Abstract
Aim
We aimed to determine the changes in TNF-α expression and Malondialdehyde (MDA) level in a short time after irradiation. Furthermore, we evaluated the effect of melatonin on the modulation of TNF-α gene expression.
Background
The radio-sensitivity of the cervical spinal cord limits the dose of radiation which can be delivered to tumors in the neck region. There is increasing evidence that TNF-α has a role in the development of the acute phase of spinal cord injury.
Materials/Methods
Four groups of rats were investigated. Group 1 (vehicle treatment) served as the control. Group 2 (radiation) was treated with the vehicle, and 30 min later, the rats were exposed to radiation. Group 3 (radiation + melatonin) was given an oral administration of melatonin (100 mg/kg body weight) and 30 min later exposed to radiation in the same manner as in group 2. Group 4 (melatonin-only) was also given an oral administration of melatonin (100 mg/kg body weight). 5 mg/kg of melatonin was administered daily to rats in groups 3 and 4, and the vehicle was administered daily to rats in groups 1 and 2.
Results
Three weeks after irradiation, TNF-α gene up-regulated almost 5 fold in the irradiated group compared to the normal group. TNF-α gene expression in the melatonin pretreatment group, compared to the radiation group, was significantly down-regulated 3 weeks after irradiation (p < 0.05). MDA levels increased after irradiation and then significantly decreased under melatonin treatment.
Conclusion
We suggest that inhibition of TNF-α expression by oral administration of melatonin may be a therapeutic option for preventing radiation-induced spinal cord injury.
Keywords: Melatonin, Radiation, Spinal cord, MDA, TNF-α
1. Background
Most patients diagnosed with head and neck malignancy will receive a course of radiotherapy for treatment, local control, or symptomatic palliation.1 In the emergency treatment of a cancer pressing on the spinal cord, radiotherapy may be used to reduce the size of the tumor and prevent nerve damage.2 The dose that can be administered without radiation myelopathy (RM) is, however, limited by the tolerance of the spinal cord to radiation.3 Pathological features of radiation myelopathy (RM) are well-defined, but the molecular mechanisms of this injury remain uncertain. Studies have shown that the response of the CNS after irradiation is a continuous, dynamic, and interacting process.4,5 Three types of reactions have been recognized based on the time of development of symptoms: acute, early delayed and late delayed.5 Recently, we have reported that a single dose of 22 Gy gamma ray to the spinal cord can cause limb paralysis with latency of 20 weeks.6 In that study, the role of VEGF in the development of RM has been shown. In another study, the roles of Bax and Bcl-2 gene expression have been studied in the radiation-induced apoptosis of the spinal cord.7 Several studies have provided evidence that the expression of tumor necrosis factor-alpha (TNF-α) is directly activated by ionizing radiation. Many researchers have suggested that TNF-α has a role in the development of the acute phase of spinal cord injury (SCI) through the induction of apoptosis.8 Melatonin administration has effectively prevented SCI due to spinal cord compression and trauma.9–11 We have recently reported the function of melatonin as a radioprotector and VEGF modulator.6 Some researchers have demonstrated the antiapoptotic effect of melatonin.7 Some in vivo studies have shown that melatonin can modulate the expression of TNF-α induced by toxic agents.11
2. Aim
This study follows two basic purposes: first, the study seeks to determine the changes in TNF-α expression in the short time after irradiation, to explore its possible contribution to the development of RM. The second purpose is to assess whether oral melatonin administration can modulate TNF-α gene and protein expression after localized irradiation of the cervical spinal cord.
3. Materials and methods
Twenty adult male Wistar rats weighing 180–220 g were selected and housed in conventional rodent facilities. They were fed and watered on a standard rodent chow diet, and were kept at a constant temperature on a 12-h light–dark cycle. The rats were divided into four groups. Group 1 (vehicle treatment) served as a control. Group 2 (radiation) was treated with the vehicle, and after 30 min, the rats were exposed to radiation, which is a process detailed in the following section. Group 3 (radiation + melatonin) was given an oral administration of melatonin (100 mg/kg body weight), and after 30 min, it was exposed to radiation in the same manner as in group 2. Group 4 (melatonin-only) was also given an oral administration of melatonin (100 mg/kg body weight). 5 mg/kg of melatonin was administered daily to rats in groups 3 and 4, and the vehicle was administered daily to rats in groups 1 and 2. The drug was administered between 4 and 5 pm. At this time of the day, melatonin is considered to be at its lowest natural concentration in the blood. The dose of melatonin was selected on the basis of our previous works.6,7 All the procedures in this study are in accordance with the guidelines for the care and use of laboratory animals as adopted by the Ethics Committee, School of Medicine, Tehran University of Medical Sciences (210/27686, Nov 3, 2002).
4. Irradiation
The animals were anesthetized with an i.p. injection of ketamin (60 mg/kg) and xylazin (20 mg/kg) and then placed in a prone position. The rats of groups 2 and 3 were irradiated with gamma beam of Cobalt-60 teletherapy unit (theratron 760-C) to the 1.8 cm cervical segment of the spinal cord (C1-T2). A single dose of 22 Gy (at the dose rate of 1.8 Gy/min and source skin distance of 79.5 cm) was delivered to the depth of 0.5 cm based on lateral simulation radiographs. This dose is proposed as an effective dose for white matter necrosis and limbs paralysis after 20 weeks of irradiation.12 Control and melatonin-only groups were also sham-irradiated. Although the rats were anesthetized, they were not irradiated.
5. Sample preparations
The animals were sacrificed under ketamine and xylazine injection chronologically 3 weeks after irradiation. Tissue sampling was carried out using a posterior approach to the cervical spinal cord. One cm of spinal cord was dissected and used for different purposes. For real time RT-PCR, the spinal cord was embedded in GITC (6 molar) for the inactivation of enzymes and RNase-free condition, and then homogenized by Heidolf Homogenizer. All samples were stored at −70 °C until they were subsequently used.
6. RNA isolation and real time RT-PCR
The total RNA from the spinal cord was isolated using highly pure RNA extraction kit (Roche), following the manufacturer's instructions. A 2 μg sample of the total RNA (treated in 8 μL diethel pyrocarbonate water in an Ependorf tube) was denatured by incubation at 65 °C for 5 min, and the tube was placed on ice for 2 min. The quality of the extracted RNA was checked using denatured agarose gel and quantified with a BioPhotometer (Eppendorf, Canada). Specific primers and SYBR Green PCR Mix were purchased from the Superarray Company. The PCRs were carried out in a reaction volume of 25 μL consisting of 20 pmol of each primer, 2.5 IU Super Taq DNA polymerase, 5 μL cDNA, 0.5 μL dNTPs (10 mM), 2.5 μL PCR buffer, 2.5 mM MgCl2, and 1/10,000 SYBR Green as fluorogenic dye. Thermal cycling was initiated with an initial denaturation step of 94 °C for 3 min, and followed by the thermal profile of 94 °C (20 s) + 55° C (30 s) + 72° C (40 s) for 40 cycles in a Stratagen real-time system. A suitable threshold was applied to amplification plots, and the resulting Ct values (threshold cycles) were used for relative quantification. The Ct values of TNF-α were normalized according to the Ct values for β-actin, and the resulting values were compared to those of the control group through a 2−ΔΔCt method.
7. Biochemical survey
MDA levels (in μg/mg of protein) as an index of lipid peroxidation were measured in each of the tissue samples; accordingly, the samples were briefly homogenized in 1 ml of 0.9% cold saline. After the addition of 200 μL TCA (25%), they were then centrifuged at 6000 rpm for 15 min, and their absorbance of supernatant at 535 nm was measured.
8. Biochemical procedure for TNF-α protein assay
Tissue samples were homogenized in 400 ml PBS (pH 7.2). The homogenates were centrifuged at 15,000 rpm for 30 min at 41 °C. The supernatant was collected and stored at −78 °C until further analysis. The TNF-α concentration was determined using an enzyme-linked immunosorbent assay (ELISA) kit specific for TNF-α (Biosource, Camarillo, CA, USA), according to the manufacturer's specifications. The TNF-α level of each sample was evaluated as the TNF-α protein concentration (mg/l) divided by the total protein concentration (g/l) dissolved in a sodium dodecyl sulfate solution. Results were expressed as picograms per milligram of tissue protein (pg/mg protein).
9. Statistical analysis
The data are presented as mean ± SEM. The differences among the groups were analyzed using the analysis of variance (ANOVA) test followed by Tukey's multiple comparisons test. p < 0.05 was considered significant.
10. Results
10.1. TNF-α gene expression
Three weeks after irradiation, TNF-α gene up-regulated almost 5 fold in the irradiated group compared to the control group. TNF-α gene expression in the melatonin pretreatment group, compared to the radiation group, was significantly down-regulated 3 weeks after irradiation (p < 0.05). There was no significant difference between the control and melatonin-only groups (Fig. 1).
Fig. 1.
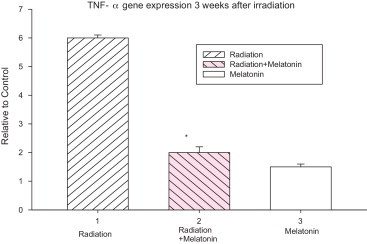
Effect of melatonin pre treatment on TNF-α gene expression at 3 weeks after exposure to 22 Gy irradiation. Vertical bars represent mean ± SEM, n = 5 for each group.
10.2. TNF-α protein expression
We next assessed whether the influence of melatonin on TNF-α mRNA levels also corresponded to a decreased production of TNF-α protein. Three weeks after irradiation, TNF-α protein levels were measured in the samples by ELISA. At this time point, TNF-α levels in the spinal cord tissue samples were found to be significantly higher in the irradiation group than in the control group (p < 0.05). The levels of TNF-α protein were notably lower in the radiation + melatonin group compared to that in the radiation-only group (p < 0.05). No significant differences in TNF-α protein levels were seen in the control and melatonin-only groups. The TNF-α protein levels for all experimental groups are shown in Fig. 2.
Fig. 2.
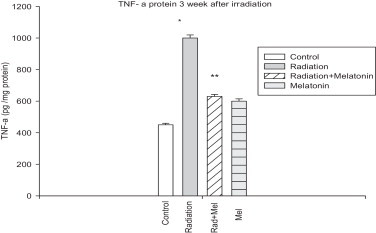
Effect of melatonin pre treatment on TNF-α protein expression at 3 weeks after exposure to 22 Gy irradiation. Vertical bars represent mean ± SEM, n = 5 for each group.
10.3. Tissue MDA levels
MDA levels of all experimental groups are shown in Fig. 3. Tissue MDA levels in the spinal cord samples were found to be significantly higher in the irradiation group than in the control group (p < 0.05). Oral administration of melatonin (100 mg/kg) significantly reduced MDA levels in the spinal cords of rats subjected to irradiation. No significant differences were observed between the levels of MDA in the spinal cord tissues of the control and melatonin-only groups.
Fig. 3.
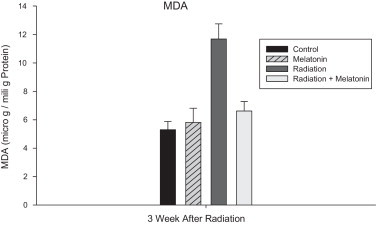
Effect of pre-treatment with melatonin on MDA levels (microgram per milligram protein) 3 weeks after irradiation. MDA levels of the irradiated group are significantly higher than that of the control group, Melatonin significantly reduced MDA levels in the spinal cords of rats subjected to irradiation. Data are given as mean ± SEM of 5 rats.
11. Conclusion
In this study, we evaluated the expression of TNF-α gene, TNF-α protein and level of MDA as an index of lipid peroxidation in the spinal cord tissue after cervical irradiation. The effects of melatonin on these factors were then investigated.
Several molecular and cellular aspects of radiation myelopathy have been explored. The histopathologic appearance of RM is similar to that of other spinal cord injuries, which may result from hypoxia or trauma. Parallel to the induction of apoptosis, the activation of TNF-α gene expression seems to be highly important for the development of radiation-induced spinal cord injury.8
TNF-α is an important mediator for inflammation and tissue injury. Stimulated granulocytes are the main source of TNF-α. Glial cells also produce TNF-α after stimulation. TNF-α activates the inflammatory cascade by stirring the production of several cytokines and chemokines, and by enhancing endothelial adhesion molecules expression on vascular endothelial cells that promote neutrophil adherence to these cells. Several studies provided evidence that the expression of TNF-α is directly activated by ionizing radiation.13–15 Furthermore, it was shown previously that after spinal cord injury, TNF-α might serve as an external signal, initiating apoptosis in neurons and oligodendrocytes. In addition, TNF-α increases the endothelial cell permeability and induces the release of other inflammatory mediators, which may contribute to the increased permeability.8,16
Lipid peroxidation is a fundamentally harmful reaction and is considered in various types of neurodegenerative disorders. MDA, the product of lipid peroxidation, is toxic to cells. The amount of lipid peroxidation is a useful parameter for evaluating radiation-induced spinal cord injury.17
Overexpression of TNF-α in the irradiated group indicates that this cytokine has a role in the radiation-induced spinal cord injury. In an animal model of lung irradiation, similar TNF-α mRNA and protein expression patterns were observed.14,15 Our results, that are in good agreement with what has been mentioned in previous studies, indicate that an increase in MDA level is an indicator of radiation injury and most likely tissue membrane damage due to the production of free radicals through ionizing radiation.10,11,17
There was a good correlation between changes in TNF-α expression and MDA level three weeks after irradiation. This result indicates that overexpression of this cytokine may result in lipid peroxidation (proinflammatory effect of TNF-α may be involved in the increased level of MDA).
Lipid peroxidation has a disturbing effect on the functional state of the membrane because of decreasing membrane fluidity and, accordingly, changing the Ion exchange process. Excessive lipid peroxidation can initiate the inflammation processes and TNF-α expression, as observed in this study.
Currently, there is a great interest in the potential value of melatonin as an inhibitor of normal tissue injury caused by radiation.18 There is no current evidence to suggest that melatonin protects cancerous cells from the destructive effects of ionizing radiation. In fact, other researchers have observed that melatonin may enhance tumor radiosensitivity through apoptosis activation.19 With respect to the amelioration of radiation myelopathy, several observations suggested that melatonin might be a useful therapeutic agent.18,20
Melatonin has also been reported to significantly reduce radiation-induced injury in rats. Genovese's study reported that melatonin attenuated the release of TNF-α in spinal cord trauma.9 In an animal model of lung irradiation, melatonin treatment of mice partially suppressed radiation-induced TNF-α expression in the lung tissue.15 Our previous study showed that intraperitonial (i.p) injection of melatonin could modulate overexpression of TNF-α due to spinal cord irradiation.21
The present data provided evidence that oral administration of melatonin modulated the TNF-α overexpression and MDA level. The results of our previous study and this study indicate that oral administration and i.p injection of melatonin have the same effect on irradiated cervical spinal cord. The exact mechanism through which melatonin decreases the levels of MDA in the spinal cord tissue has not yet been fully determined. Probably, TNF-α overexpression results in reactive oxygen species (ROS) generation, and ROS can in turn enhance the lipid peroxidation process.22 Furthermore, TNF-α directly induces the oxidative stress of the cells by depleting the GSH, which is the most abundant and vital antioxidant of the body.13 Melatonin, as a free radical scavenger, attenuates ROS generation, inhibits oxidative stress, reduces lipid peroxidation, and enhances GSH depletion.18–24 It has been suggested that melatonin may also have strong anti-inflammatory actions.11,14
Most probably, the regulation of TNF-α is a complicated process; in addition to the anti-oxidative effects of melatonin, the anti-inflammatory role of this agent was involved in the regulation of TNF-α expression.
This study found a significant radiation-induced increase of TNF-α in the spinal cord tissue during the early phase. In addition, our results indicate that melatonin down-regulates the TNF-α mRNA as well as protein production in the spinal cord tissue in response to radiation. Inhibition of the acute phase is thought to be important for the prevention of radiation myelopathy, so pharmacological regulation of the TNF-α production may thus ameliorate severe consequences of spinal cord irradiation. Therefore, melatonin may provide protection against radiation-induced cellular damage as a cytokine-mediated.
Conflict of interest
None declared.
Financial disclosure
This study was supported by grant number 92004 from the Vice Chancellor of Research at Fasa University of Medical Sciences.
Acknowledgments
We are grateful to Dr Alireza Shirazi for his financial support and Dr Zargham sepehrizadeh for his kind efforts in doing the Real time PCR procedure of this study. We also express our gratitude to the staff of the Radiotherapy Department at Cancer Institute especially Mr Amir Hooshang Sedigh for the great help.
Contributor Information
Gholam Hassan Haddadi, Email: ghadadi@gmail.com.
Reza Fardid, Email: rfardid@sums.ac.ir.
References
- 1.Bij H.P., Van luijik P., Coppes R.P. Unexpected changes of rat cervical spinal cord tolerance caused by inhomogenous dose distributions. Int J Radiat Oncol Biol Phys. 2003;57:274–281. doi: 10.1016/s0360-3016(03)00529-7. [DOI] [PubMed] [Google Scholar]
- 2.Nider C., Ataman F., Price R.E., Ang K.K. Radiation Myelopathy. New Perspective on an old problem. Radiat Oncol Investig. 1999;7(4):193–203. doi: 10.1002/(SICI)1520-6823(1999)7:4<193::AID-ROI1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Schultheiss T.E. Spinal cord radiation tolerance. Doctrine vs data. Int J Radiat Oncol Biol Phys. 1990;19:219–221. doi: 10.1016/0360-3016(90)90157-f. [DOI] [PubMed] [Google Scholar]
- 4.Bijl H., Luijk P., Coppes R.P., Schippers J.M., Konings A.W., Van der Kogel A. Unexpected changes of rat cervical spinal cord tolerance caused by inhomogeneous dose distributions. Int J Radiat Oncol Biol Phys. 2003;57:274–281. doi: 10.1016/s0360-3016(03)00529-7. [DOI] [PubMed] [Google Scholar]
- 5.Nieder C., Andratschke N., Astner S. Experimental concepts for toxicity prevention and tissue restoration after central nervous system irradiation. Radiat Oncol. 2007;2:23–27. doi: 10.1186/1748-717X-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddadi G.H., Shirazi A.R., Sepehrizadeh Z., Mahdavi S.R., Haddadi M. Radioprotective effect of melatonin on the cervical spinal cord in irradiated rats. Cell J (Yakhteh) 2013;14(4):246–253. [PMC free article] [PubMed] [Google Scholar]
- 7.Shirazi A., Haddadi G.H., Minaee B., Sepehrizadeh Z., Mahavi S.R., Jaberi E. Evaluation of melatonin for modulation of apoptosis-related genes in irradiated cervical spinal cord. Int J Low Radiat. 2010;7(6):436–445. [Google Scholar]
- 8.Belka C., Budach W., Kortmann R.D., Bamberg M. Radiation induced CNS toxicity–molecular and cellular mechanisms. Brit J Cancer. 2001;85(9):1233–1239. doi: 10.1054/bjoc.2001.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genoves T., Mazzon E., Muia C., Bramanti P., Sarro A., Cuzzocrea S. Attenuation in the evolution of experimental spinal cord trauma by treatment with melatonin. J Pineal Res. 2005;38:198–208. doi: 10.1111/j.1600-079X.2004.00194.x. [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto T., Nakamura T., Ikeda T., Takagi K. Potent protective effects of melatonin on experimental spinal cord injury. Spine. 2000;25(7):769–775. doi: 10.1097/00007632-200004010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Kaptanoglu E., Tuncel M., Palaoglu S., Konan A., Demirpençe E. Comparison of the effects of melatonin and methylprednisolone in experimental spinal cord injury. J Neurosurg. 2000;93:77–84. doi: 10.3171/spi.2000.93.1.0077. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson S., Li U.Q., Wong C.S. Changes in oligodendrocytes and myelin gene expression after radiation in the rodent spinal cord. Int J Radiat Oncol Biol Phys. 2003;57:1093–1100. doi: 10.1016/s0360-3016(03)00735-1. [DOI] [PubMed] [Google Scholar]
- 14.Mukhopadhyay S., Hoidal J.R., Mukherjee T.K. Role of TNF in pulmonary pathophysiology. Respir Res. 2006;7:125. doi: 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rube C.E., Wilfert F., Uthe D., Schmid K.W., Knoop R., Willich N. Modulation of radiation-induced tumor necrosis factor-α (TNF-α) expression in the lung tissue by pentoxifyline. Radiother Oncol. 2002;64:177–187. doi: 10.1016/s0167-8140(02)00077-4. [DOI] [PubMed] [Google Scholar]
- 16.Jangss, Kim H.G., Lee J.S., Han J.M., Park H.J., Huh G.J. Melatonin reduces X-ray radiation-induced lung injury in mice by modulating oxidative stress and cytokine expression. Int J Radiat Biol. 2013;89(2):97–105. doi: 10.3109/09553002.2013.734943. [DOI] [PubMed] [Google Scholar]
- 17.Hallahan D.E., Spriggs D.R., Beckett M.A., Kufe D.W., Weichselbaum R.R. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Nat Acad Sci USA. 1989;86:10104–10107. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirazi A., Haddadi G.H., Ghazi-Khansari M., Abolhassani F., Mahdavi S.R. Evaluation of melatonin for prevention of radiation myelopathy in irradiated cervical spinal cord. Yakhteh Med J. 2009;11(1):43–48. [Google Scholar]
- 19.Shirazi A., Ghobadi G., Ghazi-Khansari M. A radiobiological review on melatonin: a novel radioprotector. J Radiat Res (Tokyo) 2007;48:263–272. doi: 10.1269/jrr.06070. [DOI] [PubMed] [Google Scholar]
- 20.Di Bella G., Mascia F., Gualano L., Di Bella L. Melatonin anticancer effect: review. Int J Mol Sci. 2013;14(2):2410–2430. doi: 10.3390/ijms14022410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang Y., Xu S.P., Wu Y. Melatonin reduces acute lung injury in endotoxemic rats. Chin Med J. 2009;122(12):1388–1393. [PubMed] [Google Scholar]
- 22.Haddadi G.H., Shirazi A.R., Sepehrizadeh Z., Haddadi M., Refahi S. Modulation of Radiation–induced Tumor Necrosis Factor (TNF-α) Gene Expression in the Rats Spinal Cord by Melatonin. World Congress on Medical Physics and Biomedical Engineering. IFMBE Proc. 2012;39:38–40. [Google Scholar]
- 23.Tahamtan R., Shabestani Monfared A., Tahamtani A. Radio protective effect of melatonin on radiation-induced lung injury and lipid peroxidation in rat. Cell J (Yakhteh) 2015;17(1) doi: 10.22074/cellj.2015.517. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirazi A.R., Fardid R., Mihandoost E. Protective effect of low dose melatonin on radiation-induced damage to rat liver. J Biomed Phys Eng. 2012;2(2):66–71. [Google Scholar]


