Abstract
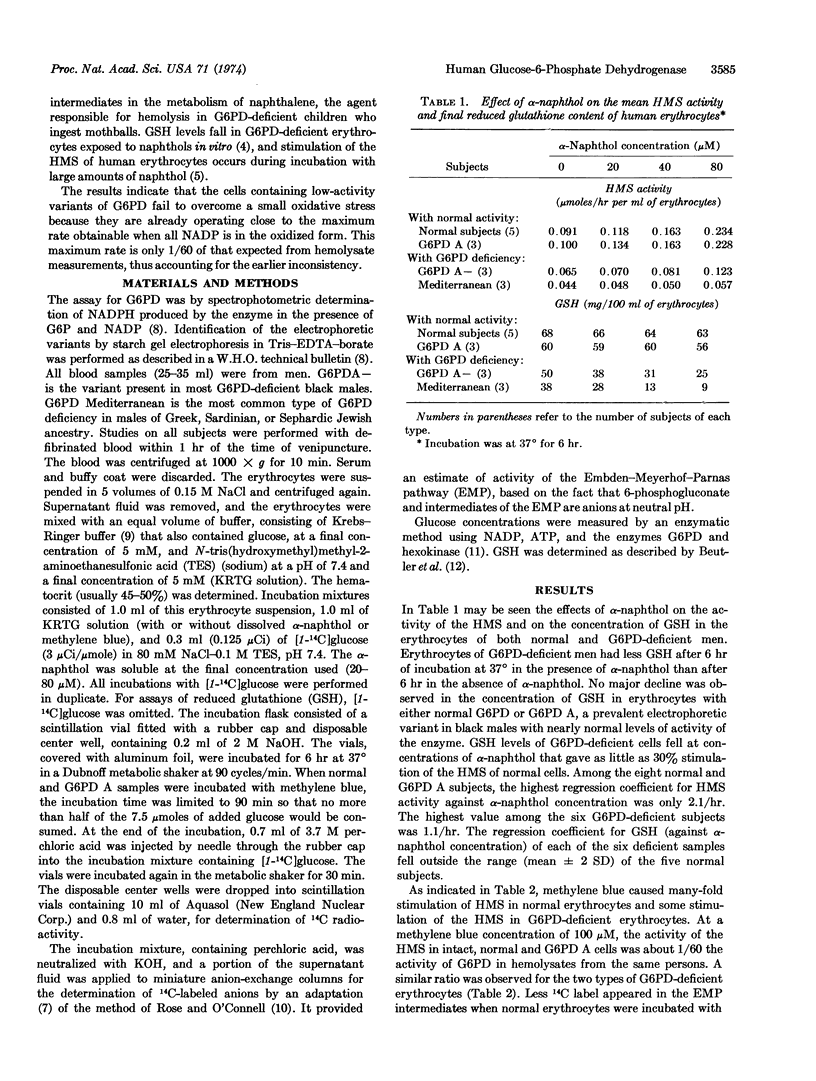
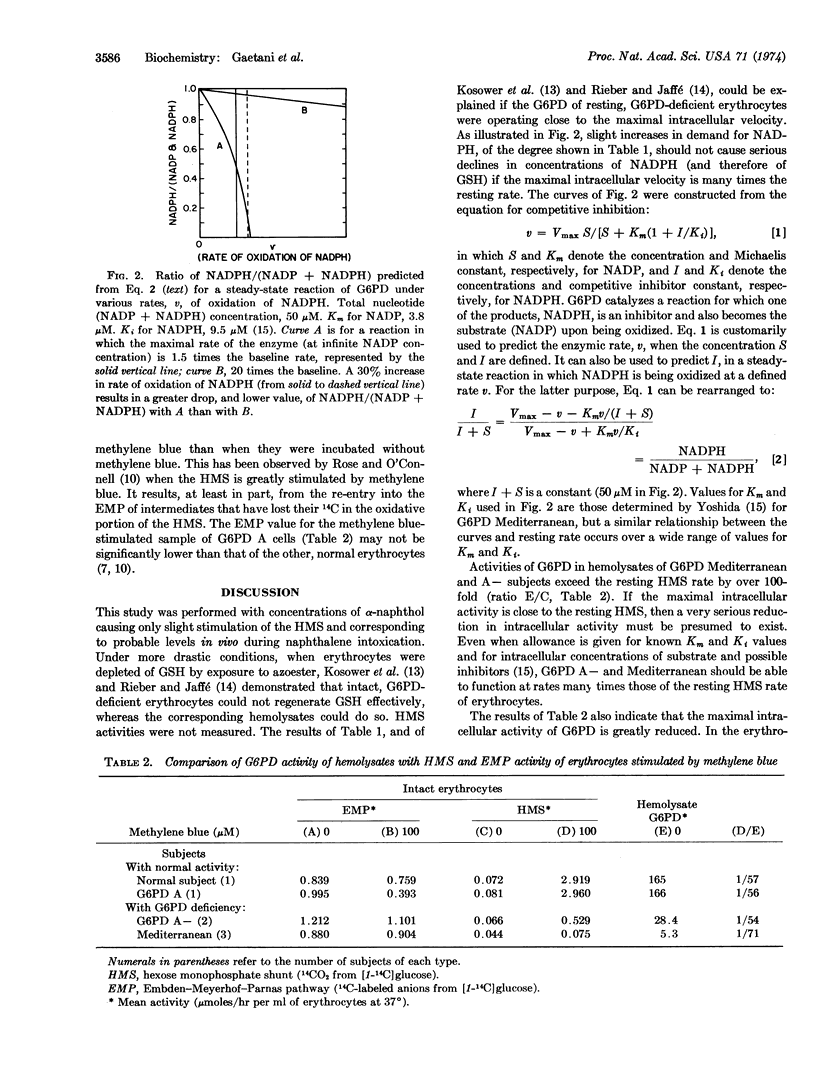
Several mechanisms recently proposed for regulation of the hexose monophosphate shunt require the concentration of NADP to be low or that of NADPH to be high. The present study indicates that the first enzyme of the hexose monophosphate shunt of human erythrocytes is under severe restraint even when these conditions do not exist. In human erythrocytes containing low-activity variants of this enzyme, glucose-6-phosphate dehydrogenase (D-glucose-6-phosphate:NADP+ 1-oxidoreductase; EC 1.1.1.49), measurements of the rate of oxidation of C-1 labeled glucose show that the enzyme is operating at a rate much closer to its maximum than in normal cells. This requires that the ratio of inhibitory NADPH to NADP be much lower in the variant cells than in normal cells. A small increase in oxidative rate, induced by naphthol, then causes a disappearance in reduced glutathione in the variant cells, presumably because a significant further decrease in NADPH occurs in these cells, whereas the same oxidative stress in normal cells would not lower the NADPH level appreciably. A low NADPH/NADP ratio in unstressed cells deficient in glucose-6-phosphate dehydrogenase is confirmed by direct measurement. The maximum activity of the variant enzyme in the cell, as measured with methylene blue to keep most of the NADP in the oxidized form, is only about 1/60 of that found in hemolysates, thus accounting for the failure to compensate for a relatively small oxidative stress in vivo in spite of an apparent sufficiency of enzyme. The reason for the limitation on maximum intracellular activity is unknown. A similar limitation is seen with normal cells incubated with methylene blue, where the maximum intracellular rate is also only about 1/60 of that found in hemolysates.
Keywords: glucose-6-phosphate dehydrogenase deficiency, hexose monophosphate shunt, NADPH
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEUTLER E., DURON O., KELLY B. M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888. [PubMed] [Google Scholar]
- BEUTLER E. The glutathione instability of drug-sensitive red cells; a new method for the in vitro detection of drug sensitivity. J Lab Clin Med. 1957 Jan;49(1):84–95. [PubMed] [Google Scholar]
- BRIN M., YONEMOTO R. H. Stimulation of the glucose oxidative pathway in human erythrocytes by methylene blue. J Biol Chem. 1958 Jan;230(1):307–317. [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GENERATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES BY HEMOLYTIC AGENTS. Biochemistry. 1964 Jul;3:895–900. doi: 10.1021/bi00895a006. [DOI] [PubMed] [Google Scholar]
- Davidson W. D., Tanaka K. R. Factors affecting pentose phosphate pathway activity in human red cells. Br J Haematol. 1972 Sep;23(3):371–385. doi: 10.1111/j.1365-2141.1972.tb08884.x. [DOI] [PubMed] [Google Scholar]
- Eggleston L. V., Krebs H. A. Regulation of the pentose phosphate cycle. Biochem J. 1974 Mar;138(3):425–435. doi: 10.1042/bj1380425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumaa K. A., McLean P. The pentose phosphate pathway of glucose metabolism. Enzyme profiles and transient and steady-state content of intermediates of alternative pathways of glucose metabolism in Krebs ascites cells. Biochem J. 1969 Dec;115(5):1009–1029. doi: 10.1042/bj1151009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower N. S., Vanderhoff G. A., London I. M. The regeneration of reduced glutathione in normal and glucose-6-phosphate dehydrogenase deficient human red blood cells. Blood. 1967 Mar;29(3):313–319. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Omachi A., Scott C. B., Hegarty H. Pyridine nucleotides in human erythrocytes in different metabolic states. Biochim Biophys Acta. 1969 Jun 17;184(1):139–147. doi: 10.1016/0304-4165(69)90108-1. [DOI] [PubMed] [Google Scholar]
- ROSE I. A., O'CONNELL E. L. THE ROLE OF GLUCOSE 6-PHOSPHATE IN THE REGULATION OF GLUCOSE METABOLISM IN HUMAN ERYTHROCYTES. J Biol Chem. 1964 Jan;239:12–17. [PubMed] [Google Scholar]
- Rattazzi M. C., Corash L. M., van Zanen G. E., Jaffé E. R., Piomelli S. G6PD deficiency and chronic hemolysis: four new mutants--relationships between clinical syndrome and enzyme kinetics. Blood. 1971 Aug;38(2):205–218. [PubMed] [Google Scholar]
- Rieber E. E., Jaffé E. R. Reduction of oxidized glutathione in normal and glucose-6-phosphate dehydrogenase deficient erythrocytes and their hemolysates. Blood. 1970 Feb;35(2):166–172. [PubMed] [Google Scholar]
- SZEINBERG A., MARKS P. A. Substances stimulating glucose catabolism by the oxidative reactions of the pentose phosphate pathway in human erythrocytes. J Clin Invest. 1961 Jun;40:914–924. doi: 10.1172/JCI104330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapag-Hagar M., Lagunas R., Sols A. Apparent unbalance between the activities of 6-phosphogluconate and glucose-6-phosphate dehydrogenases in rat liver. Biochem Biophys Res Commun. 1973 Jan 4;50(1):179–185. doi: 10.1016/0006-291x(73)91080-2. [DOI] [PubMed] [Google Scholar]
- Welt S. I., Jackson E. H., Kirkman H. N., Parker J. C. The effects of certain drugs on the hexose monophosphate shunt of human red cells. Ann N Y Acad Sci. 1971 Jul 6;179:625–635. doi: 10.1111/j.1749-6632.1971.tb46938.x. [DOI] [PubMed] [Google Scholar]
- Yoshida A. Hemolytic anemia and G6PD deficiency. Science. 1973 Feb 9;179(4073):532–537. doi: 10.1126/science.179.4073.532. [DOI] [PubMed] [Google Scholar]



