Abstract
Aim
To assess the outcomes of patients treated with postoperative RT in relation to the possible prognostic factors.
Background
Postoperative radiotherapy (RT) has been proved to reduce the risk of biochemical recurrence in high-risk prostate cancer patients. Baseline prostate specific antigen (PSA), pathological Gleason score (GS), positive surgical margins, nodal status and seminal vesicle invasion are independent predictors of biochemical relapse.
Materials and methods
The clinical records of 282 patients who underwent postoperative RT were retrospectively reviewed. The prognostic value of postoperative PSA, preoperative risk class, nodal status, pathological GS, margins status, and administration of hormonal therapy (HT) was analyzed.
Results
Postoperative RT was delivered with a median dose to the prostatic fossa of 66 Gy (range 50–72) in 1.8–2 Gy/fraction. Median follow-up was 23.1 months (range 6–119). Five-year actuarial biochemical disease-free survival (bDFS) and overall survival rates were 76% and 95%, respectively. Higher bDFS was found for patients with postoperative PSA <0.02 ng/ml (p = 0.03), low preoperative risk class (p = 0.01), pN0 (p = 0.003), GS 4–6 (p = 0.0006), no androgen deprivation therapy (p = 0.02), and irrespective of surgical margin status (p = 0.10). Multivariate analysis showed that postoperative PSA and Gleason score had a significant impact on bDFS (p = 0.039 and p = 0.05, respectively).
Conclusions
Postoperative RT with a dose of 66 Gy offers an acceptable toxicity and an optimal disease control after radical prostatectomy in patients with different risk features. A postoperative PSA >0.02 ng/ml could be considered as a prognostic factor and a tool to select patients at risk for progression.
Keywords: Postoperative radiotherapy, Prostate cancer, Biochemical recurrence, Salvage radiotherapy
1. Background
Radical prostatectomy (RP) and radiation therapy (RT) are both effective treatment alternatives for men with localized, low- and intermediate-risk prostate cancer (PCa).1,2 Approximately 15–25% of patients who undergo RP for localized PCa will experience cancer recurrence, diagnosed initially as biochemical recurrence (BCR).2,3
For high-risk localized PCa, RP is a potentially attractive treatment option, providing definitive information about pathology and an excellent loco-regional control.2 However, the risk of biochemical recurrence ranges from 30%3 to 68%4 in patients with high risk features.
Baseline prostate-specific antigen (PSA), pathological Gleason score (GS), positive surgical margins, nodal status and seminal vesicle invasion are independent predictors of biochemical relapse.5,6
Short post-RP PSA doubling time, high pathologic Gleason score (8–10), and short disease-free interval from RP to BCR were proven to be significant risk factors for progression to distant metastases and PCa-specific mortality.7,8
Recently, three large randomized controlled trials (SWOG 8794, EORTC 22911, and ARO 96-02) have demonstrated that postoperative radiotherapy reduces the risk of biochemical recurrence and improves local control in patients with adverse risk factors compared with watchful waiting.9–11 One phase 3 trial showed a reduced risk of metastasis and increased survival.12
Although the benefits of postoperative RT are clear, no data from randomized trials comparing adjuvant (ART) and salvage (SRT) radiotherapy are available.
At present there is not a clear definition of “salvage” RT since the definition of “detectable” PSA is still ambiguous: modern ultrasensitive PSA assays (measuring 0.001 ng/ml) were not available when the major trials regarding postoperative RT were conducted.13–16
2. Aim
Aim of this study was to retrospectively analyze the outcome of 282 patients treated with surgery and postoperative RT in relation to the possible prognostic factors for biochemical disease-free survival.
3. Materials and methods
Two hundred and eighty-three patients with clinically localized prostate cancer underwent postoperative RT after RP at the Department of Radiotherapy of the University Hospital “Maggiore della Carità” in Novara, Italy from 1998 to 2008. Patients were discussed in a multidisciplinary group that included urologists, radiation oncologists, and medical oncologists. This retrospective study was approved by the local Ethical Committee following the regulations of our Institution.
All clinical records were carefully reviewed. The following data were extracted from clinical records: history and physical examination, including digital rectal examination, and routine serum laboratory studies (complete blood count and biochemistry panel, including alkaline phosphatase), pre-surgery PSA, risk class according to the D’Amico classification,17 bone scan and computed tomography (CT) scan of the abdomen and pelvis according to the risk class, neoadjuvant hormonal therapy when given, time and type of surgery, pathological T and N stage (according to the American Joint Committee on Cancer 5th edition)18 pathological Gleason score, margin status, post-operative PSA one month after surgery, adjuvant HT if given, and follow-up status.
Two different cut-off values were considered (>0.2 ng/ml and >0.02 ng/ml) to define BCR after RP. Patients were clinically evaluated every 6 months for the first 5 years after RT, and annually thereafter, with PSA assessment every 6 months. Duration of clinical follow-up was defined as the interval from the last day of RT to the date of the last contact with the patient.
BCR after postoperative RT was defined as two subsequent PSA increases (each of at least 0.1 ng/ml) above the lowest value after postoperative RT, according with data of the literature.19,20 Biochemical disease-free survival (bDFS) was defined as the time from the last day of RT to the date of BCR using 2 thresholds at 0.2 ng/ml and 0.02 ng/ml. Survival was calculated from the date of surgery to the last contact with the patient. Overall survival (OS) reflected all deaths, either cancer-related or for other causes. When calculating biochemical control, patients were censored at the date of their last PSA measurement.
Acute and late toxicity was scored by using the RTOG toxicity scale.21
3.1. Statistical analysis
Statistical analysis was carried out according to different clinical aspects: preoperative risk class, margins status, pathological Gleason score, postoperative PSA and administration of concomitant/adjuvant HT.
Survival was calculated using the Kaplan–Meier method22 and comparison between groups was performed using the log-rank test.
Actuarial bDFS and OS were calculated at 5 years with 95% confidence interval (CI).
Cox regression model was carried out by STATA 11 software.
The validity of the proportionality assumption was checked by stphtest STATA command.
4. Results
All 282 patients were eligible for the analysis. Patients’ characteristics are shown in Table 1.
Table 1.
Patients’ characteristics (N = 282).
| Mean age (years) | 67 (sd = 5.89; range 44–76) |
| Median age (25th–75th) years | 68 (63–71) |
| Risk classes17 | |
| Low risk | 102 (36.2%) |
| Intermediate risk | 92 (32.6%) |
| High risk | 88 (31.2%) |
| Median pre-operative PSA (ng/ml) | 10 (25th–75th 6.6–18.4) |
| ≤10 ng/ml | 138 (48.9%) |
| 10 < ng/ml ≤ 20 | 82 (29.1%) |
| 21 < ng/ml < 40 | 38 (13.5%) |
| ≥40 ng/ml | 24 (8.5%) |
| Median pathologic Gleason score | 7 (25th–75th 7–8) |
| Pathological T stage (UICC 1992) | |
| pT2a | 3 (1.1%) |
| pT2b | 2 (0.7%) |
| pT2c | 36 (12.8%) |
| pT3a | 99 (35.1%) |
| pT3b | 119 (42.2%) |
| T4 | 23 (8.1%) |
| Pathological nodal status (UICC 1992) | |
| pN0 | 143 (50.7%) |
| pN1 | 25 (8.9%) |
| pNx | 114 (40.4%) |
| Positive margins (%) | 205 (72%) |
| Median PSA 30–60 days postoperatively (ng/ml) | 0.07 (range 0–20; 25th–75th 0.01–0.45) |
| Mean pre-RT PSA (cut-off = 0.02 ng/ml) | |
| <0.02 | 78 (27.7%) |
| ≥0.02 | 204 (72.3%) |
| Mean pre-RT PSA (cut-off = 0.2 ng/ml) | |
| <0.2 | 192 (68.1%) |
| ≥0.2 | 90 (31.9%) |
| RT dose on prostatic fossa (Gy) | |
| <60 | 13 (4.6%) |
| 60 | 49 (17.4%) |
| 66 | 217 (77.0%) |
| 70 | 2 (0.7%) |
| 72 | 1 (0.3%) |
Two-hundred and seventy-eight patients underwent an open retropubic radical prostatectomy and 4 a laparoscopic transperitoneal radical prostatectomy. A nerve sparing approach was performed in eight patients. A pelvic lymphadenectomy was performed in 166 patients.
Of the 282 patients, 57 (20%) received HT before surgery (34 received anti-androgenic drugs, 9 LHRH analogues, and 14 total androgen blockade – TAB).
Adjuvant HT (following surgery and concurrent to RT) was delivered to 148 (52%) patients: 36 (25%) low risk, 48 (32%) intermediate risk and 64 (43%) high risk class.
Median duration of postoperative HT was 23.1 months (range 1–119).
Forty patients (14%) received both neoadjuvant and adjuvant HT.
Serum PSA before RT was detectable (≥0.02 ng/ml) for 204 patients (72%) and undetectable (<0.02 ng/ml) for 78 patients (28%). Using the cut-off at 0.2 ng/ml, 192 patients (68%) had an undetectable PSA and 90 patients (32%) had a detectable PSA.
Twenty-six patients (9%) had severe incontinence 2–3 month after surgery, 67 (23%) reported a mild (1–2 pads/die) incontinence, the remaining 68% (189 patients) had no stress incontinence. For RT purposes, patients were simulated with a CT scan with 3-mm slices, with no contrast medium, in supine position and using a leg immobilization device (Combifix-Sinmed, Civco, Kalona, IA). Treatment plan was performed by the 3-dimension (3D) convolution algorithm of the Pinnacle™ treatment planning system (Philips, The Netherlands) and dose distribution was analyzed by 3-dimension maps and dose-volume histograms.
A bladder filling protocol was utilized to obtain a comfortably full bladder at the time of planning and during treatment. An empty rectum protocol was also adopted.
The anatomical limits of CTV (prostatic fossa) were the following: the inferior border was 6 mm below the vesico-urethral anastomosis; the anterior border was the posterior aspect of the pubic symphysis (in the lower portion) and encompassed the posterior 1.5 cm of the bladder in the higher portion; the posterior border was the anterior rectal wall; the lateral border was the elevator ani muscle; the superior border extended to the seminal vesicle bed including the distal portion of the vas deferens. From 2008 we referred to the available guidelines for CTV contouring.23
A uniform margin of 10 mm was added to CTV to obtain PTV, except a posterior margin of 7 mm to reduce rectal dose. No less of 95% of CTV and PTV volume received at least 95% of the prescribed dose (CTV95%, PTV95%).
Rectum, bladder and femoral heads were considered organs at risk (OARs): the rectum was contoured as a solid organ, including the rectal wall and its contents, from the sigmoid flexure to the ischeal tuberosities. The bladder was contoured as a solid organ from the dome to the bladder neck.
The following dose constraints were considered for treatment planning: the percentage of rectum receiving >40 Gy did not exceed 60% of the rectal volume, and the percentage receiving >60 Gy was less than 40% of the rectal volume. Regarding the bladder, no more than 50% of the volume received a dose >65 Gy.
The prostatic fossa was irradiated in all patients with 6–15 MV photons to a median dose of 66 Gy (range, 50–72 Gy) in 1.8–2 Gy per fraction. In 50 patients (18%), the pelvic lymph nodes were also included within the initial RT volume: in 12 of them (24%) for pN1 status, in the other 38 (76%) because of other pathologic high risk features in absence of an adequate nodal sampling. In these cases, pelvic nodes received a dose of 45 Gy in 1.8 Gy per fraction. The dose was prescribed at the isocentre according to the International Commission of Radiation Units and Measurements (ICRU) recommendations.24
Three-dimensional conformal irradiation techniques were used in all cases with a multileaf collimator: four-six static coplanar fields in 258 cases (91%) and four-fields with dynamic arcs in the other 25 (9%) patients.
RT started about 4 months after surgery (range 2–20 months).
Fifty-eight out of 282 patients (20.5%) reported a biochemical failure during follow-up.
After a median follow-up of 23.1 months (range 6–119), 260 patients (92.2%) were alive, 7 patients (2.4%) died because of prostate cancer and 15 patients (5%) died for other causes.
Median follow-up from the last day of RT to biochemical recurrence was 3.6 years (range 0.1–13). Actuarial 5-year bDFS rate was 76% (95% CI 69–82). Five year OS rate was 95% (95% CI 91–97) (Figs. 1 and 2). Three patients were not followed at our centre and were considered “lost to follow up”.
Fig. 1.
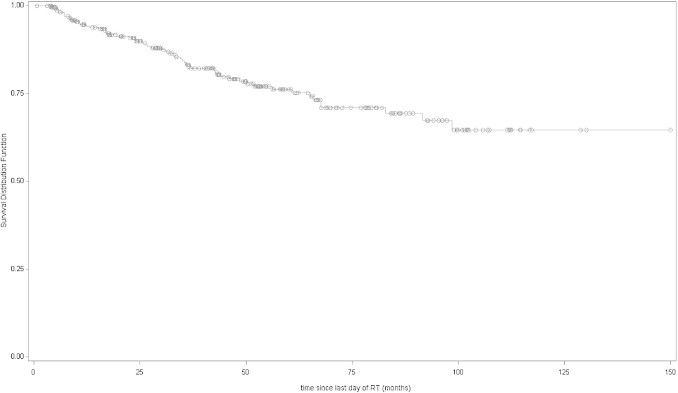
Disease-free survival (Kaplan–Meier curves) of all patients.
Fig. 2.
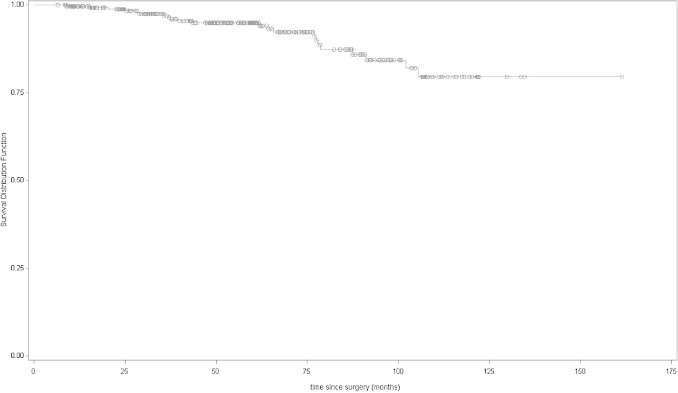
Overall survival (Kaplan–Meier curves) of all patients.
A univariate analysis was performed to assess the impact of clinical features on clinical outcomes (Table 2). None of the risk factors had a significant impact on overall survival (p = 0.11).
Table 2.
Univariate analysis for 5-year bDFS.
| 5-year bDFS % (95% CI) | p value* | |
|---|---|---|
| PSA cut-off | ||
| <0.2 ng/ml | 81% (95% CI 73–87) | 0.06 |
| ≥0.2 ng/ml | 68% (95% CI 56–77) | |
| <0.02 ng/ml | 86% (95% CI 63–95) | 0.03 |
| ≥0.02 ng/ml | 73% (95% CI 66–79) | |
| Risk class17 | ||
| Low risk | 86% (95% CI 76–92) | 0.01 |
| Intermediate risk | 75% (95% CI 61–84) | |
| High risk | 63% (95% CI 48–75) | |
| Pathological nodal status | ||
| pN0 | 71% (95% CI 61–78) | 0.003 |
| pN1 | 49% (95% CI 22–72) | |
| pNx | 89% (95% CI 80–94) | |
| Pathological Gleason score | ||
| 4–6 (61 pts, 22%) | 92% (95% CI 78–98) | 0.0006 |
| 7 (125 pts, 44%) | 81% (95% CI 72–88) | |
| 8–10 (96 pts, 34%) | 59% (95% CI 45–71) | |
| Surgical margins status | ||
| Positive | 72% (95% CI 64–79) | 0.10 |
| Negative | 85% (95% CI 74–92) | |
| Adjuvant hormonal therapy | ||
| Yes | 67% (95% CI 56–76) | 0.02 |
| No | 84% (95% CI 75–89) | |
Log-rank test.
With a PSA cut-off at 0.2 ng/ml, the 5-year bDFS was 81% (95% CI 73–87) for patients with PSA <0.2 ng/ml and 68% (95% CI 56–77) for those with PSA ≥0.2 ng/ml (p = 0.059).
With a PSA cut-off at 0.02 ng/ml, the 5-year bDFS was 86% (95% CI 63–95) for patients with PSA< 0.02 ng/ml and 73% (95% CI 66–79) for those with PSA ≥0.02 ng/ml (p = 0.034).
A statistically significant difference in 5-year bDFS was found based on the preoperative risk class (p = 0.01), on pathological nodal status (p = 0.003) and on Gleason score (4–6 vs. 7 vs. 8–10) (p = 0.0006). No significant difference in 5-year bDFS was found based on surgical margin status (p = 0.10). The 5-year bDFS rate was 84% (CI 95% 75–89) for patients who received no HT (134 patients) and 67% (95% CI 56–76) for those who received HT (148 patients) (p = 0.02).
Postoperative PSA (cut-off 0.02 ng/ml), D’Amico risk class, Gleason score, pN stage, surgical margins status and adjuvant HT were assessed at multivariate analysis (Table 3). Cox proportional hazard ratio analysis was carried out and showed a significant impact of postoperative PSA (≥ or <0.02 ng/ml) and Gleason score (3–6 vs. 7 vs. 8–10) on bDFS (p = 0.03 and p = 0.05, respectively).
Table 3.
Multivariate analysis for 5-year bDFS.
| HR (95% CI) | p | p value** | |
|---|---|---|---|
| PSA | |||
| ≥0.02 vs. <0.02 | 2.67 (1.04–6.83) | 0.03 | 0.03 |
| Risk class17 | |||
| Intermediate vs. low risk | 1.70 (0.82–3.52) | 0.15 | 0.36 |
| High vs. low risk | 1.49 (0.69–3.22) | 0.30 | |
| Pathological Gleason score | |||
| GL 7 vs. GL 3–6 | 2.89 (0.99–8.42) | 0.05 | 0.05 |
| GL 8–10 vs. GL 3–6 | 3.79 (1.28–11.16) | 0.05 | |
| Pathological nodal status | |||
| pN1 vs. pN0 | 1.14 (0.51–2.55) | 0.74 | 0.17 |
| pNx vs. pN0 | 0.53 (0.26–1.07) | 0.07 | |
| Surgical margins status | |||
| Positive vs. negative | 1.81 (0.96–3.41) | 0.06 | 0.06 |
| Adjuvant hormonal therapy | |||
| Yes vs. no | 1.54 (0.87–2.72) | 0.13 | 0.13 |
Wald chi-square statistic.
A subgroup analyses was performed for patients who did or did not receive post-operative HT. The results of univariate and multivariate analyses are shown in Tables 4 and 5.
Table 4.
Univariate analysis for 5-year bDFS of two different groups: patients treated with and without hormonal therapy (HT).
| Patients treated with HT 148 pts (52%) |
Patients treated without HT 134 pts (48%) |
|||
|---|---|---|---|---|
| 5-year bDFS % (95% CI) | p value* | 5-year bDFS % (95% CI) | p value* | |
| PSA cut-off | ||||
| <0.2 ng/ml ART | 64% (95% CI 17–89) | 0.22 | 89% (95% CI 78–94) | 0.19 |
| ≥0.2 ng/ml SRT | 66% (95% CI 55–76) | 74% (95% CI 58–85) | ||
| <0.02 ng/ml ART | 64% (95% CI 17–89) | 0.21 | 97% (95% CI 80–99) | 0.13 |
| ≥0.02 ng/ml SRT | 66% (95% CI 55–76) | 76% (95% CI 65–84) | ||
| Risk class17 | ||||
| Low risk | 84% (95% CI 65–93) | 0.20 | 88% (95% CI 75–95) | 0.12 |
| Intermediate risk | 67% (95% CI 45–81) | 69% (95% CI 46–84) | ||
| High risk | 55% (95% CI 34–71) | 76% (95% CI 50–89) | ||
| Pathological N status | ||||
| pN0 | 65% (95% CI 50–76) | 0.0022 | 80% (95% CI 67–88) | 0.28 |
| pN1 | 35% (95% CI 9–62) | – | ||
| pNx | 91% (95% CI 73–97) | 87% (95% CI 74–94) | ||
| Pathological Gleason score | ||||
| 3–6 (61 pts, 22%) | 93% (95% CI 59–99) | 0.002 | 93% (95% CI 75–98) | 0.13 |
| 7 (125 pts, 44%) | 78% (95% CI 61–89) | 81% (95% CI 68–89) | ||
| 8–10 (96 pts, 34%) | 45% (95% CI 27–62) | 77% (95% CI 56–89) | ||
| Surgical margins | ||||
| Positive | 82% (95% CI 62–91) | 0.22 | 83% (95% CI 70–96) | 0.15 |
| Negative | 60% (95% CI 45–72) | 82% (95% CI 72–89) | ||
Log-rank test.
Table 5.
Multivariate analysis for 5-bDFS of two different groups: patients treated with and without hormonal therapy (HT).
| Patients treated without HT 134 (48%) |
Patients treated with HT 148 pts (52%) |
|||
|---|---|---|---|---|
| HR | p | HR | p | |
| PSA | ||||
| ≥0.02 vs. <0.02 | 2.96 (0.68–13.0) | 0.15 | 1.91 (0.55–6.61) | 0.30 |
| Risk class17 | ||||
| Intermediate vs. low risk | 2.28 (0.78–6.70) | 0.30 | 1.36 (0.487–3.796) | 0.84 |
| High vs. low risk | 2.15 (0.61–7.69) | 1.20 (0.45–3.186) | ||
| Pathological Gleason score | ||||
| GL 7 vs. 3–6 | 3.81 (0.84–17.39) | 0.22 | 2.29 (0.48–10.99) | 0.13 |
| GL 8–10 vs. 3–6 | 3.51 (0.70–17.95) | 3.89 (0.88–17.239) | ||
| Pathological nodal status | ||||
| pN1 vs. pN0 | NC | 0.94 | 1.44 (0.62–3.70) | 0.07 |
| pNx vs. pN0 | 0.85 (0.32–2.22) | 0.35 (0.12–1.024) | ||
| Surgical margins | ||||
| Positive vs. negative | 1.73 (0.48–6033) | 0.39 | 1.63 (0.76–3.51) | 0.21 |
Acute urinary toxicity was mild except for three cases of urethral stricture, which required urethral catheterization. Most acute rectal toxicities (99%) were grade 0–2.
Overall, the rate of late toxicity was low: only four patients developed a severe late toxicity, with two still presenting frequent episodes of hematuria 24 and 60 months after RT (Table 6).
Table 6.
Acute and late toxicity.
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| Rectal | |||||
| Acute toxicity | 168 (59%) | 79 (28%) | 33 (12%) | 2 (<1%) | 0 (0%) |
| Late toxicity | 264 (94%) | 9 (3%) | 7 (2%) | 2 (<1%) | 0 (0%) |
| Urinary | |||||
| Acute toxicity | 188 (67%) | 58 (21%) | 27 (10%) | 6 (2%) | 3 (1%) |
| Late toxicity | 238 (84%) | 35 (12%) | 7 (2%) | 2 (<1%) | 0 (0%) |
5. Discussion
The outcomes of RP are suboptimal in high-risk patients in which the risk of biochemical recurrence is as high as 67% at 5 years.25
The rationale for immediate RT after surgery (adjuvant RT) is to improve disease free survival in patients at risk, while that of salvage RT is to promptly treat selected patients with recurrent disease.
According to three large, randomized, controlled trials published in the last few years, postoperative RT offers a benefit in biochemical recurrence-free survival of at least 15% at 5 years.9–12
In these trials there is some heterogeneity in the definition of “undetectable PSA” and biochemical recurrence. While in the CARO ARO trial only men with a PSA <0.1 ng/ml were eligible for randomization, the EORTC 22911 and SWOG 8794 trials did not identify a maximum PSA level as an inclusion criteria. EORTC and SWOG included 33% and 11% of the patients, respectively, having a detectable persistent PSA after surgery, representing a sort of “salvage” RT rather than a really “adjuvant” RT. This heterogeneous definition of baseline PSA strongly influenced the definition of “biochemical relapse”: in the German trial relapse was defined as PSA >0.1 ng/ml whereas in the EORTC-22911 and SWOG-S8794 trials, biochemical recurrence was defined as PSA >0.2 ng/ml and >0.4 ng/ml, respectively.9,11,12
In our study, we defined a detectable postoperative PSA using two different cut-offs: PSA <0.2 ng/ml, and PSA <0.02 ng/ml, considering that the detectable threshold has been evolving in the last decade, reaching now 0.001 ng/ml. In both cases, we used the same criteria to define biochemical recurrence: two consecutive rises in PSA of more than 0.1 ng/ml each. This is the same criterion used in the RADICALS trial,26 an ongoing randomized trial comparing adjuvant vs. salvage RT. This univocal definition of biochemical recurrence allowed us to analyze the oncological outcome of our patients independently by the evolving threshold for PSA detection.
We reported a relatively low biochemical recurrence rate, with a BCR (biochemical control rate) of 24% at 5 years: only 58 out of 282 patients (20.5%) reported a biochemical failure during follow-up. Our results are similar to BCR in the three randomized trials (28%, 21% and 30%, respectively).
All patients in our series received a 3D conformal treatment plan, with a median dose of 66 Gy. The three randomized trials on postoperative RT applied doses <66 Gy. The rate of biochemical relapse reported in the literature may be due to a relatively low RT dose, supporting the concept that an increasing dose could improve biochemical control. The results from our high dose postoperative RT series (66 Gy) seem to confirm that. However, the wide use of postoperative HT for a median of 23 months might have influenced the BCR in our patients, as we will discuss later.
In this study we retrospectively evaluated the impact of postoperative RT in patients with different risk features to find out potential prognostic factors. Regarding postoperative PSA, we used two different PSA cut-off levels (0.2 ng/ml or 0.02 ng/ml). We observed a trend of correlation of a PSA ≥ or <0.2 ng/ml with bDFS (p = 0.06), while a significant correlation of a PSA ≥ or <0.02 ng/ml with bDFS was observed (p = 0.03) (Fig. 3).
Fig. 3.
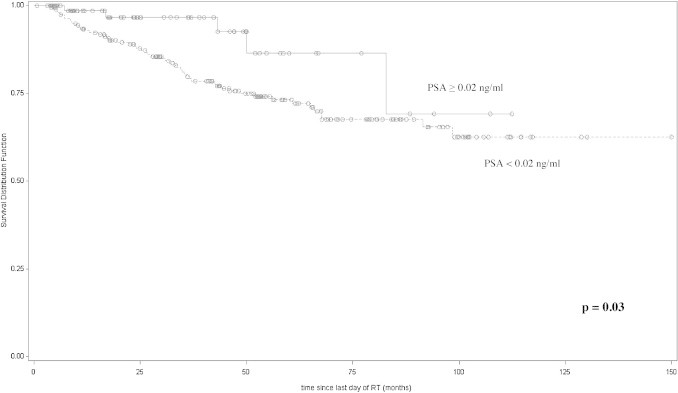
Disease-free survival (Kaplan–Meier curves) of patients stratified according with their postoperative PSA level.
A PSA ≥ of 0.02 ng/ml was found to be an independent predictor of 5-year bDFS at multivariate analysis (p = 0.03), confirming that this postoperative PSA cut-off has a prognostic significance in our series.
In an era where we discuss the opportunity of a pure “adjuvant” vs. an “early salvage” radiotherapy, the use of a lower and reliable PSA cut-off to stratify patients at risk may be of clinical relevance.14,27
Other prognostic factors were analyzed in our series. Surgical margins status did not have an impact on bDFS (p = 0.06). Patients with positive surgical margins were 72% in our series, a slightly higher proportion compared to the three randomized trials (63–68%). Positive surgical margins are considered the most important prognostic factor in PC patients: in fact the benefit of postoperative radiation in patients with negative margins (regardless of other risk factors) has not been considered significant in a sub group analysis of the EORTC 22911 series.9,27 Higher doses (≥66 Gy) and the use of postoperative HT in our series might explain the lower significance of surgical margins involvement.
Pathological Gleason score had a significant impact on bDFS (p = 0.05), regardless of other risk factors.
Nodal status was surgically assessed in 168 patients (60%). At univariate analysis nodal involvement significantly correlated with biochemical relapse (p = 0.003) but this was not confirmed at multivariate analyses (p = 0.17), likely due to the small proportion of pN1 patients (8.9%). Twelve of the 25 pN+ patients received whole pelvis irradiation, while the others did not. Fifty patients without surgical nodal staging (pNx) were also treated with a whole pelvis approach. This heterogeneity reflects the evolving technological assessment at our centre due to the acquisition of 3D conformal technique approach that allowed a safer pelvic nodes irradiation. Looking at our experience and the available literature data, the role of pelvic radiation in node-positive disease still remains a controversial issue.
The role of HT is a crucial issue. Adjuvant androgen suppression after definitive RT was shown to improve disease-free survival and overall survival. In the RTOG 85-31 trial, the biochemical failure rate (defined as PSA >0.5 ng/ml) was 42% and 65% in patients who received combination therapy and RT alone, respectively (p = 0.002).28 In the postoperative setting, a survival advantage with adjuvant androgen deprivation was reported in a small trial of lymph node–positive (N1) patients.29
In our series HT significantly influenced bDFS at univariate analysis (p = 0.02), but not at multivariate analysis (p = 0.13). A separate analysis was performed for patients who received or did not receive HT to better understand the impact of different prognostic factors in the absence of androgen deprivation therapy. We did not observe substantial differences in the two groups, except for a significant impact of nodal involvement (pN1) and high GS (8–10) on bDFS in patients receiving postoperative HT (p = 0.0022 and p = 0.002, respectively). These patients may be those who need a more aggressive adjuvant approach, in terms of radiation dose and volume, and duration of HT.
In our series, patients who did not receive postoperative HT (134) achieved a better 5-year bDFS (84% vs. 67%, p = 0.02). It should be noted that high risk features were present in 43% (64 patients) of patients who received HT group and only in 8% (23 patients) of patients who did not. It is therefore clear that the HT group was composed of patients with worse prognosis. The lack of clear guidelines on the use of HT combined with RT in ART and SRT may justify the inhomogeneous use of this treatment modality in our retrospective series.
At present no study has compared different kinds of hormonal treatment after RP. The ongoing trials RTOG 9601 and RTOG 062130,31 are comparing RT with or without hormones in the postoperative setting.
In our series the toxicity rate was quite low: acute gastrointestinal and urinary toxicity >G2 were 1% and 2%, respectively. Both gastrointestinal and genitor-urinary late toxicity were 1%. These findings may be due to our strict dose constraints. However, similar results have been reported in other large trials, with a gastrointestinal toxicity rate ranging from 1% to 5% in irradiated patients.10,12,32
Our study has some limitations. First of all, this is a retrospective analysis, with the relative bias in the accuracy and continuity of follow-up. Three patients stopped their follow-up few weeks after the end of RT and were therefore considered lost to follow up. Treatment intent (ART or SRT) did not influence any treatment feature, such as radiation dose, volume and association with HT. Furthermore, the most relevant bias in our series is likely the wide use of HT. Pre-surgical HT administration was mainly due to the long surgical waiting list at the time the patients were treated.
The wide use of HT may explain the absence of significant differences in OS and bDFS according to margin status and other high risk features. HT likely produced a sort of cosmetic effect on PSA values, masking the real impact of the risk factors.
Another potential bias is due to the fact that PSA was tested in different laboratories with different assay kits.
Finally, we did not carry out any analysis of the impact of surgery and postoperative RT on quality of life (QoL). In a collateral study of the SWOG trial,33 69% of patients receiving postoperative RT had a normal global health-related QoL at the end of the 5-year period of observation. We included urinary disorders in the late toxicity assessment, and therefore we can assume that the QoL of patients was generally good.
On the other hand, the study has some strong assets: first, all patients in our single-institutional series underwent a homogeneous surgical and radiation treatment. Furthermore, radiation dose, volumes and techniques can be still considered a standard today. Every step of treatment (from prescription to follow up) was performed by dedicated radiation oncologists, with particular expertise in genito-urinary cancers and working in a urological multidisciplinary group.
There are several open issues in postoperative RT for prostate cancer: first of all the role of HT in combination with radiotherapy following RP is yet to be defined. In our series patients receiving adjuvant HT had a worse 5-year bDFS, likely due to their worse prognostic features (high risk features were present in 43% of those patients receiving HT). The RTOG 96-01 trial34 evaluating the role of bicalutamide plus salvage RT found a reduced rate of progression to metastasis at 7 years (12.6% vs. 7.4%, p = 0.04) and an overall survival benefit (91% vs. 86%). However, further studies are needed to confirm the role of combined radiation and hormonal in the postoperative setting.
6. Conclusions
In keeping with published data our results show that postoperative RT with a median dose of 66 Gy has an acceptable toxicity and offers an optimal disease control after RP in patients with different risk features.
Postoperative PSA and high Gleason score were confirmed to be independent prognostic factors for bDFS. In patients receiving postoperative HT, positive nodal status and high GS have a significant impact on 5-year bDFS. Longer follow-up is needed to confirm our data and to evaluate the impact of adjuvant RT on overall survival.
The ongoing clinical trials (RTOG 9601, RADICALS) will help to clarify the role of post-operative RT in the adjuvant or early salvage setting, and the impact of the association of HT with RT.
Among the assessed prognostic factors, a postoperative PSA >0.02 ng/ml was found to significantly predict the risk of bDFS and may therefore represent a useful tool to select patients at risk that may benefit from tailored adjuvant treatment.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Kim M.M., Hoffman K.E., Levy L.B. Improvement in prostate cancer survival over time: a 20-year analysis. Cancer J. 2012;18:1–8. doi: 10.1097/PPO.0b013e3182467419. [DOI] [PubMed] [Google Scholar]
- 2.Gnanapragasam V.J., Mason M.D., Shaw G.L. The role of surgery in high-risk localised prostate cancer. BJU. 2011;109:648–658. doi: 10.1111/j.1464-410X.2011.10596.x. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons R.P., Cole B.S., Richardson R.G. Adjuvant radiotherapy following radical prostatectomy: results and complications. J Urol. 1986;135:65–68. doi: 10.1016/s0022-5347(17)45519-4. [DOI] [PubMed] [Google Scholar]
- 4.Anscher M.S., Prosnitz L.R. Postoperative radiotherapy for patients with carcinoma of the prostate undergoing radical prostatectomy with positive surgical margins, seminal vesicle involvement, and/or penetration through the capsule. J Urol. 1987;138:1407–1412. doi: 10.1016/s0022-5347(17)43656-1. [DOI] [PubMed] [Google Scholar]
- 5.Kupelian P.A., Katcher J., Levin H.S. Stage T1–2 prostate cancer: a multivariate analysis of factors affecting biochemical and clinical failures after radical prostatectomy. Int J Radiat Oncol Biol Phys. 1997;37:1043–1052. doi: 10.1016/s0360-3016(96)00590-1. [DOI] [PubMed] [Google Scholar]
- 6.Collette L., van Poppel H., Bolla M. Patients at risk of progression after radical prostatectomy: do they all benefit from immediate post-operative irradiation? (EORTC trial 22911) Eur J Cancer. 2005;41:2662–2672. doi: 10.1016/j.ejca.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Pound C.R., Partin A.W., Epstein J.I. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395–406. doi: 10.1016/s0094-0143(05)70386-4. [DOI] [PubMed] [Google Scholar]
- 8.Roberts S.G., Blute M.L., Bergstralh E.J. PSA doubling time as a predictor of clinical progression after biochemical failure following radical prostatectomy for prostate cancer. Mayo Clin Proc. 2001;76:576–581. doi: 10.4065/76.6.576. [DOI] [PubMed] [Google Scholar]
- 9.Bolla M., van Poppel H., Collette L. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572–578. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 10.Bolla M., Van Poppel H., Tombal B. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380:2018–2027. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 11.Wiegel T., Bottke D., Steiner U. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–2930. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 12.Thompson I.M., Jr., Tangen C.M., Paradelo J. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296:2329–2335. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 13.Cookson M.S., Aus G., Burnett A.L. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association prostate guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 14.Collette L., van Poppel H., Bolla M. Patients at high risk of progression after radical prostatectomy: do they all benefit from immediate post-operative irradiation? (EORTC trial 22911) Eur J Cancer. 2005;41:2662–2672. doi: 10.1016/j.ejca.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Valicenti R.K., Thompson I., Jr., Albertsen P. Adjuvant and salvage radiation therapy after prostatectomy: American Society for Radiation Oncology/American Urological Association guidelines. Int J Radiat Oncol Biol Phys. 2013;86:822–828. doi: 10.1016/j.ijrobp.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Rossi C., Hsu J., Abdel-Wahab M. ACR Appropriateness Criteria® postradical prostatectomy irradiation in prostate cancer. Am J Clin Oncol. 2011;34:92–98. doi: 10.1097/COC.0b013e3182005319. [DOI] [PubMed] [Google Scholar]
- 17.D’Amico A.V., Whittington R., Malkowicz S.B. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 18.5th ed. Lippincott Raven; Philadelphia: 1998. American Joint Committee on Cancer staging manual. [Google Scholar]
- 19.Stephenson A.J., Shariat S.F., Zelefsky M.J. Salvage radiotherapy for recurent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325–1332. doi: 10.1001/jama.291.11.1325. [DOI] [PubMed] [Google Scholar]
- 20.Wiegel, Lohm, Bottke Achieving an undetectable PSA after radiotherapy for biochemical progression after radical prostatectomy is an independent predictor of biochemical outcome – results of a retrospective study. Int J Radiat Oncol Biol Phys. 2009;73:1009–1016. doi: 10.1016/j.ijrobp.2008.06.1922. [DOI] [PubMed] [Google Scholar]
- 21.Cox J.D., Stetz T., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 23.Sidhom M.A., Kneebone A.B., Lehman M. Post-prostatectomy radiation therapy: consensus guidelines of the Australian and New Zealand Radiation Oncology Genito-Urinary Group. Radiother Oncol. 2008;88:10–19. doi: 10.1016/j.radonc.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 24.International Commission on Radiation Units and Measurements . International Commission on Radiation Units and Measurements; Bethesda: 1999. ICRU report 62: prescribing, recording and reporting photon beam therapy (supplement to ICRU report 50) [Google Scholar]
- 25.Han M., Partin A., Zahurak M. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517–523. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 26.Parker C., Clarke N., Logue J. RADICALS (radiotherapy and androgen deprivation in combination after local surgery) Clin Oncol. 2007;19:167–171. doi: 10.1016/j.clon.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Van der Kwast T.H., Bolla M., Van Poppel H. EORTC 22911. Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol. 2007;25:4178–4186. doi: 10.1200/JCO.2006.10.4067. [DOI] [PubMed] [Google Scholar]
- 28.Pilepich M.V., Winter K., Lawton C.A. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma – long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 29.Messing E.M., Manola J., Yao J. Immediate versus deferred androgren deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–479. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 30.Denham J.W., Steigler A., Kumar M.L. Measuring time to biochemical failure in the TROG 96.01 trial: when should the clock start ticking? Int J Radiat Oncol Biol Phys. 2009;75:1008–1012. doi: 10.1016/j.ijrobp.2008.12.085. [DOI] [PubMed] [Google Scholar]
- 31.RTOG 0621: adjuvant 3DCRT/IMRT in combination with androgen suppression and docetaxel for high risk prostate cancer patients post-prostatectomy: a phase II trial. http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0621 [last access 18.11.13].
- 32.Fuentes-Raspalla R., Inorizac G.M., Rosello-Serrano A. Late rectal and bladder toxicity following radiation therapy for prostate cancer: predictive factors and treatment results. Rep Pract Oncol Radiother. 2013;18:298–303. doi: 10.1016/j.rpor.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moinpour C.M., Hayden K.A., Unger J.M. Health-related quality of life results in pathologic stage C prostate cancer from a Southwest Oncology Group trial comparing radical prostatectomy alone with radical prostatectomy plus radiation therapy. J Clin Oncol. 2008;26:112–120. doi: 10.1200/JCO.2006.10.4505. [DOI] [PubMed] [Google Scholar]
- 34.Shipley W.U., Hunt D., Lukka H. Initial report of RTOG 9601: a phase III trial in prostate cancer anti-androgen therapy (AAT) with bicalutamide during and after radiation therapy (RT) improves freedom from progression and reduces the incidence of metastatic disease in patients following radical prostatectomy (RP) with pT2–3, N0 disease, and elevated PSA level. Int J Radiat Oncol Biol Phys. 2010;78:S27. [Google Scholar]


