Abstract
About 5% of patients admitted to acute-care hospitals acquire nosocomial infections. A variety of factors contribute: increasing age of patients; availability, for treatment of formerly untreatable diseases, of extensive surgical and intensive medical therapies; and frequent use of antimicrobial drugs capable of selecting a resistant microbial flora. Nosocomial infections due to resistant organisms have been a problem ever since infections due to penicillinase-producing Staphylococcus aureus were noted within a few years of the introduction of penicillin. By the 1960s aerobic Gram-negative bacilli had assumed increasing importance as nosocomial pathogens, and many strains were resistant to available antimicrobials. During the 1980s the principal organisms causing nosocomial bloodstream infections were coagulase-negative staphylococci, aerobic Gram-negative bacilli, S. aureus, Candida spp., and Enterococcus spp. Coagulase-negative staphylococci and S. aureus are often methicillin-resistant, requiring parenteral use of vancomycin. Prevalence of vancomycin resistance among enterococcal isolates from patients in intensive care units has increased, likely due to increased use of this drug. Plasmid-mediated gentamicin resistance in up to 50% of enterococcal isolates, along with enhanced penicillin resistance in some strains, leaves few therapeutic options. The emergence of Enterobacteriaceae with chromosomal or plasmid-encoded extended spectrum beta-lactamases presents a world-wide problem of resistance to third generation cephalosporins. Control of these infections rests on (i) monitoring infections with such resistant organisms in an ongoing fashion, (ii) prompt institution of barrier precautions when infected or colonized patients are identified, and (iii) appropriate use of antimicrobials through implementation of antibiotic control programs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur M., Courvalin P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother. 1993 Aug;37(8):1563–1571. doi: 10.1128/aac.37.8.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. N., Emori T. G., Culver D. H., Gaynes R. P., Jarvis W. R., Horan T., Edwards J. R., Tolson J., Henderson T., Martone W. J. Secular trends in nosocomial primary bloodstream infections in the United States, 1980-1989. National Nosocomial Infections Surveillance System. Am J Med. 1991 Sep 16;91(3B):86S–89S. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- Bortin M. M., Horowitz M. M., Rimm A. A. Increasing utilization of allogeneic bone marrow transplantation. Results of the 1988-1990 survey. Ann Intern Med. 1992 Mar 15;116(6):505–512. doi: 10.7326/0003-4819-116-6-505. [DOI] [PubMed] [Google Scholar]
- Boyce J. M. Patterns of methicillin-resistant Staphylococcus aureus prevalence. Infect Control Hosp Epidemiol. 1991 Feb;12(2):79–82. doi: 10.1086/646290. [DOI] [PubMed] [Google Scholar]
- Burchall J. J., Elwell L. P., Fling M. E. Molecular mechanisms of resistance to trimethoprim. Rev Infect Dis. 1982 Mar-Apr;4(2):246–254. doi: 10.1093/clinids/4.2.246. [DOI] [PubMed] [Google Scholar]
- Carlisle P. S., Gucalp R., Wiernik P. H. Nosocomial infections in neutropenic cancer patients. Infect Control Hosp Epidemiol. 1993 Jun;14(6):320–324. doi: 10.1086/646750. [DOI] [PubMed] [Google Scholar]
- Cookson B. D., Phillips I. Epidemic methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1988 Apr;21 (Suppl 100):57–65. doi: 10.1093/jac/21.suppl_c.57. [DOI] [PubMed] [Google Scholar]
- Emori T. G., Banerjee S. N., Culver D. H., Gaynes R. P., Horan T. C., Edwards J. R., Jarvis W. R., Tolson J. S., Henderson T. S., Martone W. J. Nosocomial infections in elderly patients in the United States, 1986-1990. National Nosocomial Infections Surveillance System. Am J Med. 1991 Sep 16;91(3B):289S–293S. doi: 10.1016/0002-9343(91)90384-a. [DOI] [PubMed] [Google Scholar]
- FINLAND M. Changing patterns of resistance of certain pathogenic bacteria to antimicrobial agents. N Engl J Med. 1955 Apr 7;252(14):570–580. doi: 10.1056/NEJM195504072521404. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana R., Amalfitano G., Rossi L., Satta G. Mechanisms of resistance to growth inhibition and killing by beta-lactam antibiotics in enterococci. Clin Infect Dis. 1992 Sep;15(3):486–489. doi: 10.1093/clind/15.3.486. [DOI] [PubMed] [Google Scholar]
- Galetto D. W., Johnston J. L., Archer G. L. Molecular epidemiology of trimethoprim resistance among coagulase-negative staphylococci. Antimicrob Agents Chemother. 1987 Nov;31(11):1683–1688. doi: 10.1128/aac.31.11.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley R. W., Culver D. H., White J. W., Morgan W. M., Emori T. G. The nationwide nosocomial infection rate. A new need for vital statistics. Am J Epidemiol. 1985 Feb;121(2):159–167. doi: 10.1093/oxfordjournals.aje.a113988. [DOI] [PubMed] [Google Scholar]
- Handwerger S., Pucci M. J., Volk K. J., Liu J., Lee M. S. The cytoplasmic peptidoglycan precursor of vancomycin-resistant Enterococcus faecalis terminates in lactate. J Bacteriol. 1992 Sep;174(18):5982–5984. doi: 10.1128/jb.174.18.5982-5984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan T. C., Culver D. H., Gaynes R. P., Jarvis W. R., Edwards J. R., Reid C. R. Nosocomial infections in surgical patients in the United States, January 1986-June 1992. National Nosocomial Infections Surveillance (NNIS) System. Infect Control Hosp Epidemiol. 1993 Feb;14(2):73–80. doi: 10.1086/646686. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bieth G., Delbos F. High-level, plasmid-borne resistance to gentamicin in Streptococcus faecalis subsp. zymogenes. Antimicrob Agents Chemother. 1979 Nov;16(5):686–689. doi: 10.1128/aac.16.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Medeiros A. A. More extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1991 Sep;35(9):1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawetz E., Gunnison J. B., Coleman V. R. The Combined Action of Penicillin with Streptomycin or Chloromycetin on Enterococci in Vitro. Science. 1950 Mar 10;111(2880):254–256. doi: 10.1126/science.111.2880.254. [DOI] [PubMed] [Google Scholar]
- Karanfil L. V., Murphy M., Josephson A., Gaynes R., Mandel L., Hill B. C., Swenson J. M. A cluster of vancomycin-resistant Enterococcus faecium in an intensive care unit. Infect Control Hosp Epidemiol. 1992 Apr;13(4):195–200. doi: 10.1086/646509. [DOI] [PubMed] [Google Scholar]
- Kayser F. H. Methicillin-resistant staphylococci 1965-75. Lancet. 1975 Oct 4;2(7936):650–653. doi: 10.1016/s0140-6736(75)90129-4. [DOI] [PubMed] [Google Scholar]
- Livornese L. L., Jr, Dias S., Samel C., Romanowski B., Taylor S., May P., Pitsakis P., Woods G., Kaye D., Levison M. E. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med. 1992 Jul 15;117(2):112–116. doi: 10.7326/0003-4819-117-2-112. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987 Mar;51(1):88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
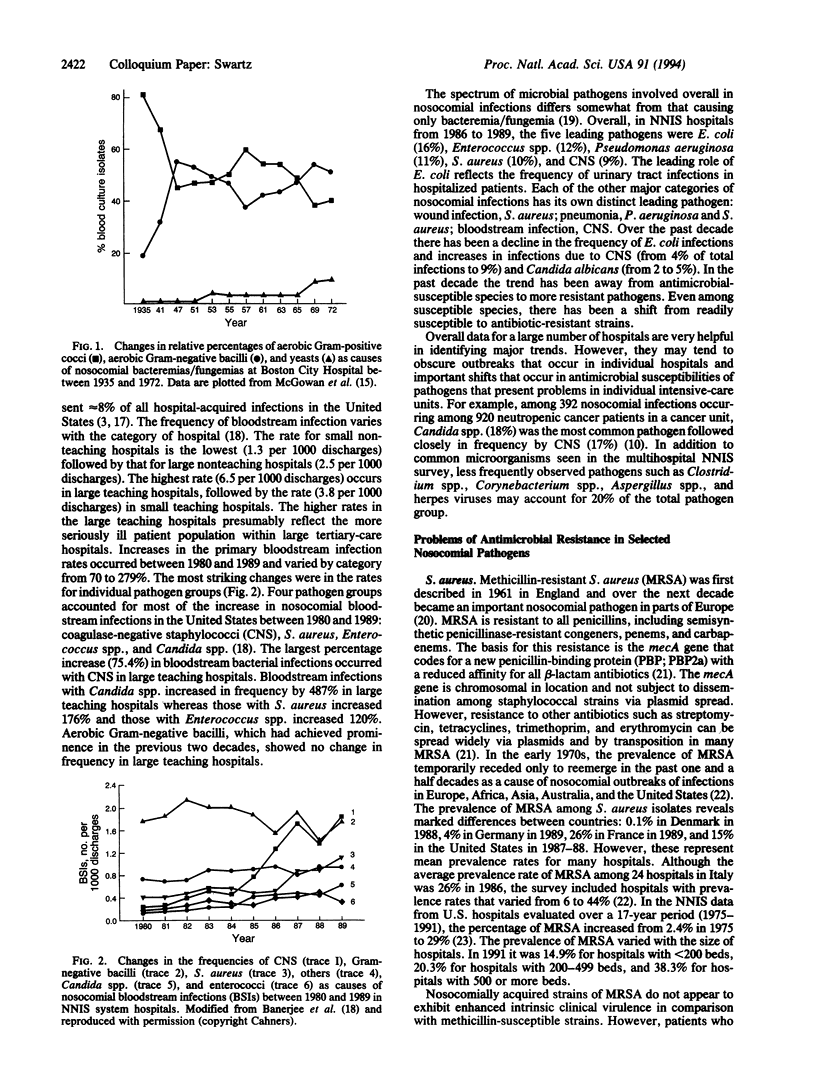
- McGowan J. E., Jr, Barnes M. W., Finland M. Bacteremia at Boston City Hospital: Occurrence and mortality during 12 selected years (1935-1972), with special reference to hospital-acquired cases. J Infect Dis. 1975 Sep;132(3):316–335. doi: 10.1093/infdis/132.3.316. [DOI] [PubMed] [Google Scholar]
- McGowan J. E., Jr Changing etiology of nosocomial bacteremia and fungemia and other hospital-acquired infections. Rev Infect Dis. 1985 Jul-Aug;7 (Suppl 3):S357–S370. doi: 10.1093/clinids/7.supplement_3.s357. [DOI] [PubMed] [Google Scholar]
- McMurry L. M., Park B. H., Burdett V., Levy S. B. Energy-dependent efflux mediated by class L (tetL) tetracycline resistance determinant from streptococci. Antimicrob Agents Chemother. 1987 Oct;31(10):1648–1650. doi: 10.1128/aac.31.10.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. S., Urban C., Eagan J. A., Berger B. J., Rahal J. J. Nosocomial outbreak of Klebsiella infection resistant to late-generation cephalosporins. Ann Intern Med. 1993 Sep 1;119(5):353–358. doi: 10.7326/0003-4819-119-5-199309010-00001. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr The Garrod Lecture. The enterococcus: a classic example of the impact of antimicrobial resistance on therapeutic options. J Antimicrob Chemother. 1991 Jul;28(1):1–12. doi: 10.1093/jac/28.1.1. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Weinberg A. N. Studies on antibiotic syngerism against enterococci. II. Effect of various antibiotics on the uptake of 14 C-labeled streptomycin by enterococci. J Clin Invest. 1971 Dec;50(12):2580–2584. doi: 10.1172/JCI106758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muder R. R., Brennen C., Wagener M. M., Vickers R. M., Rihs J. D., Hancock G. A., Yee Y. C., Miller J. M., Yu V. L. Methicillin-resistant staphylococcal colonization and infection in a long-term care facility. Ann Intern Med. 1991 Jan 15;114(2):107–112. doi: 10.7326/0003-4819-114-2-1-107. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Quinolone antimicrobial agents. Annu Rev Med. 1992;43:465–486. doi: 10.1146/annurev.me.43.020192.002341. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The crisis in antibiotic resistance. Science. 1992 Aug 21;257(5073):1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- Noble W. C., Virani Z., Cree R. G. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol Lett. 1992 Jun 1;72(2):195–198. doi: 10.1016/0378-1097(92)90528-v. [DOI] [PubMed] [Google Scholar]
- Nosocomial infection rates for interhospital comparison: limitations and possible solutions. A Report from the National Nosocomial Infections Surveillance (NNIS) System. Infect Control Hosp Epidemiol. 1991 Oct;12(10):609–621. [PubMed] [Google Scholar]
- Pallares R., Dick R., Wenzel R. P., Adams J. R., Nettleman M. D. Trends in antimicrobial utilization at a tertiary teaching hospital during a 15-year period (1978-1992). Infect Control Hosp Epidemiol. 1993 Jul;14(7):376–382. doi: 10.1086/646765. [DOI] [PubMed] [Google Scholar]
- Panlilio A. L., Culver D. H., Gaynes R. P., Banerjee S., Henderson T. S., Tolson J. S., Martone W. J. Methicillin-resistant Staphylococcus aureus in U.S. hospitals, 1975-1991. Infect Control Hosp Epidemiol. 1992 Oct;13(10):582–586. doi: 10.1086/646432. [DOI] [PubMed] [Google Scholar]
- Peters G. New considerations in the pathogenesis of coagulase-negative staphylococcal foreign body infections. J Antimicrob Chemother. 1988 Apr;21 (Suppl 100):139–148. doi: 10.1093/jac/21.suppl_c.139. [DOI] [PubMed] [Google Scholar]
- Philippon A., Labia R., Jacoby G. Extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1989 Aug;33(8):1131–1136. doi: 10.1128/aac.33.8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice L. B., Eliopoulos G. M., Wennersten C., Goldmann D., Jacoby G. A., Moellering R. C., Jr Chromosomally mediated beta-lactamase production and gentamicin resistance in Enterococcus faecalis. Antimicrob Agents Chemother. 1991 Feb;35(2):272–276. doi: 10.1128/aac.35.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosdahl V. T., Knudsen A. M. The decline of methicillin resistance among Danish Staphylococcus aureus strains. Infect Control Hosp Epidemiol. 1991 Feb;12(2):83–88. doi: 10.1086/646291. [DOI] [PubMed] [Google Scholar]
- Sanders C. C. Chromosomal cephalosporinases responsible for multiple resistance to newer beta-lactam antibiotics. Annu Rev Microbiol. 1987;41:573–593. doi: 10.1146/annurev.mi.41.100187.003041. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr beta-Lactam resistance in gram-negative bacteria: global trends and clinical impact. Clin Infect Dis. 1992 Nov;15(5):824–839. doi: 10.1093/clind/15.5.824. [DOI] [PubMed] [Google Scholar]
- Schaberg D. R., Culver D. H., Gaynes R. P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991 Sep 16;91(3B):72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- Scheckler W. E., Scheibel W., Kresge D. Temporal trends in septicemia in a community hospital. Am J Med. 1991 Sep 16;91(3B):90S–94S. doi: 10.1016/0002-9343(91)90350-7. [DOI] [PubMed] [Google Scholar]
- Schwalbe R. S., Stapleton J. T., Gilligan P. H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987 Apr 9;316(15):927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- Trucksis M., Wolfson J. S., Hooper D. C. A novel locus conferring fluoroquinolone resistance in Staphylococcus aureus. J Bacteriol. 1991 Sep;173(18):5854–5860. doi: 10.1128/jb.173.18.5854-5860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban C., Go E., Mariano N., Berger B. J., Avraham I., Rubin D., Rahal J. J. Effect of sulbactam on infections caused by imipenem-resistant Acinetobacter calcoaceticus biotype anitratus. J Infect Dis. 1993 Feb;167(2):448–451. doi: 10.1093/infdis/167.2.448. [DOI] [PubMed] [Google Scholar]
- Walsh C. T. Vancomycin resistance: decoding the molecular logic. Science. 1993 Jul 16;261(5119):308–309. doi: 10.1126/science.8392747. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Siddhikol C., Lai C. J., Demohn V. Erythromycin-inducible resistance in Staphylococcus aureus: requirements for induction. J Bacteriol. 1971 Jun;106(3):835–847. doi: 10.1128/jb.106.3.835-847.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells V. D., Wong E. S., Murray B. E., Coudron P. E., Williams D. S., Markowitz S. M. Infections due to beta-lactamase-producing, high-level gentamicin-resistant Enterococcus faecalis. Ann Intern Med. 1992 Feb 15;116(4):285–292. doi: 10.7326/0003-4819-116-4-285. [DOI] [PubMed] [Google Scholar]
- Westh H., Jarløv J. O., Kjersem H., Rosdahl V. T. The disappearance of multiresistant Staphylococcus aureus in Denmark: changes in strains of the 83A complex between 1969 and 1989. Clin Infect Dis. 1992 Jun;14(6):1186–1194. doi: 10.1093/clinids/14.6.1186. [DOI] [PubMed] [Google Scholar]




