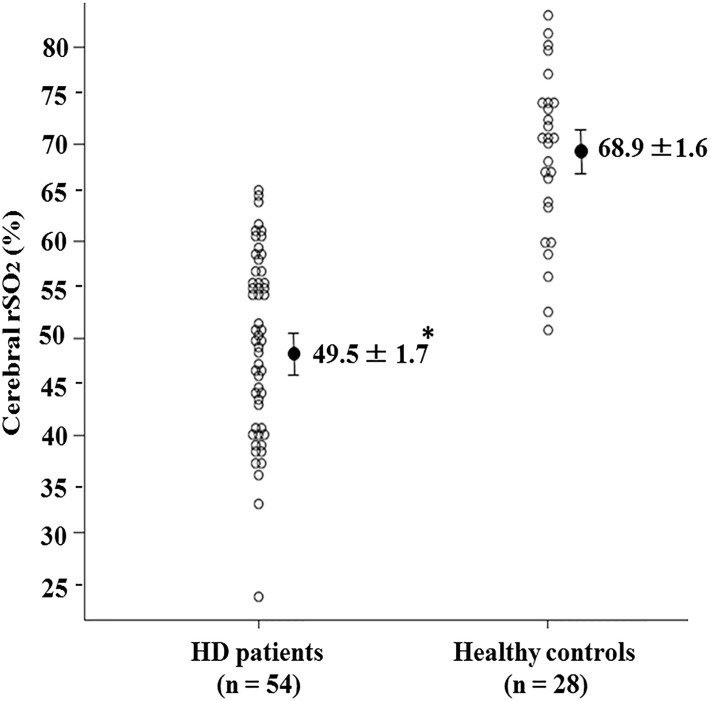
Cerebral rSO2 at rest in HD patients was compared with that in healthy controls, and there was a significant difference between the 2 groups (HD patients: 49.5 ± 1.7%, healthy controls: 68.9 ± 1.6%, p < 0.001) (Fig. 1). Recently, cerebral rSO2 was reported to be significantly lower in HD patients than in healthy controls [1, 8], and our results are consistent with these reports.
Fig 1. Comparison between cerebral rSO2 in hemodialysis patients and healthy controls.
Table 1 shows patients’ characteristics, and correlations between the cerebral rSO2 and clinical parameters. Cerebral rSO2 showed significant positive correlations with CaO2, hemoglobin (Hb) level, serum sodium concentration, serum potassium concentration, serum inorganic phosphate concentration, serum albumin concentration, and plasma glucose level. A simple linear regression analysis revealed that cerebral rSO2 was negatively correlated with pH, serum calcium concentration, sOsm, HD duration, and C-reactive protein.
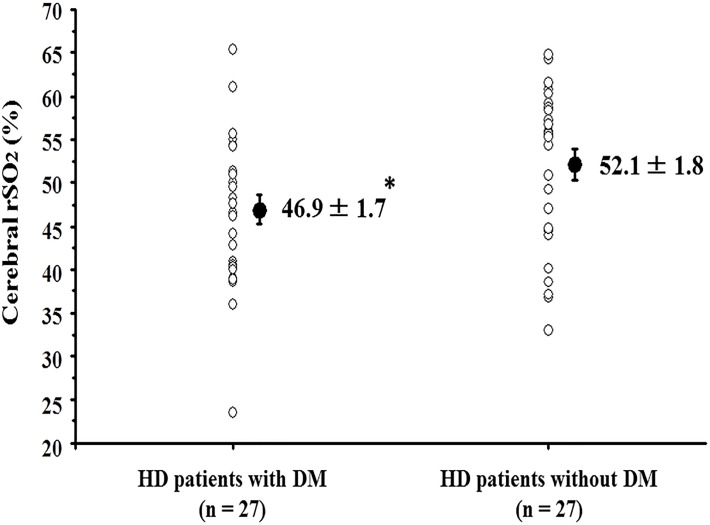
We performed a multivariate linear regression analysis using variables that showed a significant correlation with the cerebral rSO2 in a simple linear regression analysis (Table 2). The multivariate regression analysis found that the cerebral rSO2 was independently associated with pH (standardized coefficient: -0.35), HD duration (standardized coefficient: -0.33), and serum albumin concentration (standardized coefficient: 0.28). On the other hand, the cerebral rSO2 was independent of Hb and CaO2. We also evaluated the influence of DM on the cerebral rSO2 values (Table 3). The cerebral rSO2 was significantly lower in patients with DM than in those without DM (46.8 ± 1.7% vs. 52.1 ± 1.8, p < 0.05) (Fig. 2). In addition to the difference in cerebral rSO2, there were significant differences in serum sodium concentration, plasma glucose level, and HbA1c levels between the 2 groups.
Table 2. Multivariate linear regression analysis: Independent factors of cerebral rSO2 in hemodialysis patients.
| Variables | Coefficient | Standardized coefficient | p |
|---|---|---|---|
| pH | -62.5 | -0.35 | 0.012 |
| HD duration | -0.47 | -0.33 | 0.006 |
| Serum albumin | 4.12 | 0.28 | 0.041 |
Table 3. Different clinical parameters for hemodialysis patients with and without diabetes mellitus.
| with DM | without DM | p | |
|---|---|---|---|
| rSO2 (%) | 46.9 ± 1.7 | 52.1 ± 1.8 | <0.05 |
| Age (years) | 66.3 ± 1.4 | 69.1 ± 2.0 | NS |
| Male/Female | 18 / 9 | 20 / 7 | NS |
| HD duration (years) | 7.7 ± 1.4 | 5.4 ± 1.1 | NS |
| Weight gain (%) | 3.6 ± 0.4 | 3.3 ± 0.3 | NS |
| Systolic blood pressure (mmHg) | 144.8 ± 3.7 | 134.6 ± 4.1 | NS |
| Diastolic blood pressure (mmHg) | 74.0 ± 2.6 | 72.4 ± 2.3 | NS |
| pH | 7.37 ± 0.01 | 7.38 ± 0.01 | NS |
| pCO2 (mmHg) | 38.3 ± 0.6 | 36.9 ± 1.1 | NS |
| pO2 (mmHg) | 80.1 ± 3.2 | 83.7 ± 2.9 | NS |
| HCO3- (mEq/L) | 21.8 ± 0.4 | 21.3 ± 0.5 | NS |
| Sat O2 (%) | 94.5 ± 0.6 | 95.1 ± 1.0 | NS |
| Hb (g/dL) | 10.2 ± 0.2 | 9.6 ± 0.2 | NS |
| Arterial O2 content (mL/dL) | 13.1 ± 0.3 | 12.5 ± 0.3 | NS |
| ESAs dose (U/week) | 5287 ± 797 | 5463 ± 651 | NS |
| BUN (mg/dL) | 48.1 ± 3.0 | 56.0 ± 3.2 | NS |
| Na (mEq/L) | 136.1 ± 0.6 | 138.1 ± 0.7 | NS |
| K (mEq/L) | 4.4 ± 0.1 | 4.5 ± 0.2 | NS |
| Ca (mg/dL) | 8.9 ± 0.1 | 9.0 ± 0.1 | NS |
| P (mg/dL) | 4.7 ± 0.3 | 4.8 ± 0.2 | NS |
| Serum albumin (g/dL) | 3.3 ± 0.1 | 3.2 ± 0.1 | NS |
| Serum osmolarity (mosm/kg-H2O) | 299.6 ± 2.0 | 303.5 ± 2.0 | NS |
| Plasma glucose (mg/dL) | 181.9 ± 14.5 | 131.5 ± 6.2 | <0.01 |
| HbA1c (%) | 6.6 ± 0.2 | 5.1 ± 0.1 | <0.01 |
Fig 2. Comparison of cerebral rSO2 in hemodialysis patients (HD) with and without diabetes mellitus (DM).
* < 0.05 at HD patients with DM vs. those without DM.
Discussion
Regional saturation of oxygen (rSO2) is widely used for monitoring cerebral function during cerebral surgery, as rSO2 measured using NIRS can provide accurate yet non-invasive information on cerebral oxygen saturation, and can be easily performed in the clinical setting. [1, 4–11] Recently, cerebral rSO2 was reported to be significantly lower in HD patients than in healthy controls. [1,8] The reasons for this however, remain uncertain, and the factors affecting the deterioration of cerebral rSO2 in HD patients have not been determined. In this study, we identified modifiable factors, including pH, HD duration, and serum albumin concentration, as being independently associated with cerebral rSO2; we also demonstrated that cerebral rSO2 was significantly lower in HD patients with DM than in those without DM.
Among the modifiable factors identified as being independently associated with cerebral rSO2, pH was the factor most strongly affecting cerebral rSO2 in HD patients. A decrease of extracellular pH, even without partial pressure of carbon dioxide (pCO2) increase, was previously reported to induce a dilation of the cerebral artery, [16] and therefore, regional cerebral blood flow (rCBF) would increase in response to the decrease in pH. Thus, in the brain, it is possible that cerebral rSO2 increases via the increase of arterial oxygen delivery accompanying rCBF increase induced by pH decrease. This mechanism could explain why cerebral rSO2 shows an inverse relationship with pH change. Furthermore, changes in cerebral rSO2 were recently shown to be independently and negatively associated with changes in pH in patients undergoing liver transplantation, [17] and our results, which indicated an inverse relationship between cerebral rSO2 and pH, were consistent with this report. Thus far, however, the change of rSO2 affected by pH remains unclear, so further examination would be required regarding the association between cerebral rSO2 change and pH.
Serum albumin concentration also showed a significant positive correlation with cerebral rSO2. Serum albumin concentration was previously reported to be a prognostic marker of survival in HD patients, similar to nutritional status. [18,19] The decrease in its concentration has often been observed in patients with protein-energy malnutrition, leading to prognostic aggravation in HD patients. In general, serum albumin concentration contributes to the formation of colloid osmotic pressure in vessels and associates with body-fluid movement, mainly between the vessels and the interstitium. In addition, serum albumin concentration was recently shown to positively correlate with the regional cerebral blood flow in patients with liver cirrhosis. [20] Based on our results, we propose that an increase in serum albumin concentration might lead to increased rCBF and improved cerebral oxygenation, which can then be measured as cerebral rSO2. Therefore, serum albumin concentration would appear to associate not only with nutritional status and prognosis, but also with cerebral oxygenation in HD patients, although its precise mechanism remains uncertain.
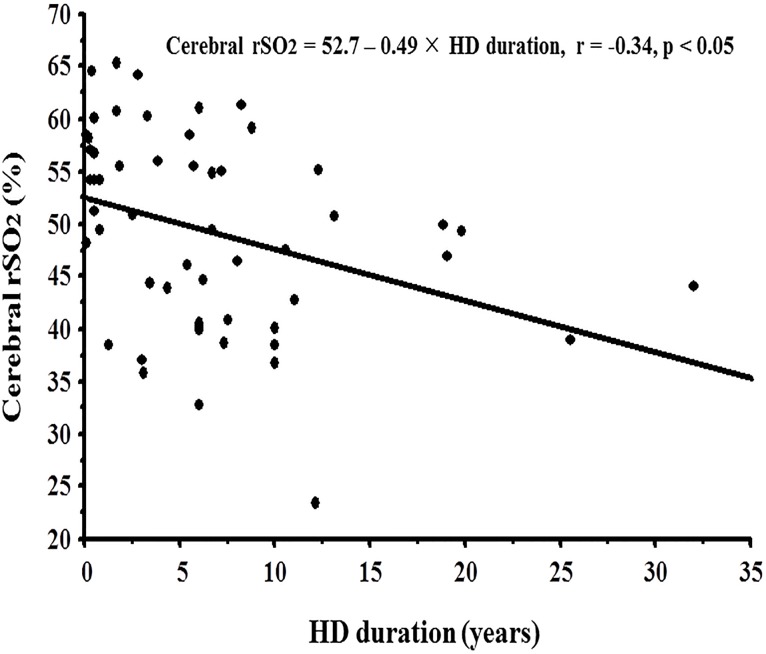
Furthermore, in this study, the cerebral rSO2 was negatively affected by HD duration, and the annual rSO2 decline in HD patients was predicted to be -0.49%/year by simple linear regression analysis (Fig. 3). It was previously reported that, in HD patients, rCBF to the frontal cortex decreased with an increase in HD duration, resulting in white matter lesions. [21] As the cerebral rSO2 mainly indicates the condition of rCBF, the negative impact of HD duration on cerebral rSO2 might be due to a decrease in the rCBF.
Fig 3. Correlation between hemodialysis duration and rSO2.
On the other hand, although CaO2 and Hb levels significantly correlated with cerebral rSO2 in a simple linear regression analysis, these associations disappeared upon multivariate linear regression analysis. Hemoglobin is an important factor in oxygen supply to the peripheral tissues and organs, including the brain; CaO2 is a marker for oxygen supply. Thus, cerebral rSO2 might be expected to show a strong correlation with Hb and CaO2 levels; however, no significant correlations were observed in the present study. Positron emission tomography analysis has revealed an association between the rCBF and Hb levels in HD patients. [22] In this report, regional cerebral blood flow decreased significantly, and oxygen metabolism was disrupted despite the increase in Hb levels. Decreased blood cell deformability and increased plasma viscosity resulted in decreased erythrocyte velocity in the cerebral capillaries, leading to an increase in Hb levels. Furthermore, in HD patients, CaO2 was reported to be the most important determinant of inter-individual middle cerebral artery (MCA) blood flow velocity variance and variation; in addition, an increase in CaO2 could induce the decrease of MCA blood flow velocity via vasoconstrictions in small intracerebral vessels to maintain oxygen delivery to the brain. [23,24] Indeed, in an experimental study, cerebral O2 transport (CBF × CaO2) was regulated at constant levels, independently of alterations in Hb levels and CaO2 values. [25] Therefore, it is unlikely that oxygen metabolism including rSO2 could directly associate with Hb and CaO2 levels. Furthermore, ESA administration was reported to reduce the development of brain edema, and preserve the local brain oxygen saturation and brain tissue oxygenation after traumatic brain injury. [26,27] However, there was no association between cerebral rSO2 values and ESA doses in HD patients.
This study included the results obtained from 1 patient with DM, which showed an extremely low cerebral rSO2 value. Thus, we analyzed the cerebral rSO2 values using the Grubbs-Smirnov rejection test to clarify whether the extremely low rSO2 value should be excluded. The test results were not significant; therefore, based on the data obtained in this study, a comparison of cerebral rSO2 values was performed for cohorts of HD patients with, and without, DM. The results, which showed significant differences in cerebral rSO2 in HD patients with DM compared with those without DM, are interesting, because it was recently suggested that dementia and cognitive impairment were related to DM and chronic kidney disease in patients, including those that underwent HD. [28,29] In particular, DM has previously been reported to be a significant risk factor for Alzheimer’s disease and vascular dementia. [30] In addition, an increase in fasting plasma glucose was associated with functional impairment of regional cerebral perfusion; moreover, an improvement in glycemic control led to a reduction in cerebral perfusion deficits. [31] In this report, impaired regional cerebral perfusion could be induced by hyperglycemia-induced endothelial dysfunction. Furthermore, dynamic cerebral autoregulation was reported to be impaired even during an early phase in type 2 DM patients. [32] In this study, plasma glucose and HbA1c levels were significantly higher in patients with DM than in those without DM; therefore, the significant decrease in cerebral rSO2 with DM might be induced by the dysregulation of regional cerebral perfusion due to hyperglycemia-induced endothelial dysfunction. In the present study, there was a difference of ~5% in the cerebral rSO2 between HD patients with and without DM, and this value corresponds to cerebral rSO2 decline for nearly 10 years, at an annual rate of decline of -0.49%/year for rSO2 in HD patients; calculations for the rate of decline were based on the negative correlation between cerebral rSO2 and HD duration. Therefore, these results might explain the mechanisms underlying the frequent occurrence of cerebral complications such as dementia and cognitive impairment in HD patients with DM.
This study faced the limitation of a relatively small sample size; therefore, further study is required to fully elucidate the correlation of cerebral rSO2 with various clinical parameters.
In conclusion, cerebral rSO2 was affected by multiple factors in HD patients, including pH, HD duration, and serum albumin concentration. Furthermore, rSO2 was significantly lower in HD patients with DM than in those without DM.
Acknowledgments
We thank the study participants and the clinical engineers, Katsunobu Ando, Takayuki Uchida, Masaya Kofuji, and Tsukasa Higuchi, for their day-to-day clinical care about hemodialysis.
Data Availability
All relevant data are within the paper.
Funding Statement
These authors have no support or funding to report.
References
- 1. Prohovnik I, Post J, Uribarri J, Lee H, Sandu O, et al. (2007) Cerebrovascular effects of hemodialysis in chronic kidney disease. J Cereb Blood Flow Metab 27: 1861–1869. [DOI] [PubMed] [Google Scholar]
- 2. Nakai S, Watanabe Y, Masakane I, Wada A, Shoji T, et al. (2013) Overview of regular dialysis treatment in Japan (as of 31 December 2011). Ther Apher Dial 17: 567–611. 10.1111/1744-9987.12147 [DOI] [PubMed] [Google Scholar]
- 3. Naganuma T, Uchida J, Tsuchida K, Takemoto Y, Tatsumi S, et al. (2005) Silent cerebral infarction predicts vascular events in hemodialysis patients. Kidney Int 67: 2434–2439. [DOI] [PubMed] [Google Scholar]
- 4. Parnia S, Nasir A, Ahn A, Malik H, Yang J, et al. (2014) A feasibility study of cerebral oximetry during in-hospital mechanical and manual cardiopulmonary resuscitation. Crit Care Med 42: 930–933. 10.1097/CCM.0000000000000047 [DOI] [PubMed] [Google Scholar]
- 5. Ono M, Arnaoutakis GJ, Fine DM, Brady K, Easley RB, et al. (2013) Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 41: 464–471. 10.1097/CCM.0b013e31826ab3a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCusker K, Chalafant A, de Foe G, Gunaydin S, Vijay V (2006) Influence of hematocrit and pump prime on cerebral oxygen saturation in on-pump coronary revascularization. Perfusion 21: 149–155. [DOI] [PubMed] [Google Scholar]
- 7. Calderon-Arnulphi M, Alaraj A, Amin-Hanjani S, Mantulin WW, Polzonetti CM, et al. (2007) Detection of cerebral ischemia in neurovascular surgery using quantitative frequency-domain near-infrared spectroscopy. J Neurosurg 106: 283–290. [DOI] [PubMed] [Google Scholar]
- 8. Hoshino T, Ookawara S, Miyazawa H, Ito K, Ueda Y, et al. (2014) Evaluation of cerebral oxygenation in patients undergoing long-term hemodialysis. Nephron Clin Pract 126: 57–61. 10.1159/000358432 [DOI] [PubMed] [Google Scholar]
- 9. Tobias JD (2006) Cerebral oxygenation monitoring: near-infrared spectroscopy. Expert Rev Med Devices 3:235–243. [DOI] [PubMed] [Google Scholar]
- 10. Ferrari M, Mottola L, Quaresima V (2004) Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol 29: 463–487. [DOI] [PubMed] [Google Scholar]
- 11. Lemmers PMA, Toet MC, van Bel F (2008) Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics 121: 142–147. 10.1542/peds.2007-0925 [DOI] [PubMed] [Google Scholar]
- 12. Nielsen AL, Thunedborg P, Brinkrnfeldt H, Hegbrant J, Jensen HA, et al. (1999) Assessment of pH and oxygen status during hemodialysis using the arterial blood line in patients with an arteriovenous fistula. Blood Purif 17: 206–212. [DOI] [PubMed] [Google Scholar]
- 13. Roach RC, Koskolou MD, Calbert JAL, Saltin B (1999) Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol Heart Circ Physiol 276: H438–H445. [DOI] [PubMed] [Google Scholar]
- 14. Gennari FJ (1984) Serum osmolality. Uses and limitation. N Engl J Med 310: 102–105. [DOI] [PubMed] [Google Scholar]
- 15. Portoles JM, de Francisco ALM, Gorriz JL, Martines-Castelao A, Lopes-Gomez JM, et al. (2008) Maintenance of target hemoglobin level in stable hemodialysis patients constitutes a theoretical task: a historical prospective study. Kidney Int Suppl 111: S82–87. 10.1038/ki.2008.524 [DOI] [PubMed] [Google Scholar]
- 16. Kontos HA, Raper AJ, Patterson JL (1977) Analysis of vasoactivity of local pH, PCO2 and bicarbonate on pial vessels. Stroke 8: 358–360. [DOI] [PubMed] [Google Scholar]
- 17. Jun IG, Shin WJ, Park YS, Song JG, Kim YK, et al. (2012) Factors affecting intraoperative changes in regional cerebral oxygen saturation in patients undergoing liver transplantation. Transplant Proc 45: 245–50. [DOI] [PubMed] [Google Scholar]
- 18. Lowrie EG, Lew NL (1990) Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis 15: 458–482. [DOI] [PubMed] [Google Scholar]
- 19. Stenvinkel P, Heimbürger O, Lindholm B, Kaysen GA, Bergström J (2000) Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol Dial Transplant 15: 953–960. [DOI] [PubMed] [Google Scholar]
- 20. Tanaka H, Maeshima S, Ueda H, Shigekawa Y, Fukuchi H, et al. (2007) Reduction of regional cerebral blood flow of patients with liver cirrhosis and its correlation with serum albumin. International Medical Journal 14: 35–39. [Google Scholar]
- 21. Kanai H, Hirakata H, Nakane H, Fujii K, Hirakata E, et al. (2001) Depressed cerebral oxygen metabolism in patients with chronic renal failure: a positron emission tomography study. Am J Kidney Dis 38: S129–33. [DOI] [PubMed] [Google Scholar]
- 22. Metry G, Wikström B, Valind S, Sandhagen B, Linde T, et al. (1999) Effect of normalization of hematocrit on brain circulation and metabolism in hemodialysis patients. J Am Soc Nephrol 10: 854–863. [DOI] [PubMed] [Google Scholar]
- 23. Stefanidis I, Bach R, Mertens PR, Liakopoulos V, Liapi G, et al. (2005) Influence of hemodialysis on the mean blood flow velocity in the middle cerebral artery. Clin Nephrol 64: 129–137. [DOI] [PubMed] [Google Scholar]
- 24. Macko RF, Ameriso SF, Akmal M, Paganini-Hill A, Mohler JG, et al. (1993) Arterial oxygen content and age are determinants of middle cerebral artery blood flow velocity. Stroke 24: 1025–1028. [DOI] [PubMed] [Google Scholar]
- 25. Ulatowski JA, Bucci E, Razynska A, Traystman RJ, Koehler RC (1998) Cerebral blood flow during hypoxic hypoxia with plasma-based hemoglobin at reduced hematocrit. Am J Physiol 274: H1933–1942. [DOI] [PubMed] [Google Scholar]
- 26. Verdonck O, Lahrech H, Francony G, Carle R, Farion R, et al. (2007) Erythropoietin protects from post-traumatic edema in the rat brain. J Cereb Blood Flow Metab 27: 1369–1376. [DOI] [PubMed] [Google Scholar]
- 27. Bouzat P, Millet A, Boue Y, Pernet-Gallary K, Trouve-Buisson T, et al. (2013) Changes in brain tissue oxygenation after treatment of diffuse traumatic brain injury by erythropoietin. Crit Care Med 41: 1316–1324. 10.1097/CCM.0b013e31827ca64e [DOI] [PubMed] [Google Scholar]
- 28. Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, et al. (1999) Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 53: 1937–1942. [DOI] [PubMed] [Google Scholar]
- 29. Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, et al. (2006) Cognitive impairment in hemodialysis patients is common. Neurology 67: 216–23. [DOI] [PubMed] [Google Scholar]
- 30. Ohara T, Doi Y, Ninomiya T, Hirakawa Y, Hata J, et al. (2011) Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology 77: 1126–1134. 10.1212/WNL.0b013e31822f0435 [DOI] [PubMed] [Google Scholar]
- 31. Cosentino F, Battista R, Scuteri A, De Sensi F, De Siati L, et al. (2009) Impact of fasting glycemia and regional cerebral perfusion in diabetic subjects: a study with technetium-99m-ethyl cysteinate dimer single photon emission computed tomography. Stroke 40: 306–308. 10.1161/STROKEAHA.108.520627 [DOI] [PubMed] [Google Scholar]
- 32. Kim YS, Immink RV, Stok WJ, Karemaker JM, Secher NH, et al. (2008) Dynamic cerebral autoregulatory capacity is affected early in type 2 diabetes. Clin Sci 115: 255–262. 10.1042/CS20070458 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.