Abstract
Introduction
Organophosphate (OP) pesticides remain widely used in agriculture. Previously, we reported that PON1 genotype was directly associated with neurodevelopment at age two, and that PON1 genotype may increase susceptibility to OP exposure.
Objectives
We examined the relationships of maternal and child PON1 genotype and enzyme activity levels and neurodevelopment at school age and examined their interaction with maternal dialkyl phosphate (DAP) metabolite levels to investigate differential susceptibility to OP-related neurotoxicity.
Methods
Participants were from the CHAMACOS longitudinal birth cohort of Latino families in an agricultural region of California. We measured DAP metabolites of OP pesticides in maternal and child urine samples, and analyzed PON1192 and PON1−108 genotypes and enzyme activity [arylesterase (ARYase), paraoxonase (POase)] in maternal and child blood. We examined their association with children’s performance on the Conners’ Kiddie Continuous Performance Test (K-CPT) at 5 years (n=296) and the Wechsler Intelligence Scale for Children (WISC-IV) at 7 years (n=327).
Results
Maternal and child PON1 genotype was not related to performance on K-CPT or WISC, although WISC scores tended to be lowest in children and children of mothers who carried the PON−108TT genotype. Pregnancy ARYase levels were positively associated with all WISC subscales (e.g., 4.0 point increase in Full Scale IQ per standard deviation increase in ARYase, 95% CI = 1.6, 6.4), while pregnancy POase levels were positively associated with WISC Processing Speed only. Maternal PON1−108 weakly modified the relationship of maternal DAPS and K-CPT scores (pinteraction = 0.21) and WISC verbal IQ (pinteraction = 0.71). The association between DAPs and Full-Scale IQ was strongest for children of mothers with lowest-tertile ARYase levels (pinteraction = 0.27). This relationship held for both diethyl and dimethyl DAPs and for all subscales of the WISC.
Conclusions
We extend our previous findings that PON1 genotype and enzyme levels may be directly related to performance on certain domains of neurodevelopment in school-age children. Lower maternal PON1 enzyme levels during pregnancy may also increase susceptibility of children to neurotoxicity from OP pesticide exposure.
Keywords: Attention; Bilingual; Conners’ Continuous Performance Test (CPT, KCPT, K-CPT); DAPs; Farmworker; Genotype; Mexican Americans; Neurodevelopment; Organophosphates; Pesticides; PON1; Wechsler Intelligence Scale for Children (WISC); Paraoxonase; Arylesterase
1. Introduction
Organophosphate (OP) pesticides are widely used in agriculture and until the early 2000 were frequently used for household pest control in the United States (U.S.) (Williams et al., 2008). National biomonitoring efforts indicate that OP pesticide exposure, as measured by the presence of dialkyl phosphate (DAP) metabolites in urine, is nearly ubiquitous in the U.S. population (Barr et al., 2004). Results of exposure modeling (McKone et al., 2007) and organic diet replacement studies (Lu et al., 2008) suggest that diet is a key exposure pathway for adults and children alike.
Two factors that may influence human susceptibility to adverse effects of OP pesticide exposure are genetics and age at time of exposure. The paraoxonase 1 (PON1) enzyme plays an important role in the detoxification and elimination of several oxon derivatives of OP pesticides, thereby preventing the inhibition of acetylcholinesterase (James, 2006; Li et al., 2003). Polymorphisms in the coding (e.g., PON1192) and promoter (e.g., PON1−108) regions of the PON1 gene have demonstrated particular influence on the production of this detoxifying enzyme. Specifically, individuals with PON1−108TT genotype have lower levels of detoxifying enzyme activity than those with PON1−108CT or PON1−108CC genotypes, suggesting that the CC genotype is the most protective and TT the most vulnerable genotype with regards to susceptibility to OP pesticides (Huen et al., 2010). Similarly, evidence suggests that PON1192RR genotype may exert protective influence, whereas QQ genotype may underlie heightened vulnerability to OP pesticides (Huen et al., 2010).
Developmental immaturity may also heighten susceptibility to OP pesticide exposures. Exposures to OP pesticides occur in utero as a consequence of ready passage through the placenta and blood-brain barrier (Bradman et al., 2003; Whyatt et al., 2003). In infancy and early childhood, exposure may come through breastmilk (Weldon, 2010), diet, ambient or airborne exposures, or hand-to-mouth activity following contact with contaminated surfaces or house dust (Zartarian et al., 2000). Early life exposure may be particularly detrimental given the rapid and formative brain development occurring during these periods. In addition, children do not have adult level of enzymes to detoxify OP pesticides until after age 7 (Huen et al., 2009a).
We have previously demonstrated associations between women’s pregnancy OP pesticide exposure as measured by urinary dialkyl phosphate metabolites (DAPs) and their children’s performance on the Bayley Scales of Infant Development at age 2 years (Eskenazi et al., 2007), on measures of attention at age 5 (Marks et al., 2010), and on the Wechsler Intelligence Scale for Children at age 7 (Bouchard et al., 2011). We have also presented evidence that the adverse association between maternal DAPs and Bayley mental development was strongest in children with PON1−108TT genotype (Eskenazi et al., 2010). The purpose of the current study is to follow up on our previous research by assessing the potential modifying effects of PON1 genotype and enzyme activity on associations between maternal DAP levels during pregnancy and children’s cognitive function and attention at early school age.
2. Materials and methods
2.1. Participants and recruitment
CHAMACOS, which is the acronym for The Center for the Health Assessment of Mothers and Children of Salinas, is a longitudinal birth cohort study aimed at studying the association of pesticides and other environmental exposures with children’s health and development. Participants in CHAMACOS are primarily Mexican–American immigrant families residing in the Salinas Valley, an agricultural region of Monterey County, California. Methods for the CHAMACOS study have been described elsewhere (Eskenazi et al., 2004, 2006). Briefly, 1800 pregnant women were screened for eligibility between October 1999 and 2000 at community clinics serving primarily farmworker families. Eligible women were ≥ 18 years old, < 20 weeks gestation, Spanish- or English-speaking, eligible for Medi-Cal, receiving prenatal care, and planning to deliver at Natividad Medical Center. Of 1130 eligible women, 601 enrolled in the study. We followed 531 of these women to delivery of a liveborn, surviving neonate, and have completed follow-up study visits with families at child ages 6 months and 1, 2, 3.5, 5, and 7 years. All mothers provided written informed consent to participate and, beginning at age seven, children provided verbal assent. The Institutional Review Board at University of California, Berkeley approved this study.
Included in the current analysis are 343 families who participated in either or both of the 5- and 7-year visits for whom PON1 genotype and/or enzyme activity data were available. We excluded from the present analysis 6 twins and 1 child with autism. PON1 genotypes and prenatal enzyme activity and DAP concentrations did not differ significantly between individuals included in this analysis and those lost to follow-up. Compared to mothers lost to follow-up, mothers in this analysis were slightly older at enrollment, were more likely to have lived in the U.S. for 6–10 years at time of enrollment than either less or more time, and were less likely to have smoked during pregnancy. Children in this analysis were less likely to have been born preterm or of low birthweight than their peers who were lost to follow up, but were raised in households with slightly lower home stimulation scores at age 6 months.
2.2. Maternal interviews
Women were interviewed twice during pregnancy (mean = 14.0 and 26.6 weeks gestation), shortly after delivery, and at every postnatal visit. Interviews were conducted in Spanish or English by bilingual, bicultural interviewers. At the 6-month postnatal visit, mothers were administered either the Peabody Picture Vocabulary Test (PPVT) (Dunn and Dunn, 1981) or its Spanish equivalent, the Test de Vocabulario en Imagenes Peabody (TVIP) (Dunn et al., 1986), as an indicator of maternal scholastic abilities and general intelligence. At 1, 3.5, and 7 year visits, mothers completed the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977). The Home Observation for Measurement of the Environment (HOME) (Caldwell and Bradley, 1984) was completed at 6 months and 1, 2, 3.5, and 7 years; at ages 3.5 and 7, the short form of the HOME scale (HOME-SF) was used (Sugland et al., 1995). Data on family structure and household income were collected at every visit, on child’s daycare or school attendance at every postnatal visit, and on children’s video game usage at ages 5 and 7 years. Child birthweight was abstracted from medical records by a registered nurse.
2.3. Neurodevelopmental outcomes
Neurodevelopmental assessments were conducted by bilingual, bicultural psychometricians, who were trained and supervised for quality assurance by a clinical neuropsychologist; all were blind to children’s pesticide exposures and PON1 genotype or enzyme levels. Assessments were performed in a private room at the CHAMACOS research office or in a recreational vehicle outfitted as a mobile testing facility. We have focused our analyses on two neurodevelopmental outcomes, which we previously found to be related to maternal DAPs, namely, attention at age 5 years and cognition at age 7 years (Marks et al., 2010; Bouchard et al., 2011).
Attention
Children’s attention was evaluated at age 5 years using the Conners’ Continuous Performance Test—Kiddie Version (K-CPT) (Conners and Staff, 2001), a 7.5-minute computerized vigilance task designed for pre-literate children. The K-CPT presents children with images of familiar objects, which flash briefly and in random succession on the computer screen. Children are instructed to respond to these images by pressing the spacebar as quickly as possible for all images except the ball, and the computer records variables such as response time and errors of omission and commission. For the present analysis, we used the summary Attentional Deficit and Hyperactivity Disorder (ADHD) Confidence Index score, which reflects the degree to which a child’s overall pattern of performance corresponds to that of a same-age, same-sex child clinically diagnosed with ADHD (theoretical range = 0– 100%; 70% would indicate that 70 of 100 children with that performance pattern would be clinically diagnosed as having ADHD).
Cognition
At age 7, children’s cognition was assessed in English or Spanish using the Wechsler Intelligence Scale for Children—Fourth Edition (WISC-IV) (Wechsler, 2003). Eight subtests were administered, yielding age-standardized Perceptual Reasoning Index (PRI), Verbal Comprehension Index (VCI), Working Memory Index (WMI), Processing Speed Index (PSI), and Full Scale Intelligence Quotient (FSIQ) scores (mean 100, SD 15 for each).
2.4. Pesticide exposure measurement
We measured six non-specific DAP metabolites in maternal urine collected twice during pregnancy, typically at the time of the prenatal interviews. Urine specimens were immediately aliquotted and stored at −80 °C until shipment on dry ice to the Centers for Disease Control and Prevention. DAP metabolites were measured using gas chromatography-tandem mass spectrometry and quantified using isotope dilution calibration (Bravo et al., 2002). These DAP metabolites were comprised of three dimethyl (DM) phosphate metabolites (dimethylphosphate, dimethylthiophosphate, dimethyldithiophosphate) and three diethyl (DE) phosphate metabolites (diethylphosphate, diethylthiophosphate, and diethyldithiophosphate) (Bradman et al., 2005). Details of urine collection, analysis, and quality control procedures, including detection limits and use of blanks and spikes, have been described elsewhere (Bradman et al., 2005).
Concentrations below the limit of detection (LOD) were randomly imputed based on a log-normal probability distribution, estimated using maximum likelihood estimation, and molar concentrations were summed to yield total DM, total DE, and total DAP concentrations (nmoles/L).
2.5. PON1 genotype and enzyme measurements
We collected maternal blood during pregnancy (mean 26.7, SD ± 2.8 weeks), fetal umbilical cord blood, and child blood samples at 5 years of age. Heparinized whole blood was collected in BD vacutainers® (Becton, Dickinson and Company, Franklin Lakes, NJ), centrifuged, divided into plasma, buffy coats and red blood cells, and stored at −80 °C. Serum and blood clots were collected in vacutainers containing no anticoagulant.
Maternal and child genotyping was conducted using DNA isolated from blood clots as previously described (Holland et al., 2006). The coding polymorphism, PON1192, was genotyped using the Taqman real-time PCR method (Eskenazi et al., 2010; Holland et al., 2006). We used a two-part nested PCR strategy to genotype the promoter SNP PON1−108. The region surrounding the SNP was pre-amplified using non-allelic flanking primers and then the amplicon was diluted and used as the template for the subsequent Amplifluor assay.
PON1 enzyme activity levels were measured in heparinized plasma. The PON1 enzyme activities towards phenyl acetate (ARYase) and paraoxon (POase) were determined using spectrophotometric methods as described by Huen et al. (2009b). The ARYase assay provides an indirect measure of PON1 enzyme quantity that is strongly correlated with other ELISA and Western blot-based methods of PON1 quantitation (Connelly et al., 2008; Kujiraoka et al., 2000) and is genetically influenced by the promoter polymorphism PON1−108. In contrast, the measure of POase from the substrate-specific assay is reflective both of quantity and catalytic efficiency of the PON1 enzyme and is primarily affected by the coding polymorphism PON1192. Quality assurance of genotype and enzyme activity data are detailed elsewhere (Huen et al., 2009b).
2.6. Data analysis
We first determined whether PON1 genotype (maternal and child) or enzyme activity (maternal, cord, and child) had a direct association with the neurodevelopmental outcomes of interest, controlling for maternal DAP levels and other covariates. We then examined the effect modification of PON1 genotype and enzyme activity on the previously reported (Marks et al., 2010; Bouchard et al., 2011) relationship between exposure as measured by maternal DAPs and neurodevelopment (attention and cognition).
Total DAP, DM, and DE metabolite concentrations, including mean pregnancy concentrations, were transformed to the log10 scale and analyzed as continuous variables. PON1192 and PON1−108 genotypes were analyzed primarily as indicator variables, with the non-susceptible genotypes (e.g., PON1192RR and PON1−108CC) as the respective reference groups. In separate analyses, PON1 genotypes were analyzed as discrete numerical variables reflecting the number of susceptible alleles (0, 1, 2). PON1 enzyme activity was also analyzed primarily as indicator variables, with ARYase and POase values at each time point categorized as tertiles and with the lowest tertile as the reference group, though in separate analyses, ARYase and POase were analyzed as continuous values (standard deviation units). K-CPT ADHD Confidence Index percentage scores were analyzed as a censored continuous variables, and WISC outcome variables were analyzed as continuous variables.
To maintain consistency with our previous publications, we controlled for all covariates identified in the papers examining the relation of maternal DAP levels and child attention (Marks et al., 2010) and cognition (Bouchard et al., 2011), though we added one new covariate to attention analyses: video game usage at age 5 years. Thus, age 5 attention (K-CPT) scores were adjusted for psychometrician; child sex and age at testing (continuous months); maternal education at enrollment (categorical), PPVT score at 6 months post-partum (continuous), and CES-D depression status at 3.5 years post-partum (dichotomous; ≥ 16 symptom points versus fewer); and child’s duration of breastfeeding (continuous months), time in childcare between ages 3.5 and 5 (dichotomous; 15+ hours in out-of-home care versus less), and video game usage at age 5 (dichotomous any versus none). For the measures of WISC performance at age 7 years, we controlled for maternal education at enrollment (categorical), maternal PPVT score at 6 months post-partum (continuous), and HOME score at 6 months (continuous), and for VCIQ and FSIQ analyses, we also controlled for language of assessment (dichotomous English versus Spanish). In addition, all analyses assessing ARYase adjusted for the individual’s PON1192 genotype as a comprehensive measure of PON1 status; pregnancy ARYase analyses adjusted for maternal PON1192 genotype while cord blood and child age 5 ARYase analyses adjusted for child PON1192 genotype. PON1 status accounts for both PON1 catalytic efficiency and enzyme quantity and can be more informative than looking at PON1 genotype alone in epidemiologic studies (Richter and Furlong, 1999).
We tested for effect modification by maternal or child PON1 genotype on the association between average prenatal DAP levels and neurodevelopmental outcomes. We also assessed effect modification by maternal and child enzyme levels using maternal enzyme levels and DAPs in specimens collected at ~26 weeks gestation or child enzyme levels in cord blood and average maternal prenatal DAPs. This allowed us to assess “real-time” interactions between individuals’ OP exposure at a given point in time and the enzymes they had available to detoxify these exposures. We constructed separate linear regression models within each genotype or enzyme level category (i.e. low, medium, high tertiles). This allowed for a visual comparison of associations between maternal DAP metabolite levels and neurodevelopmental outcome under different genotype or gene activity conditions. We tested the statistical significance of interactions using both categorical and continuous interaction terms. Interaction was assessed for categorical variables as follows: we constructed linear regression models which each included two interaction terms per model (e.g., ARYaseMedium* DAP and ARYaseHigh* DAP versus the reference, ARYaseLow* DAP), and the interaction p-value was calculated using a post-estimation combined F-test (or chi-square test) for the two interaction variables. To assess interaction based on continuous variables, we constructed parallel linear regression models using discrete numerical genotype (0, 1, 2) or continuous enzyme activity (standard deviation units) and a single continuous interaction term (e.g. ARYaseSD*DAP), and report the p-value for that interaction term. Statistical significance for interactions was based on a p-value of 0.15. Data analyses were completed using Stata version 11.2 software (StataCorp, 2009).
3. Results
At the time of pregnancy enrollment, mothers averaged 26.7 years of age (SD = 5.2) with 86% having been born in Mexico, and 79% having less than a high school education. Almost all lived within 200% of poverty (Table 1). Only 4% of children were born at < 2500 g and 7% were born at < 37 weeks (data not shown). Children were assessed on average (mean ± standard deviation (SD)) at 5.0 ± 0.2 years, and 7.1 ± 0.2 years. Approximately two-thirds of the children were tested in Spanish at age 7 years.
Table 1.
Sociodemographic characteristics of the CHAMACOS cohort (n = 343).
| N | (%) | |
|---|---|---|
| Child’s sex | ||
| Male | 165 | (48.1) |
| Female | 178 | (51.9) |
| Maternal country of birth | ||
| Mexico | 295 | (86.0) |
| U.S. | 43 | (12.5) |
| Other | 5 | (1.5) |
| Maternal age at baseline | ||
| 18-24 | 145 | (42.3) |
| 25–29 | 114 | (33.2) |
| 30–34 | 55 | (16.0) |
| 35–45 | 29 | (8.5) |
| Marital status at baseline | ||
| Married/living as married | 280 | (81.6) |
| Not married | 63 | (18.4) |
| Poverty level during pregnancya | ||
| < Poverty level | 213 | (62.1) |
| Within 200% of poverty level | 118 | (34.4) |
| > 200% of poverty level | 12 | (3.5) |
| Maternal education | ||
| < 6th grade | 152 | (44.3) |
| 7th–12th grade | 119 | (34.7) |
| Completed high school | 72 | (21.0) |
| Maternal intelligence (PPVT Score) | ||
| ≤ 74 | 112 | (32.7) |
| 75–99 | 123 | (35.9) |
| ≥ 100 | 108 | (31.5) |
| HOME score at 6 months | ||
| ≤ 31.0 | 145 | (42.3) |
| 31.1–33.3 | 89 | (26.0) |
| ≥ 33.4 | 109 | (31.8) |
| Language of age 7 WISC testing | ||
| English | 107 | (32.7) |
| Spanish | 220 | (67.3) |
U.S. Census Bureau Year 2000 poverty thresholds based on household income and number of people supported.
The children averaged 45.9% (SD = 17.5) on the Confidence Index of the K-CPT at 5 years, with 8.4% of children scoring above a 70%. The mean WISC Full-Scale IQ was 103.8 (SD = 14.3) at 7 years (Supplementary Table 1). The geometric mean (GM) (95% Confidence Interval, CI) of the average total DAP, DM and DE concentrations in maternal urine during pregnancy was 123.5 nmoles/L (110.3, 238.3), 90.8 nmoles/L (79.7, 103.3) and 18.4 nmoles/L (16.1, 21.0), respectively (Supplementary Table 2). Average prenatal urinary DAP concentrations in this population are higher than general U.S. population levels (Bradman et al., 2005).
We found that 24.6% of children had the PON1192QQ genotype, 23.4% had PON1192RR, and the remainder were heterozygous (see Table 2). For PON1−108, 30.2% had PON1−108CC and 18.6% had PON1−108TT, and the remainder was heterozygous. Supplementary Table 3 presents the descriptive statistics for ARYase and POase levels in the maternal blood, cord blood, and child blood at age 5 years, which confirm our previous reports (Huen et al., 2010) of lowest enzyme activity levels in cord blood, followed by child age 5 and adult maternal blood (ARYase GM = 30.6, 80.5, and 124.5 U/mL, respectively; POase GM = 195.4, 602.5, and 728.9 U/L, respectively). As in previous analyses (Huen et al., 2010), ARYase and POase levels varied widely by genotype; mothers and children with PON1108TT and PON1192RR genotype tended to have the lowest ARYase levels, and mothers and children with PON1108TT but with PON1192QQ genotype had the lowest POase levels (not shown).
Table 2.
Adjusted association between maternal and child genotype and measures of child attention at age 5 on the Kiddie Continuous Performance test (K-CPT) and cognition at age 7 on the Wechsler Intelligence Scale for Children-IV (WISC).
| Genotype | Gene frequencies
|
Age 5
|
Age 7
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | (%) | KCPT ADHD indexa
|
p trendd |
WISC PRIb
|
p trendd |
WISC VCIc
|
p trendd |
WISC WMIb
|
p trendd |
WISC PSIb
|
p trendd |
WISC FSIQc
|
p trendd |
|||||||
| β | (95% CI) | β | (95% CI) | β | (95% CI) | β | (95% CI) | β | (95% CI) | β | (95% CI) | |||||||||
| Mother | ||||||||||||||||||||
| PON1−108 | ||||||||||||||||||||
| CC | 92 | (28.4) | Reference | 0.41 | Reference | 0.69 | Reference | 0.22 | Reference | 0.37 | Reference | 0.32 | Reference | 0.28 | ||||||
| CT | 156 | (48.1) | 2.9 | (−1.9, 7.8) | −2.6 | (−6.8, 1.6) | −0.2 | (−3.8, 3.3) | 0.6 | (−3.1, 4.4) | 0.8 | (−2.9, 4.5) | −0.5 | (−4.3, 3.3) | ||||||
| TT | 76 | (23.5) | 2.1 | (−3.6, 7.9) | −0.8 | (−5.8, 4.2) | −2.8 | (−7.0, 1.5) | − 2.2 | (−6.6, 2.3) | − 2.4 | (−6.8, 2.0) | −2.5 | (−7.0, 1.9) | ||||||
| PON1192 | ||||||||||||||||||||
| RR | 93 | (28.6) | Reference | 0.86 | Reference | 0.65 | Reference | 0.29 | Reference | 0.30 | Reference | 0.72 | Reference | 0.54 | ||||||
| QR | 144 | (44.3) | 2.1 | (−2.9, 7.1) | −2.6 | (−7.0, 1.7) | 0.2 | (−3.5, 3.9) | − 0. 3 | (−4.2,3.6) | − 0.2 | (−4.0, 3.7) | −0.9 | (−4.8, 3.0) | ||||||
| 88 | (27.1) | − 0.5 | (−6.1, 5.1) | 1.0 | (−3.7, 5.8) | 2.1 | (−1.9, 6.2) | 2. 3 | (−2.1,6.6) | − 0.8 | (−5.0, 3.5) | 1.3 | (−3.0, 5.6) | |||||||
| Child | ||||||||||||||||||||
| PON1−108 | ||||||||||||||||||||
| CC | 99 | (30.2) | Reference | 0.88 | Reference | 0.52 | Reference | 0.27 | Reference | 0.94 | Reference | 0.28 | Reference | 0.41 | ||||||
| CT | 168 | (51.2) | − 0.2 | (−4.9, 4.6) | −0.5 | (−4.6, 3.6) | 0.5 | (−3.0, 4.0) | 0. 5 | (−3.1,4.1) | − 2.1 | (−5.6, 1.5) | 0.0 | (−3.7, 3.7) | ||||||
| TT | 61 | (18.6) | 0.6 | (−5.4, 6.5) | −1.8 | (−7.0, 3.4) | −2.9 | (−7.3, 1.5) | − 0.3 | (−4.9, 4.3) | − 2.2 | (−6.8, 2.3) | −2.2 | (−6.8, 2.5) | ||||||
| PON1192 | ||||||||||||||||||||
| RR | 77 | (23.4) | Reference | 0.96 | Reference | 0.21 | Reference | 0.68 | Reference | 0.69 | Reference | 0.36 | Reference | 0.70 | ||||||
| QR | 171 | (52.0) | 5.0 | (0.1, 9.9)* | −4.6 | (−9.0, −0.2)** | 0.8 | (−2.9, 4.6) | − 4.2 | (−8.1, −0.3)** | − 4.8 | (−8.5, −1.0)** | −4.7 | (−8.6, −0.8)** | ||||||
| 81 | (24.6) | 0.1 | (−5.8, 5.9) | −3.4 | (−8.5, 1.7) | −0.9 | (−5.3, 3.5) | 0.7 | (−3.8, 5.3) | 1.9 | (−2.5, 6.2) | −1.0 | (−5.6, 3.6) | |||||||
Adjusted for mean prenatal total DAPs; psychometrician; child sex and age at testing; maternal education, PPVT, and depression; duration of breastfeeding; time in childcare; and video game usage.
Adjusted for mean prenatal total DAPs, maternal education, maternal PPVT, HOME score at 6 months of age, and language of neurological assessment at 7 years of age.
Adjusted for mean prenatal total DAPs, maternal education, maternal PPVT, HOME score at 6 months of age, and language of neurological assessment at 7 years of age.
p-value on trend based on discrete variable representing the number (0, 1, 2) of protective alleles (C for PON108, R for PON192).
p < 0.10.
p < 0.05.
The ARYase and POase levels in maternal blood (r = 0.26, p < 0.001), cord blood (r = 0.59, p < 0.001) and 5-year old blood (r = 0.23, p < 0.001) were weakly to moderately positively correlated (Supplementary Table 4). ARYase levels in cord and in 5-year blood (r = 0.52, p < 0.001) and POase in cord and in 5-year blood (r = 0.76, p < 0.001) were strongly correlated. Maternal pregnancy ARYase levels were poorly correlated with fetal (r = −0.01, p = 0.94) and weakly correlated with child levels of ARYase (r = 0.25, p = 0.005); maternal POase levels were weakly to moderately correlated with both fetal (r = 0.29, p < 0.001) and child POase (r = 0.42, p < 0.001) levels.
In Table 2, we examine the main effect of maternal and child genotype on child’s performance on the K-CPT and WISC IQ and show no clear association of maternal or child PON1192 genotype with either attention or cognition. Child IQ scores tended to be lowest in mothers and in children with the PON1−108TT genotype, though this trend was not statistically significant. Children of mothers with PON1−108CT and PON1−108TT genotypes also showed higher (i.e. worse) K-CPT scores, though this trend was also not statistically significant. In Table 3, we examine the main effect of maternal and child enzyme activity on child neurodevelopment. We observe no association of POase levels and performance on the K-CPT or the WISC IQ, except for a positive relationship of POase levels in pregnancy and the Processing Speed Index at age 7 (β = 2.6 per standard deviation increase in enzyme levels, 95% CI = 0.2, 5.0), and a borderline-significant negative (i.e. protective) relationship of POase levels in pregnancy and the age 5 ADHD Confidence Index score (β = −2.8 per standard deviation increase in enzyme levels, 95% CI = −5.7, 0.1). In contrast, ARYase levels in pregnancy were positively associated with all subscales on the WISC. Specifically, a standard deviation increase in maternal ARYase levels during pregnancy was related to a 3.9 point increase in Perceptual Reasoning (95% CI = 1.4, 6.4), 2.6 points increase in Verbal Comprehension (95% CI = 0.5, 4.6), a 3.6 point increase in Working Memory (95% CI = 1.3, 5.9), a 2.1 point increase in Processing Speed (95% CI = −0.4, 4.5), and an overall 4.0 point increase in Full Scale IQ (95% CI = 1.6, 6.4) in the children at 7 years. The associations between ARYase levels in cord or child age 5 blood and IQ, though positive, were usually less strong. For example, only the relationship between cord blood ARYase levels and age 7 Processing Speed (β = 1.9, 95% CI = 0.1, 3.8) and between age 5 ARYase levels and Verbal Comprehension (β = 2.3, 95% CI = 0.3, 4.2) and Processing Speed (β = 1.8, 95% CI = 0.8, 3.6) were statistically significant (Table 3). ADHD Confidence index was somewhat associated with ARYase levels in cord blood ARYase levels (β = −2.1 per standard deviation increase in enzyme levels, 95% CI = −4.6, 0.3).
Table 3.
Adjusted association of arylesterase (ARYase) and paraoxonase(POase) enzyme activity level (per SD increase) with measures of attention at age 5 on the Kiddie-Continuous Performance test (K-CPT) and cognition at age 7 on the Wechsler Intelligence Scale for Children-IV (WISC), CHAMACOS.
| Age 5
|
Age 7
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KCPT ADHD Indexb
|
WISC PRIc
|
WISC VCId
|
WISC WMIc
|
WISC PSIc
|
WISC FSIQd
|
|||||||
| β | (95% CI) | β | (95% CI) | β | (95% CI) | β | (95% CI) | β | (95% CI) | β | (95% CI) | |
| ARYasea | ||||||||||||
| Pregnancy | −0.5 | (−3.6, 2.7) | 3.9 | (1.4, 6.4)** | 2.6 | (0.5, 4.6)** | 3.6 | (1.3, 5.9)** | 2.1 | (−0.4, 4.5)* | 4.0 | (1.6, 6.4)** |
| Cord blood | −2.1 | (−4.6, 0.3)* | 0.1 | (−2.0, 2.3) | 0.2 | (−1.7, 2.2) | 1.1 | (−0.8, 2.9) | 1.9 | (0.1, 3.8)** | 0.1 | (−1.0, 2.9) |
| Child age 5 | −1.1 | (−3.4, 1.3) | 0.7 | (−1.6, 3.0) | 2.3 | (0.3, 4.2)** | 1.1 | (−1.0, 3.1)) | 1.8 | (0.8, 3.6)** | 1.6 | (−0.5, 3.7) |
| POase | ||||||||||||
| Pregnancy | −2.8 | (−5.7, 0.1)* | 1.1 | (−1.3, 3.5) | 0.4 | (−1.6, 2.3) | 1.0 | (−1.3, 3.3) | 2.6 | (0.2, 5.0)** | 1.2 | (−1.3, 3.7) |
| Cord blood | −0.9 | (−3.3, 1.5) | − 0.1 | (−2.3, 2.2) | − 0.1 | (−2.1, 1.8) | 1.2 | (−0.6, 3.0) | 1.0 | (−0.9, 2.8) | 0.9 | (−1.1, 2.8) |
| Child age 5 | 1.5 | (−0.7, 3.7) | 0.4 | (−1.8, 2.7) | 0.9 | (−1.0, 2.8) | 0.0 | (−1.9, 2.0) | − 0.6 | (−2.3, 1.1) | 0.3 | (−1.7, 2.3) |
All ARYase analyses adjusted for individual’s PON192 genotype (maternal genotype for pregnancy, child genotype for cord blood and age 5) in addition to other specified covariates.
Adjusted for mean prenatal total DAPs (pregnancy, cord blood) or age 5 total DAPS (age 5); psychometrician; child sex and age at testing; maternal education, PPVT, and depression; duration of breastfeeding; time in childcare; and video game usage.
Adjusted for mean prenatal total DAPs (pregnancy, cord blood) or age 5 total DAPS (age 5), maternal education, maternal PPVT, and HOME score at 6 months of age.
Adjusted for mean prenatal total DAPs (pregancy, cord blood) or age 5 total DAPS (age 5), maternal education, maternal PPVT, HOME score at 6 months of age, and language of neurological assessment at 7 years of age.
p < 0.10.
p < 0.05.
We next investigated whether maternal or child genotype modified the relationship of maternal DAP levels during pregnancy and performance on the K-CPT (Marks et al. 2010) and WISC IQ (Bouchard et al. 2011). Overall, we found weak evidence of effect modification on K-CPT performance and WISC IQ. Specifically, children of mothers carrying the T allele of PON−108 showed a positive relationship (i.e. poorer attention) of DAPS and ADHD Confidence Index (PON−108CT β = 5.9, 95% CI = −1.2,13.0; PON−108CT β = 5.9, 95% CI = −3.3,15.2) whereas children of mothers with PON−108CC did not (β = −4.7, 95% CI = −14.0,4.5; pinteraction = 0.21 categorical). Amond WISC scales, most notably we found that a 10-fold increase in maternal total DAP levels during pregnancy was associated with an 8.3 point decrease in the child’s Verbal Comprehension Index (95% CI = −15.7, −1.0) among mothers with PON1−108TT genotype, a 4.9 point decrease (95% CI = −9.3, −0.5) among heterozygous mothers, and a 3.2 decrease (95% CI = −11.5, 5.1) among mothers with PON1−108CC genotype, although the p-value for interaction was not significant (pinteraction = 0.71 categorical) (Table 4). This difference in association by maternal genotype was primarily with maternal DM DAP concentration. We found similar suggestive evidence by child genotype (Supplementary Table 5).
Table 4.
Adjusted associations of average maternal prenatal dialkyl phosphate matabolites (DAPs) and neurodevelopment outcomes at ages 5 and 7, stratified by maternal PON1−108 and PON1−192 genotype, CHAMACOS.
| Age 5
|
Age 7
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal genotype |
KCPT ADHD indexa
|
p-value interactiond,e |
WISC PRIb
|
p-value interactiond,e |
WISC VCIc
|
p-value interactiond,e |
WISC WMIb
|
p-value interactiond,e |
WISC PSIb
|
p-value interactiond,e |
WISC FSIQc
|
p-value interactiond,e |
||||||
| β | (95% CI) | β | (95% CI) | β | (95% CI) | β | (95% CI) | β | (95% CI) | β | (95% CI) | |||||||
| Total DAPs | ||||||||||||||||||
| PON1–108 | ||||||||||||||||||
| CC | −4.7 | (−14.0, 4.5) | 0.21d | −1.0 | (−10.2, 8.1) | 0.74 | −3.2 | (−11.5, 5.1) | 0.71 | −1.5 | (−9.3, 6.2) | 0.67 | −4.2 | (−11.5, 3.1) | 0.96 | −3.0 | (−11.4, 5.3) | 0.76 |
| CT | 5.9 | (−1.2,13.0) | 0.25e | −3.9 | (−8.8, 0.9) | 0.97 | −4.9 | (−9.3, −0.5)** | 0.41 | −5.1 | (−10.0,−0.2)** | 0.42 | −3.7 | (−8.4, 1.0) | 0.99 | −6.1 | (−10.5, −1.7)** | 0.66 |
| TT | 5.9 | (−3.3, 15.2) | −2.5 | (−13.0, 8.0) | −8.3 | (−15.7, −1.0)** | −3.6 | (−11.3, 4.1) | −2.3 | (−10.7,6.0) | −5.0 | (−13.8, 3.9) | ||||||
| PON1192 | ||||||||||||||||||
| RR | 2.8 | (−6.5, 12.2) | 0.92 | −2.7 | (−10.5, 5.2) | 0.88 | −5.6 | (−13.2, 2.0) | 0.88 | −2.3 | (−8.3, 3.7) | 0.49 | −5.8 | (−13.2, 1.7) | 0.71 | −5.3 | (−12.7, 2.0) | 0.96 |
| QR | 5.1 | (−1.6, 11.8) | 0.87 | −4.4 | (−10.7, 1.9) | 0.95 | −4.1 | (−9.3, 1.0) | 0.95 | −2.7 | (−8.2, 2.8) | 0.29 | −2.0 | (−7.4, 3.3) | 0.52 | −4.3 | (−9.6, 0.9) | 0.99 |
| 3.7 | (−6.0, 13.4) | −1.9 | (−9.5, 5.8) | −4.0 | (−9.7, 1.6) | −5.9 | (−13.3, 1.6) | −1.8 | (−8.3, 4.7) | −4.1 | (−10.9, 2.7) | |||||||
| Dimethyl DAPs | ||||||||||||||||||
| PON1–108 | ||||||||||||||||||
| CC | −4.1 | (−11.9, 3.8) | 0.22 | −1.7 | (−9.9, 6.6) | 0.79 | −3.2 | (−10.6, 4.3) | 0.65 | −2.4 | (−9.3, 4.6) | 0.65 | −0.4 | (−7.0, 6.3) | 0.64 | −2.6 | (−10.1, 5.0) | 0.69 |
| CT | 5.3 | (−1.2, 11.7) | 0.33 | −3.3 | (−7.7, 1.1) | 0.94 | −4.3 | (−8.3, −0.3)** | 0.38 | −4.6 | (−9.0, −0.1)** | 0.35 | −2.7 | (−7.0, 1.6) | 0.52 | −5.1 | (−9.0, −1.1)** | 0.47 |
| TT | 2.6 | (−5.4, 10.7) | −2.1 | (−11.3, 7.1) | −7.9 | (−14.3, −1.5)** | −4.7 | (−11.3, 1.9) | −1.7 | (−9.0, 5.5) | −5.2 | (−12.9, 2.5) | ||||||
| PON1192 | ||||||||||||||||||
| RR | 2.0 | (−6.4, 10.3) | 0.76 | −2.8 | (−10. 0,4.4) | 0.88 | −5.4 | (−12.3, 1.6) | 0.88 | −2.2 | (−7.7, 3.4) | 0.58 | −4.2 | (−11.2, 2.8) | 0.60 | −4.8 | (−11.6, 2.1) | 0.91 |
| QR | 4.5 | (−1.4, 10.4) | 0.73 | −3.4 | (−9.1, 2.3) | 0.87 | −3.9 | (−8.6, 0.8) | 0.84 | −3.0 | (−8.0, 2.0) | 0.31 | 0.1 | (−4.7, 5.0) | 0.66 | −3.4 | (−8.1, 1.4) | 0.89 |
| 1.9 | (−6.8, 10.7) | −1.0 | (−7.7, 5.8) | −3.6 | (−8.7, 1.4) | −5.3 | (−11.9, 1.3) | −1.2 | (−6.9, 4.6) | −3.3 | (−9.3, 2.7) | |||||||
| Diethyl DAPs | ||||||||||||||||||
| PON1–108 | ||||||||||||||||||
| CC | −2.3 | (−12.2, 7.6) | 0.55 | 0.5 | (−7.6, 8.5) | 0.76 | 0.9 | (−6.5, 8.2) | 0.54 | 0.9 | (−5.8, 7.5) | 0.71 | −7.0 | (−13.1, −0.9) | 0.49 | −0.8 | (−7.9, 6.3) | 0.56 |
| CT | 3.0 | (−3.4, 9.4) | 0.60 | −2.1 | (−6.9, 2.7) | 0.65 | −3.6 | (−7.9, 0.6)* | 0.69 | −1.3 | (−6.1, 3.5) | 0.70 | −2.5 | (−7.0, 2.0) | 0.42 | −4.0 | (−8.3, 0.2)* | 0.73 |
| TT | 4.5 | (−2.9, 11.9) | −0.3 | (−9.3, 8.7) | −1.5 | (−8.1, 5.0) | 4.3 | (−2.4, 10.9) | −2.9 | (−10.1, 4.3) | 0.8 | (−7.0, 8.7) | ||||||
| PON1192 | ||||||||||||||||||
| RR | 5.0 | (−3.4, 13.5) | 0.53 | −2.9 | (−10.1, 4.3) | 0.995 | −3.8 | (−10.8, 3.2) | 0.56 | −4.3 | (−9.6, 1.1) | 0.18 | −7.0 | (−13.5, −0.5)** | 0.40 | −5.9 | (−12.5, 0.7)* | 0.60 |
| QR | −0.5 | (−6.3, 5.4) | 0.96 | −2.4 | (−7.8, 3.0) | 0.99 | −0.9 | (−5.4, 3.6) | 0.999 | 2.7 | (−2.0, 7.4) | 0.69 | −2.7 | (−7.2, 1.9) | 0.24 | −1.8 | (−6.3, 2.7) | 0.73 |
| 4.6 | (−4.6, 13.8) | −1.4 | (−9.1, 6.2) | −1.7 | (−7.5, 4.1) | −0.2 | (−7.8, 7.3) | −1.9 | (−8.3, 4.5) | −1.8 | (−8.6, 5.0) | |||||||
Adjusted for psychometrician; child sex and age at testing; maternal education, PPVT, and depression; duration of breastfeeding; time in childcare; and video game usage.
Adjusted for maternal education, maternal PPVT, HOME score at 6 months of age, and language of neurological assessment at 7 years of age.
Adjusted for maternal education, maternal PPVT, HOME score at 6 months of age, and language of neurological assessment at 7 years of age.
Interaction p-value based on categorical interaction terms; a postestimation combined F-test (or chi-square test) was used to test the two genotype*DAPs interaction terms (e.g. PON108CT × DAPs and PON108TT × DAPs versus PON108CC × DAPs).
Interaction p-value based on continuous interaction terms.
p < 0.10.
p < 0.05.
Next, we considered whether maternal ARYase and POase levels during pregnancy modified the relationship of DAP levels in prenatal urine samples with child K-CPT and WISC IQ scores. There was no clear pattern of effect modification on K-CPT. However, the inverse associations of DAPs and Full-Scale IQ were most pronounced for children whose mothers had the lowest ARYase levels and absent in those with the highest. Specifically, we observed a 10.9 point decrease (95% CI = −19.7, −2.0) for lowest-, a 5.3 point decrease (95% CI = −14.2, 3.6) for middle-, and a 0.9 point increase (95% CI = −7.6, 9.4) for highest-tertile ARYase, respectively, though the interaction was not statistically significant (pinteraction = 0.27 categorical) (Fig. 1; Supplementary Table 6). For all WISC subscales, a similar pattern was observed with the strongest inverse association between maternal DAP levels and WISC performance in children of mothers with lowest-tertile ARYase levels, but the only statistically-significant association was seen for Verbal Comprehension (β = −9.2, 95% CI = −17.3, −1.1). This trend usually held for both DM and DE metabolites but was more consistent for DM metabolites. Though there was some evidence of interaction between both total DAPs and DMs and POase levels on WISC subscales, the greatest deficits were usually seen in offspring of mothers with middle-tertile POase levels. As shown in Supplementary Table 7, the relationship between pre-natal average maternal DAPs, DMs and DEs and attention and IQ usually showed the greatest decrements in those with the lowest levels of cord ARYase levels; however, the interactions were not significant and the trend is less clear than for maternal ARYase levels. For cord POase levels, the greatest IQ decrements were often observed in children with the highest level of POase.
Fig. 1.
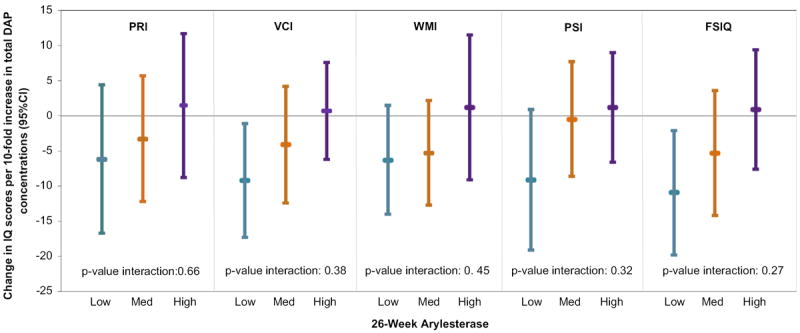
Association between maternal prenatal (26-week) total DAP concentration (log10 scale) and child age 7 WISC IQ scores as modified by maternal 26-week arylesterase activity.
4. Discussion
We have previously reported that prenatal exposure to OP pesticides as measured by maternal DAP levels was negatively associated with attention and cognition in school-age children (Marks et, al., 2010; Bouchard et al., 2011). We now present evidence that this association with IQ is strongest in children whose mothers had the lowest levels of PON1 ARYase enzyme activity during pregnancy.
When the children were two years old, we found a relationship between DAPs and the Mental Development Index (MDI) and, to a lesser extent, the Psychomotor Development Index (PDI) on the Bayley Scales (Eskenazi et al., 2007). We also reported a main effect relationship of child PON1−108 with MDI and PDI test performance. However, maternal and child PON1192 genotype and ARYase and POase in maternal, cord, and child blood were not related to MDI or PDI, though children of mothers within the highest-tertile ARYase did have the highest MDI scores. Although interactions were not significant, the relationship between DAPs and mental development was strongest in toddlers with PON1−108TT polymorphism; results were similar albeit muted by maternal genotype. In addition, the relationship of DAP levels and MDI was strongest in children within the lowest tertiles of cord ARYase and POase, although there was no clear pattern across tertiles; again, results were similar but weaker for maternal levels.
In the present study, we examined these associations between DAPs, PON1 and neurobehavioral development when the children reached school-age. Although we continue to observe a relationship between maternal pregnancy DAP levels and child neurobehavioral deficits, the relationship between maternal and child PON1−108 genotype and neurodevelopment was weaker than at the younger ages. We find only limited evidence that child or maternal PON1−108 genotype modifies the relation of DAP levels and neurodevelopment. However, at this older age, enzyme activity, in particular ARYase, appears to have a more important main effect than genotype on neurodevelopment and to show somewhat stronger evidence of effect modification. We note that ARYase level is a measure of the enzyme quantity that is influenced by the PON1−108 promotor polymorphism. We found that the relationship between ARYase enzyme activity in maternal blood during pregnancy and IQ is stronger than that for child enzyme activity.
The differences in results between toddler and school age in CHAMACOS children can be attributed to a number of factors: (1) not all the same children were assessed at these intervals; (2) we used different tools to assess development at these ages, and though the WISC and Bayley scales may both assess cognition and other higher cortical functions, the Bayley scales are not strongly predictive of school age IQ (Bornstein and Colombo, 2012; McGrath et al., 2004); and (3) neurobehavioral developmental functioning is multifactorial and has numerous known and unknown determinants, of which PON1 genotype and enzyme activity represent only one factor. Nevertheless, we find that PON1 genotype and/or enzyme activity seem to be directly related to cognitive functioning and may modify to some extent the association of OP pesticide exposure as measured by urinary DAPs and neurodevelopment.
To our knowledge, only one other study has examined the effect modification of PON on neurodevelopment. Engel et al. (2011) reported that in 6- to 9-year-olds, increasing DAP levels, and in particular increasing DM levels, were associated with decreases in Full Scale IQ (not significant) and Perceptual Reasoning IQ, but only in children of mothers carrying the PON1192QQ genotype. They did not detect any effect modification by PON1 enzyme activity and concluded that perhaps genotype was a more stable long-term predictor of metabolism potential. However, we have previously reported great variability in expression of genotype, with a very wide range of enzyme activity among people with the same genotype (Huen et al., 2010; Furlong et al., 2006).
Differences between our study and the study by Engel et al. may be due to racial/ethnic and other genetic differences in the two populations. In addition, both studies used nonspecific urinary metabolites (DAPS) to assess exposure to OP pesticides, and these metabolites derive from multiple parent compounds (CDC 2010) that may vary in the degree to which they interact with PON1 and influence neurodevelopment. The pesticides contributing to the DAP levels and their relative toxicities may vary considerably between the CHAMACOS agricultural population and the urban New York City population of the study by Engel et al., and DM levels were considerably higher in the CHAMACOS population (Bradman et al., 2005; Wolff et al., 2007).
PON1 is a multifactorial enzyme and it is not clear exactly how it may interact with OPs to modify their relationship with neurodevelopment. Although our key hypothesized mechanism was that PON1 genotype and enzyme activity would affect the individual’s abilities to detoxify the potentially neurotoxic OP pesticides, PON1’s function of protecting individuals from oxidative stress may play an equally, if not more important, role on neurodevelopmental function. The fact that our results, in many cases, seem stronger for DM levels supports this hypothesis, since DEs but not DMs are derived from known PON1 substrates (e.g., chlopyrifos-oxon and diazoxon). Furthermore, other OP pesticides not hydrolyzed by PON1, such as malathion, which devolves to DM DAPs, have been shown to induce oxidative stress (Durak et al., 2009; Franco et al., 2009).
Our research is limited in a number of ways. Despite the large sample size, this study lacked power for examining interaction by genotype or enzyme concentrations. We have limited our analysis to only two main PON1 polymorphisms; although there are other polymorphisms associated with ARYase and POase activity in this cohort, they are in strong linkage disequilibrium with those we examined here (Huen et al., 2011). It is also possible that genetic variants in other genes from related pathways (e.g. cholinesterase) could be involved. In addition, DAP metabolites are an imperfect measure of OP pesticide exposure given the short half-life of OP pesticides in the body and environment, the variability in exposure over time, the potential for exposure to preformed DAPs in the environment (Quirós-Alcalá et al., 2012), and differences in metabolism that may affect excretion (Wessels et al., 2003). It is not clear whether DAP metabolite levels differ as a function of PON status, even among individuals with similar environmental exposures. We have previously reported that whereas mothers with lowest- and middle-tertile ARYase levels showed positive, albeit non-significant, relationships between plasma levels of diazinon and chlorpyrifos and urinary levels of DE metabolites, for mothers with highest-tertile ARYase levels, there was an inverse association between these parent compounds in plasma and urinary DE metabolites (Huen et al., 2012). Lastly, our results may not be generalizable given this unique cohort of primarily Mexican American children. PON1 allelic distributions are known to vary by ethnic/racial groups (Huen et al., 2011; Chen et al., 2003; Rojas-Garcia et al., 2005), although the relationship between genotype and enzyme activities appears to be comparable (Costa et al., 2005).
In summary, in this follow-up study of Mexican–American children in the CHAMACOS cohort, we extend previous findings of associations between PON1 genotype and enzyme levels and certain domains of neurodevelopment through early school age, presenting new evidence that adverse associations between DAP levels and IQ may be strongest in children of mothers with the lowest levels of PON1 enzyme. Future policies regarding protection from OP pesticide exposure should consider that some children may be more vulnerable to early life exposures by virtue of their or their mother’s genetic predispositions and resulting PON1 enzyme activity.
Supplementary Material
Acknowledgments
We acknowledge the CHAMACOS staff, students, community partners, and the CHAMACOS participants and families, without whom this study would not be possible.
Grant information
This publication was made possible by research supported by grant numbers R82670901, RD 83171001, and RD-83273401 from EPA and PO1 ES009605, R01 ES012503, and R01 ES023067-01A1 from NIEHS. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIOSH, NIH or EPA.
Abbreviations
- 95% CI
95% confidence interval
- ARYase
arylesterase
- CHAMACOS
Center for Health Analysis of Mothers and Children of Salinas
- DAP
dialkyl phosphates
- DE
diethyl phosphates
- DM
dimethyl phosphates
- FSIQ
Full Scale Intelligence Quotient
- GM
geometric mean
- HOME
Home Observation for Measurement of the Environment
- HOME-SF
Home Observation for Measurement of the Environment—Short Form
- K-CPY
Conners’ Continuous Performance Test—Kiddie Version
- LOD
limit of detection
- n
number (total)
- nmol/L
nanomoles per liter
- OP
organophosphate
- POase
paraoxonase
- PPVT
Peabody Picture Vocabulary Test
- PRI
Perceptual Reasoning Index (WISC-IV)
- PSI
Processing Speed Index (WISC-IV)
- SD
standard deviation
- TVIP
Test de Vocabulario en Imágenes (PPVT in Spanish)
- μg/L
micrograms per liter
- US
United States
- VCI
Verbal Comprehension Index (WISC-IV)
- WISC-IV
Wechsler Intelligence Scale for Children—Fourth Edition
- WMI
Working Memory Index (WISC-IV)
Footnotes
Appendix A. Supplementary materials
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.envres.2014.07.001.
None of the authors has a competing financial interest.
References
- Barr D, Bravo R, Weerasekera G, Caltabiano L, Whitehead R. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population. Environ Health Perspect. 2004;112(2):186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Colombo J. Infant cognitive functioning and child mental development. In: Pauen S, Bornstein M, editors. Early Childhood Development and Later Achievement. Cambridge University Press; New York: 2012. pp. 118–147. [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. Prenatal exposure to organophosphate pesticides and iq in 7-year-old children. Environ Health Perspect. 2011;119(8):1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Barr DB, Claus Henn BG, Drumheller T, Curry C, Eskenazi B. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: a validation study. Environ Health Perspect. 2003;111(14):1779–1782. doi: 10.1289/ehp.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Eskenazi B, Barr D, Bravo R, Castorina R, Chevrier J, et al. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Perspect. 2005;113(12):1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Driskell WJ, Whitehead RD, Jr, Needham LL, Barr DB. Quantitation of dialkyl phosphate metabolites of organophosphate pesticides in human urine using GC–MS–MS with isotopic internal standards. J Anal Toxicol. 2002;26(5):245–252. doi: 10.1093/jat/26.5.245. [DOI] [PubMed] [Google Scholar]
- Caldwell B, Bradley R. Home Observation For Measurement Of The Environment. University of Arkansas; Little Rock, AR: 1984. [Google Scholar]
- Chen J, Kumar M, Chan W, Berkowitz G, Wetmur JG. Increased influence of genetic variation on PON1 activity in neonates. Environ Health Perspect. 2003;111(11):1403–1409. doi: 10.1289/ehp.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly PW, Maguire GF, Picardo CM, Teiber JF, Draganov D. Development of an immunoblot assay with infrared fluorescence to quantify paraoxonase 1 in serum and plasma. J Lipid Res. 2008;49(1):245–250. doi: 10.1194/jlr.D700022-JLR200. [DOI] [PubMed] [Google Scholar]
- Conners CK, Staff M. Conners’ Kiddie Continuous Performance Test (K-CPT): Computer Program for Windows Technical Guide and Software Manual. MHS; Toronto, ON: 2001. [Google Scholar]
- Costa LG, Cole TB, Vitalone A, Furlong CE. Measurement of paraoxonase (PON1) status as a potential biomarker of susceptibility to organophosphate toxicity. Clin Chim Acta. 2005;352(1-2):37–47. doi: 10.1016/j.cccn.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Dunn L, Dunn L. Peabody Picture Vocabulary Test, Revised. American Guidance Service; Circle Pines, MN: 1981. [Google Scholar]
- Dunn L, Padilla E, Lugo D, Dunn L. Test de Vocabulario en Imagenes Peabody: Adaptacion Hispanoamericana (Peabody Picture Vocabulary Test: Hispanic-American Adaptation) Dunn Educational Services, Inc; Circle Pines, MN: 1986. [Google Scholar]
- Durak D, Uzun FG, Kalender S, Ogutcu A, Uzunhisarcikli M, Kalender Y. Malathion-induced oxidative stress in human erythrocytes and the protective effect of vitamins C and E in vitro. Environ Toxicol. 2009;24(3):235–242. doi: 10.1002/tox.20423. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, et al. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011;119(8):1182–1188. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, et al. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2004;112(10):1116–1124. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Huen K, Marks A, Harley KG, Bradman A, Barr DB, et al. PON1 and neurodevelopment in children from the CHAMACOS study exposed to organophosphate pesticides in utero. Environ Health Perspect. 2010;118(12):1775–1781. doi: 10.1289/ehp.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Fenster L, Johnson C, Barr DB, et al. In utero exposure to dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) and neurodevelopment among young Mexican American children. Pediatrics. 2006;118(1):233–241. doi: 10.1542/peds.2005-3117. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican–American children. Environ Health Perspect. 2007;115(5):792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco JL, Posser T, Mattos JJ, Trevisan R, Brocardo PS, Rodrigues AL, et al. Zinc reverses malathion-induced impairment in antioxidant defenses. Toxicol Lett. 2009;187(3):137–143. doi: 10.1016/j.toxlet.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Furlong CE, Holland N, Richter RJ, Bradman A, Ho A, Eskenazi B. PON1 status of farmworker mothers and children as a predictor of organophosphate sensitivity. Pharmacogenet Genomics. 2006;16(3):183–190. doi: 10.1097/01.fpc.0000189796.21770.d3. [DOI] [PubMed] [Google Scholar]
- Holland N, Furlong C, Bastaki M, Richter R, Bradman A, Huen K, et al. Paraoxonase polymorphisms, haplotypes, and enzyme activity in latino mothers and newborns. Environ Health Perspect. 2006;114(7):985–991. doi: 10.1289/ehp.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, Barcellos L, Beckman K, Rose S, Eskenazi B, Holland N. Effects of PON polymorphisms and haplotypes on molecular phenotype in Mexican–American mothers and children. Environ Mol Mutagen. 2011;52(2):105–116. doi: 10.1002/em.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, Bradman A, Harley K, Yousefi P, Boyd Barr D, Eskenazi B, et al. Organophosphate pesticide levels in blood and urine of women and newborns living in an agricultural community. Environ Res. 2012;117:8–16. doi: 10.1016/j.envres.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, Harley K, Bradman A, Eskenazi B, Holland N. Longitudinal changes in PON1 enzymatic activities in Mexican–American mothers and children with different genotypes and haplotypes. Toxicol Appl Pharmacol. 2010;244(2):181–189. doi: 10.1016/j.taap.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, Harley K, Brooks J, Hubbard A, Bradman A, Eskenazi B, et al. Developmental changes in PON1 enzyme activity in young children and effects of PON1 polymorphisms. Environ Health Perspect. 2009a;117(10):1632–1638. doi: 10.1289/ehp.0900870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, Richter R, Furlong C, Eskenazi B, Holland N. Validation of PON1 enzyme activity assays for longitudinal studies. Clin Chim Acta. 2009b;402(1-2):67–74. doi: 10.1016/j.cca.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RW. A long and winding road: defining the biological role and clinical importance of paraoxonases. Clin Chem Lab Med. 2006;44(9):1052–1059. doi: 10.1515/CCLM.2006.207. [DOI] [PubMed] [Google Scholar]
- Kujiraoka T, Oka T, Ishihara M, Egashira T, Fujioka T, Saito E, et al. A sandwich enzyme-linked immunosorbent assay for human serum paraoxonase concentration. J Lipid Res. 2000;41(8):1358–1363. [PubMed] [Google Scholar]
- Li HL, Liu DP, Liang CC. Paraoxonase gene polymorphisms, oxidative stress, and diseases. J Mol Med (Berl) 2003;81(12):766–779. doi: 10.1007/s00109-003-0481-4. [DOI] [PubMed] [Google Scholar]
- Lu C, Barr DB, Pearson MA, Waller LA. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ Health Perspect. 2008;116(4):537–542. doi: 10.1289/ehp.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and attention in young Mexican–American children: the CHAMACOS study. Environ Health Perspect. 2010;118(12):1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath E, Wypij D, Rappaport LA, Newburger JW, Bellinger DC. Prediction of IQ and achievement at age 8 years from neurodevelopmental status at age 1 year in children with D-transposition of the great arteries. Pediatrics. 2004;114(5):e572–e576. doi: 10.1542/peds.2003-0983-L. [DOI] [PubMed] [Google Scholar]
- McKone TE, Castorina R, Harnly ME, Kuwabara Y, Eskenazi B, Bradman A. Merging models and biomonitoring data to characterize sources and pathways of human exposure to organophosphorus pesticides in the Salinas Valley of California. Environ Sci Technol. 2007;41(9):3233–3240. doi: 10.1021/es0618447. [DOI] [PubMed] [Google Scholar]
- Quirós-Alcalá L, Bradman A, Smith K, Weerasekera G, Odetokun M, Barr DB, et al. Organophosphorous pesticide breakdown products in house dust and children’s urine. J Expo Sci Environ Epidemiol. 2012;22(6):559–568. doi: 10.1038/jes.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- Richter RJ, Furlong CE. Determination of paraoxonase (PON1) status requires more than genotyping. Pharmacogenetics. 1999;9(6):745–753. [PubMed] [Google Scholar]
- Rojas-Garcia AE, Solis-Heredia MJ, Pina-Guzman B, Vega L, Lopez-Carrillo L, Quintanilla-Vega B. Genetic polymorphisms and activity of PON1 in a Mexican population. Toxicol Appl Pharmacol. 2005;205(3):282–289. doi: 10.1016/j.taap.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Stata Corporation. Stata Statistical Software: Release. Stata Corp LP; College Station, TX: 2009. p. 11. [Google Scholar]
- Sugland BW, Zaslow M, Smith JR, Brooks-Gunn J, Coates D, Blumenthal C, et al. The early childhood home inventory and home-short form in differing racial/ethnic groups. J Fam Issues. 1995;16(5):632–663. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children—Fourth Edition (WISC-IV) Administration and Scoring Manual. Harcourt Assessment Incorporated; San Antonio, TX: 2003. [Google Scholar]
- Weldon RH. Biomonitoring Persistent and Non-persistent Chemicals in Human Breast Milk and Endocrine Disruption of Lactation. University of California; Berkeley, Berkeley: 2010. [Google Scholar]
- Wessels D, Barr DB, Mendola P. Use of biomarkers to indicate exposure of children to organophosphate pesticides: implications for a longitudinal study of children’s environmental health. Environ Health Perspect. 2003;111(16):1939–1946. doi: 10.1289/ehp.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111(5):749–756. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MK, Rundle A, Holmes D, Reyes M, Hoepner LA, Barr DB, et al. Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000–2001 U.S. Environmental Protection Agency restriction of organophosphates. Environ Health Perspect. 2008;116(12):1681–1688. doi: 10.1289/ehp.11367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Engel S, Berkowitz G, Teitelbaum S, Siskind J, Barr DB, et al. Prenatal pesticide and PCB exposures and birth outcomes. Pediatr Res. 2007;61(2):243–250. doi: 10.1203/pdr.0b013e31802d77f0. [DOI] [PubMed] [Google Scholar]
- Zartarian VG, Ozkaynak H, Burke JM, Zufall MJ, Rigas ML, Furtaw EJ., Jr A modeling framework for estimating children’s residential exposure and dose to chlorpyrifos via dermal residue contact and nondietary ingestion. Environ Health Perspect. 2000;108(6):505–514. doi: 10.1289/ehp.00108505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


