Abstract
Objectives
Since vitamin D insufficiency is common worldwide in people with HIV, we explored safety and efficacy of high dose cholecalciferol (D₃) in Botswana, and evaluated potential modifiers of serum 25 hydroxy vitamin D change (Δ25D).
Design
Prospective randomized double-blind 12-week pilot trial of subjects ages 5.0–50.9 years.
Methods
Sixty subjects randomized within five age groups to either 4000 or 7000IU per day of D₃ and evaluated for vitamin D, parathyroid hormone, HIV, safety and growth status. Efficacy was defined as serum 25 hydroxy vitamin D (25D) ≥32ng/mL, and safety as no simultaneous elevation of serum calcium and 25D. Also assessed were HIV plasma viral RNA viral load (VL), CD4%, anti-retroviral therapy (ART) regime, and height-adjusted (HAZ), weight-adjusted (WAZ) and Body Mass Index (BMIZ) Z scores.
Results
Subjects were 50% male, age (mean±SD) 19.5±11.8 years, CD4% 31.8±10.4, with baseline VL log₁₀ range of <1.4 to 3.8 and VL detectable (>1.4) in 22%. From baseline to 12 weeks, 25D increased from 36±9ng/ml to 56±18ng/ml (p<0.0001) and 68% and 90% had 25D ≥32ng/ml, respectively (p = 0.02). Δ25D was similar by dose. No subjects had simultaneously increased serum calcium and 25D. WAZ and BMIZ improved by 12 weeks (p<0.04). HAZ and CD4% increased and VL decreased in the 7000IU/d group (p<0.04). Younger (5–13y) and older (30–50y) subjects had greater Δ25D than those 14–29y (26±17 and 28±12 vs. 11±11ng/ml, respectively, p≤0.001). Δ25D was higher with efavirenz or nevirapine compared to protease inhibitor based treatment (22±12, 27±17, vs. 13±10, respectively, p≤0.03).
Conclusions
In a pilot study in Botswana, 12-week high dose D₃ supplementation was safe and improved vitamin D, growth and HIV status; age and ART regimen were significant effect modifiers.
Trial Registration
ClinicalTrials.gov NCT02189902
Introduction
Suboptimal vitamin D status is common in people with HIV [1,2,3]. Inadequate sunlight, low dietary intake, increased utilization, drug therapies, malabsorption, or unknown HIV-associated factors may contribute [1,2,4,5]. Observational studies suggest that vitamin D status may impact HIV disease severity [1,6,7,8,9]. Serum 25-hydroxy vitamin D (25D) concentration was positively correlated with CD4+ counts in Norway [6], Germany [7,8] and in the USA [1]. Vitamin D supplementation was associated with changes in CD4 T-cell phenotype in Italy [9].
In Africa, vitamin D insufficiency and rickets are prevalent despite adequate sunlight exposure [10]. Insufficient 25D status (<32ng/ml) in Gambia was noted in 23% children and 55% of young women [11]. Data on vitamin D status in HIV-infected people from sub-Saharan Africa are limited. Among HIV-infected pregnant women in Tanzania, 39% had low 25D (<32ng/ml) with more rapid HIV progression, higher all-cause mortality and a higher incidence of anemia than women with higher 25D [12]. In HIV-infected adults from Botswana a quarter of subjects were vitamin D insufficient [13]. Despite these data suggesting that vitamin D insufficiency is prevalent in parts of Africa, the cholecalciferol (D3) dose needed to improve vitamin D status in this setting is unknown.
The objective of this pilot study was to test the safety and efficacy of two oral daily doses of D3 over 12 weeks in children and adults with HIV in Botswana.
Patients and Methods
The protocol for this trial and supporting CONSORT checklist are available as supporting information; see S1 CONSORT Checklist and S1–S4 Protocols. Patients were recruited from 13th December 2011 to 18th April, 2012 from an outpatient clinic at Princess Marina Hospital in Gaborone, Botswana. The last 12 week follow-up visit was completed on 12th July 2012. Eligible participants were aged 5.0 to 50.9 years, HIV infected, on first line antiretroviral therapy (ART), and in usual state of good health. “In usual state of good health” was defined as “no visit to a doctor, nurse or clinic for an acute medical condition over the 2 weeks prior to enrollment.” Exclusion criteria included HIV-unrelated chronic conditions that may affect growth, dietary intake or nutritional status. These chronic conditions were diarrhea for more than 14 days, malabsorption, renal disease requiring vitamin D supplementation, renal disease with a creatinine greater than 2.0mg/dL or oral steroid use for more than 14 days.
Sixty subjects were enrolled, 12 in each age group (5.0–8.9, 9.0–13.9, 14.0–18.9, 19.0–30.9, 31.0–50.9 years), and randomized to either 4000 or 7000IU/d of D3 for 12 weeks. Clinical data were extracted from medical records and a structured questionnaire. HIV was classified using the Centers for Disease Control and Prevention (CDC) clinical classification [14,15] and CD4 count.
ART regimens were characterized as tenofovir-containing, and either protease inhibitor-based (PI), or non-nucleoside reverse transcriptase inhibitor-based (NNRTI), being efavirenz or nevirapine. All subjects receiving tenofovir were also on NNRTIs.
Research nurses were trained in research quality anthropometric measurement (by JIS, APS). Height was measured by stadiometer (Seca, UK) and weight by digital standing scale (Adam Medical) for adults and a wheel chair digital scale (Seca, UK) in children. Body mass index (BMI) was calculated. Scales were calibrated weekly. Measurements were done in triplicate [16] and the mean used for analysis. For subjects under 20 years, weight, height and BMI were compared to reference standards to generate age- and sex-specific Z scores [17].
Laboratory investigations
Tests performed at baseline, 6 and 12 weeks included: serum 25D using liquid chromatography tandem mass spectrometry (Clinical Laboratory, the Children’s Hospital of Philadelphia [CHOP], Philadelphia, PA, USA) with intra- and inter-assay coefficients of variation (CV) below 8%; serum 1,25-dihydroxyvitamin D (1,25D) and intact parathyroid hormone (PTH) by radioimmunoassay using a radio-iodinated tracer (Heartland Assays, Ames, IA, USA) with intra- and inter-assay CV of 9.8% and 12.6% for 1,25D and 2.7% and 4.3% for PTH; vitamin D binding protein (DBP) by enzyme linked immunosorbent assay (R&D Systems, Minneapolis, MN) with intra- and inter-assay CVs below 10%. Bioavailable 25D (ng/mL) was calculated as that unbound to DBP or albumin, and bioavailable/total 25(OH)D (%) derived [18]. Safety was assessed by serum albumin, calcium (corrected for albumin), magnesium, phosphorus and whole blood lead (Diagnofirm Diagnostic Laboratory, Gaborone, Botswana) using standard techniques. CD4 count, and HIV-1 RNA viral load (VL) were measured at baseline and 12 weeks at either Botswana Harvard or Diagnofirm Laboratories. Undetectable VL was defined as <25 copies/mL (RNA log ≤1.4). Per protocol analysis required assessment of serum cathelicidin antimicrobial protein activity—however the volume of blood collected from all subjects was insufficient to perform all per protocol tests. Hence the study team elected not to perform this test.
Treatment and adherence
Study medication consisted of 4000IU/d (Vitacost Vitamin D3, Boca Raton, FL, USA) or 7000IU/d (Life Extension, Ft. Lauderdale, FL, USA) D3 tablets. Doses were verified (Tampa Bay Analytical Research, Largo, FL, USA). The randomization sequence assigned subjects in a 1:1 ratio within each age group, was generated using STATA 12.0 (College Station, TX) and given to a research pharmacist who labeled the medications. Clinicians, investigators and participants were blinded to randomization. Adherence was assessed by pill count from returned bottles and determined as a percent.
Assessment of outcome
Efficacy was defined as serum 25D ≥32ng/mL at 12-weeks; safety as a low incidence (<5%) of subjects with a simultaneously elevated serum calcium (using age and sex specific ranges) and 25D (>160ng/mL). Nurses interviewed participants after 2 weeks and at each visit for the occurrence of adverse events. Laboratory results were reviewed within 24 hours.
Statistical analysis
All variables were tested for normality and nonparametric tests were used as appropriate. Means, medians and other parameters as appropriate described outcome variables at study visits. Differences between groups at baseline or over time were assessed using unpaired t tests or Mann Whitney U tests as appropriate and chi-squared tests for categorical variables. Significance of change from baseline over time was assessed using longitudinal mixed effects (LME) models adjusting for baseline values, age and sex. Age group and ART regimen were explored as effect modifiers of 25D change (Δ25D). Models were adjusted for potential predictors of 25D including baseline 25D, sex, adherence, season, and years of ART, with age group x time or ART regime x time interaction terms for differences between groups in Δ25D. The five age groups used for enrollment stratification were collapsed to 3 groups for analyses for children (5–13y), adolescents/young adults (14–29y) and older adults (30–50y). Statistical analyses were performed using STATA 12.0 (College Station, TX), and results considered significant at p<0.05.
Ethics statement
Ethics Boards of the Botswana Ministry of Health, Princess Marina Hospital, Baylor College of Medicine, the University of Pennsylvania and CHOP approved the study. Adults gave written informed consent. For those under 21 years, a legal guardian provided written informed consent, and written assent was given by those 7 to 21 years. Due to an oversight error, this trial was not registered in a clinical trials registry before it was completed—it was however registered on clinicaltrials.gov before manuscript submission (ClinicalTrials.gov Identifier: NCT02189902). The authors confirm that all ongoing and related trials for this drug/intervention are registered.
Results
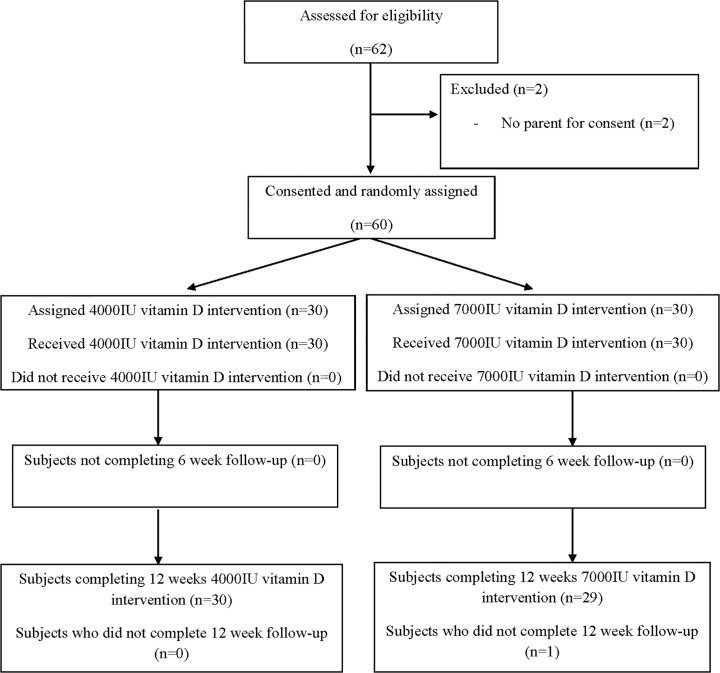
After prescreening, 62 subjects were assessed for eligibility and 60 subjects were enrolled (Fig. 1 shows the consort diagram). Of 60 subjects enrolled, 30 received 4000IU/d and 30 received 7000IU/d D3, evenly by age group. Fifty-nine completed all study visits. Subjects were 50% male, 68% perinatally infected, age 19.5±11.8 years (mean±SD) with predominantly moderate to severe AIDS defining illness (Table 1). At enrollment, 23 to 43% of those under 20 years had height adjusted Z score (HAZ), weight adjusted Z score (WAZ) or body mass index Z score (BMIZ) more than two standard deviations below the reference median. More males were in the 7000IU/d group, but groups did not otherwise differ at baseline. The mean (SD) duration of ART for the whole cohort was 5.0 years (±2.7) and did not differ between D3 dose groups.
Fig 1. Consort flow diagram for subjects randomized, drop-outs, and completing the trial of daily 4000IU or 7000IU vitamin D3 supplementation in HIV-infected children and young adults.
Table 1. Characteristics of Subjects at Baseline by D3 Dose Group.
| All | 4,000 IU | 7,000 IU | |
|---|---|---|---|
| N | 60 | 30 | 30 |
| Age, y | 19.5 ± 11.8 | 19.5 ± 11.8 | 19.5 ± 12.0 |
| Sex, % male | 50 | 37 | 63* |
| Perinatally acquired a , % | 68 | 66 | 70 |
| Season, % | |||
| Summer: Nov—Jan | 37 | 33 | 40 |
| Autumn: Feb—Apr | 63 | 67 | 60 |
| Pediatric nutritional status (n = 40) b | |||
| Height Z score≤2, % | 28 | 35 | 20 |
| Weight Z score≤2, % | 43 | 45 | 40 |
| BMI Z score≤2, % | 23 | 20 | 25 |
| BMI > 85th percentile, % | 3 | 1 (5 | 0 (0) |
| Adult nutritional status (n = 20) b | |||
| Height, cm | 163.0 ± 9.4 | 162.0 ± 10.9 | 164.1 ± 8.2 |
| Weight, kg | 58.3 ± 13.9 | 59.4 ± 18.3 | 57.2 ± 8.4 |
| BMI, kg/cm2 | 22.0 ± 5.8 | 22.8 ± 7.7 | 21.3 ± 3.4 |
| BMI<18, % | 20 | 10 | 10 |
| BMI>30, % | 5 | 10 | 0 |
| BMI>25, % | 20 | 30 | 10 |
| Years since HIV diagnosis c | 6.2 ± 2.7 | 6.5 ± 2.5 | 5.8 ± 2.9 |
| Duration of ART treatment d , y | 5.0 ± 2.7 | 5.2 ± 2.6 | 4.9 ± 2.8 |
| CDC HIV Classification e (%) | |||
| N | 14 | 14 | 14 |
| A | 20 | 21 | 18 |
| B | 11 | 11 | 11 |
| C | 55 | 54 | 57 |
| Immunity category: worst (%) | |||
| CD4 count ≥ 500 | 29 | 22 | 35 |
| CD4 count 200–499 | 34 | 44 | 24 |
| CD4 count < 200 | 38 | 33 | 41 |
| Immunity category: current (%) | |||
| CD4 count ≥ 500 | 68 | 81 | 55 |
| CD4 count 200–499 | 27 | 19 | 34 |
| CD4 count < 200 | 5 | 0 | 10 |
| CD4 f % | 31.8 ± 10.4 | 33.8 ± 8.3 | 30.0 ± 11.9 |
| Viral load, RNAlog g | 1.54 ± 0.42 | 1.44 ± 0.18 | 1.64 ± 0.56 |
| Viral load, detectable % | 19 | 12 | 26 |
| ART Regimens, % | |||
| PI | 25 | 27 | 23 |
| NNRTI: ALL | 75 | 73 | 77 |
| Efavirenz | 33 | 30 | 37 |
| Nevirapine | 42 | 43 | 40 |
| Tenofovir h | 13 | 17 | 10 |
| & NNRTI | 13 | 17 | 10 |
| & PI | 0 | 0 | 0 |
*Dose groups significantly different p<0.05
a n = 59, one subject had unknown HIV acquisition
b n = 40 for subjects <20y with calculated Z scores for body size variables (20 in 4000 and 20 in 7000IU/d group) n = 20 for subject ≥20y (10 in 4000 and 10 in 7000IU/d group)
c n = 54 subjects with date of diagnosis for HIV (28 in 4000 and 26 in 7000IU/d group)
d n = 59 subjects with date of initiation of ART treatment (30 in 4000 and 29 in 7000IU/d group)
e n = 56 subjects with HIV CDC classification data and CD4 counts from medical record review (27 in 4000 and 29 in 7000IU/d group)
f n = 59 with CD4% baseline (29 in 4000 and 30 in 7000IU/d group)
g n = 53 RNA viral load at baseline (26 in 4000 and 27 in 7000IU/d group)
h Tenofovir was used only in combination with NNRTI-based regimens.
HIV, human immunodeficiency virus; BMI, body mass index; CDC, Center for Disease Control; Clinical categories: N = asymptomatic, A = mildly symptomatic, B = moderately symptomatic, and C = severely symptomatic with AIDS defining illness; CD4 cluster of differentiation; PI, protease inhibitor-based anti-retroviral treatment (ART); NNRTI, non-nucleoside reverse transcriptase-based ART.
D3 supplementation was effective as both serum 25D and bioavailable 25(OH)D increased from baseline in more than 80% of the participants (Table 2). The proportion of bioavailable to serum 25D did not change. Using LME models adjusted for baseline 25D, age, and sex, Δ25D at 6 and 12-weeks of treatment did not differ between groups (Fig. 2A). Two subjects at 6 weeks and two at 12 weeks (total 3 subjects), all in the 7000IU/d dose group, had 25D levels >90ng/ml [19].
Table 2. Clinical and laboratory values for subjects over time by D3 dose group.
| Baseline | 6 weeks | 12 weeks | ||||
|---|---|---|---|---|---|---|
| 4,000 IU | 7,000 IU | 4,000 IU | 7,000 IU | 4,000 IU | 7,000 IU | |
| N | 30 | 30 | 30 | 30 | 30 | 29 |
| Total 25(OH)D, ng/ml | 36.5 ± 9.3 | 34.5 ± 9.5 | 53.4 ± 11.7*** | 56.4 ± 21.0*** | 54.8 ± 13.0*** | 56.5 ± 22.4*** |
| ≤ 20 ng/ml, % | 3 | 7 | 0 | 3 | 0 | 3 |
| 20–31 ng/ml, % | 23 | 30 | 10 | 3 | 7 | 10 |
| ≥ 32 ng/ml, % | 73 | 63 | 90 | 94* | 93 | 87 |
| Bioavailable 25(OH)D, ng/ml a | 9.4 ± 2.7 | 8.5 ± 2.6 | - | - | 15.7 ± 6.1*** | 16.1 ± 8.2*** |
| Bioavailable/Total 25(OH)D, % a | 26.1 ± 3.7 | 26.2 ± 4.9 | - | - | 27.9 ± 7.0 | 29.1 ± 6.4 |
| 1,25D, pg/ml | 66.8 ± 33.1 | 57.2 ± 18.8 | - | - | 72.0 ± 34.0 | 71.1 ± 27.5*** |
| PTH, pg/ml± | 33.6 ± 18.9 | 29.8 ± 17.0 | - | - | 25.6 ± 10.4** | 24.4 ± 13.7* |
| DBP, umol/L a | 1.8 ± 0.4 | 1.7 ± 0.5 | - | - | 1.8 ± 0.7 | 1.7 ± 0.8 |
| Magnesium, mmol/L | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.2 | 0.9 ± 0.1* | 0.9 ± 0.1 |
| Phosphorous, mmol/L | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.2 ± 0.2 | 1.3 ± 0.3 | 1.2 ± 0.3 | 1.3 ± 0.3 |
| Calcium, mmol/L | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 |
| Albumin, g/L | 39.7 ± 3.3 | 38.9 ± 3.9 | 39.3 ± 3.4 | 38.9 ± 3.6 | 39.8 ± 3.2 | 39.5 ± 3.5 |
| Corrected Calcium, mmol/L | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.2 | 2.3 ± 0.1 | 2.3 ± 0.1 |
| Blood Lead, μg/dl | 2.2 ± 1.3 | 2.6 ± 2.6 | 2.4 ± 2.1 | 3.2 ± 5.3 | 2.8 ± 2.5* | 2.8 ± 3.2 |
| CD4 b , mm3 | 820 ± 421 | 758 ± 540 | __ | __ | 765 ± 334 | 693 ± 451* |
| CD4, % | 33.2 ± 8.0 | 29.7 ± 12.0 | __ | __ | 33.4 ± 7.2 | 31.5 ± 12.6** |
| Viral load c , copies/mL | 34 ± 39 | 367 ± 1324 | __ | __ | 776 ± 3348 | 351 ± 1478 |
| Viral load, RNA log | 1.46 ± 0.20 | 1.70 ± 0.62 | __ | __ | 1.57 ± 0.62 | 1.55 ± 0.54* |
| Viral load, % detectable ≥25 | 15 | 29 | __ | __ | 15 | 14 |
| Height Z Score d | -1.38 ± 1.14 | -1.48 ± 1.02 | -1.39 ± 1.16 | -1.42 ± 1.02* | -1.38 ± 1.13 | -1.42 ± 1.03** |
| Weight Z score | -1.60 ± 1.53 | -1.84 ± 1.42 | -1.61 ± 1.57 | -1.84 ± 1.42 | -1.50 ± 1.49* | -1.73 ± 1.42* |
| BMI Z score | -1.12 ± 1.48 | -1.20 ± 1.16 | -1.13 ± 1.51 | -1.28 ± 1.12 | -0.99 ± 1.43* | -1.11 ± 1.09 |
*Significantly different from baseline within the dose group at p<0.05 (LME model adjusted for age, sex and baseline value)
**p≤0.01
***p<0.001
a n = 49, Bioavailable 25(OH)D and DBP at baseline (24 at 4000 and 25 at 7000IU/d D3) and n = 51 12 weeks visit (26 at 4000 and 25 at 7000IU/d D3)
b n = 56, CD4 baseline and 12 weeks visit (27 at 4000 and 29 at 7000IU/d D3)
c n = 41, RNA viral load analysis baseline and 12-week visit (20 at 4000 and 21 at 7000IU/d vit D3)—as these 41 subjects had VL run at the same laboratory both times—baseline and 12 weeks
d n = 40 subjects <20y with calculated Z scores for body size variables (20 in 4000 and 20 in 7000IU/d group). HIV, human immunodeficiency virus; BMI, body mass index
CD4, cluster of differentiation; PTH, parathyroid hormone; DBP, vitamin D binding protein.
Fig 2. Serum 25D Before and After High Dose D3 Supplementation.
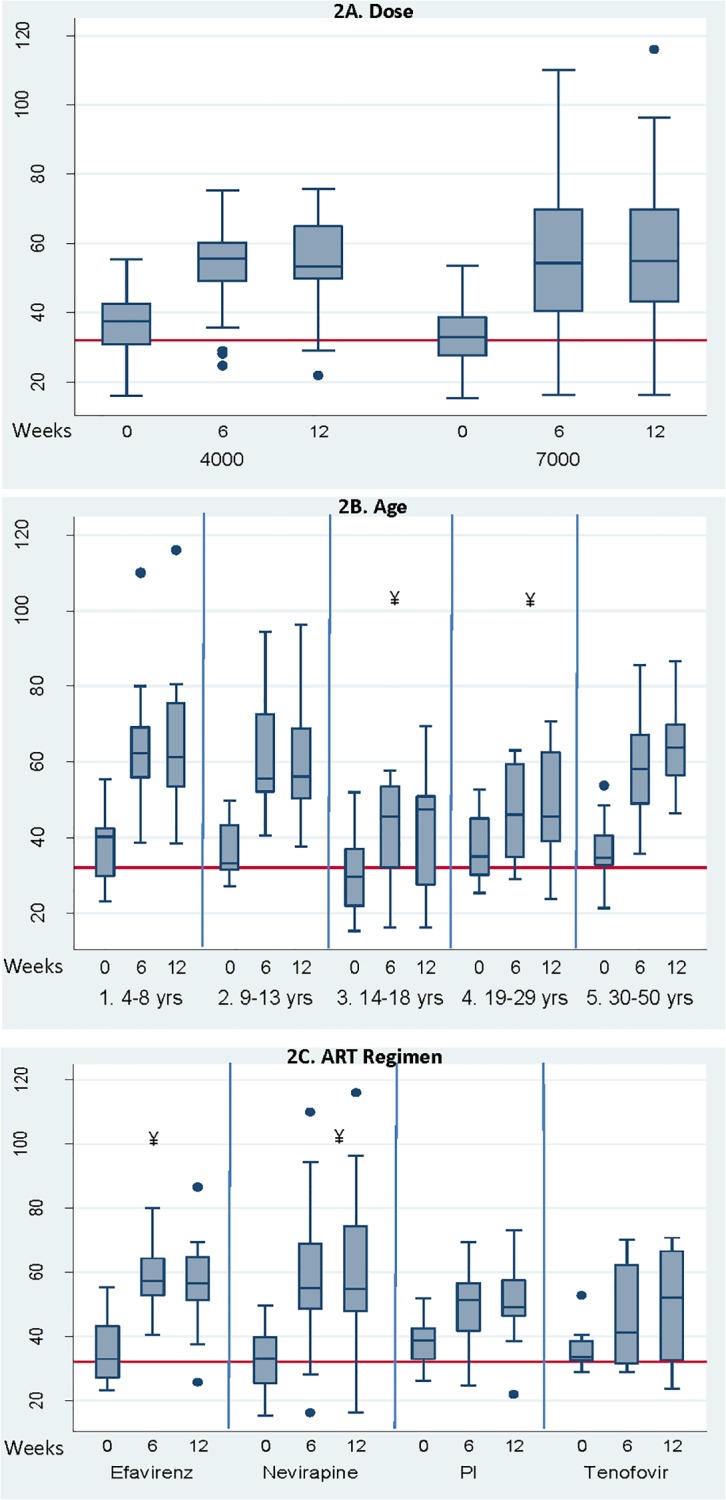
A. By Dose group (4000 IU/d or 7000IU/d). * Change in 25D significantly different from baseline at both 6 and 12 weeks, p<0.01. B. By age group. ¥ Change in 25D significantly less in subjects ages 14–29 y than those ages 4–13 y and 30–50 y age groups, p<0.004. C. By anti-retroviral therapy (ART) regime. ¥ Change in 25D significantly greater in Efavirenz and Nevirapine groups than in PI (protease inhibitor) or tenofovir groups, p<0.03
The increase in 25D with D3 supplementation was associated with decreased PTH (both groups), increased 1,25D in the 7000IU/d group and no change in DBP. WAZ and BMIZ increased in those under 20 years in both groups, and HAZ increased in the 7000IU/d group. For subjects older than 20 years, BMI increased 0.4 units at 12 weeks (p = 0.02).
Adherence to D3 was 84±17%; and did not differ by dose—4000 and 7000IU/d dose group’s adherence was 86±15% vs. 81±15%, respectively. D3 supplementation was safe with no simultaneous elevations in serum calcium and 25D or adverse clinical or laboratory events recorded. There was a 5% incidence of high serum calcium at 6 weeks (2/30 [7%] and 1/30 [3%] in the 4000 and 7000 IU/d dose groups, respectively) which required no clinical intervention. The groups did not differ in the incidence of high serum calcium and there was no one with high serum calcium at either baseline or 12 weeks. Outcomes did not differ by HIV acquisition.
Both age group and ART regimen were significant effect modifiers of 25D response to D3 supplementation (Fig. 2B and 2C, respectively). From unpaired t tests, younger children (5 to 13 years) and older adults (30 to 50 years) had greater Δ25D than adolescents and younger adults (14 to 29 years), with Δ25D of 26±17, 28±12 and 11±11ng/ml, respectively (p≤0.001) (Fig. 1B). Using LME models adjusted for baseline 25D, sex, adherence, season, and years of ART, with age group x time interaction, the 14 to 29 year age group had a lower Δ25D than the other age groups (Coefficient [95% confidence intervals], -1.3ng/mL/wk [-2.0,-0.6], R2 = 0.60, p<0.001). Only 75% of adolescents and young adults achieved 25D >32ng/mL compared to 100% of younger and older groups (p<0.05). Adherence did not differ between age groups (81 to 87%) or ART regimen (82 to 85%) and did not explain differences in Δ25D in any model.
For ART regimen (Fig. 2C), Δ25D was two-fold higher in subjects receiving efavirenz (22±12) or nevirapine (27±17) compared to those receiving PIs (13±10) (p≤0.03). At 6 weeks, both NNRTI regimens resulted in greater Δ25D than those receiving PIs (p<0.03). LME models adjusted for baseline 25D, age, sex, adherence, season and years of ART, with ART regimen x time interaction, showed a greater Δ25D in NNRTI regimens compared to PIs (0.8ng/mL/wk [0.1,1.5], R2 = 0.57, p<0.044). Those on nevirapine experienced significantly greater Δ25D over time than those treated with PI-based regimens (1.1ng/mL/wk [0.3, 2.0] R2 = 0.58, p = 0.007). Subjects receiving tenofovir (n = 8, all on NNRTI regimens) did not differ in Δ25D from subjects on other regimens.
Discussion
In this pilot study, daily 4000 and 7000 IU D3 supplementation over 12 weeks was safe and improved vitamin D status. Age and ART regimen modified the response, and mode of HIV acquisition did not. Of note, weight status improved in children and adolescents receiving either dose and height status improved in those receiving 7000IU/d after 12-weeks. Both doses resulted in more than 80% of subjects achieving a 25D >32ng/mL. These high vitamin D doses were safe with no treatment-related clinical or laboratory adverse events. Previous pediatric and young adult vitamin D supplementation studies were based in North America and utilized lower doses and longer dose intervals than used here [20,21,22].
In the present study, vitamin D supplementation was associated with improved weight and BMI status in children and adolescents, with improved height in those on 7000IU/d. BMI increased in adults (20 to 50 years), despite the relatively short 12-week duration. Other vitamin D studies of children with HIV have either not reported on growth [20,21] or did not find a change [22,23]. The findings of improved WAZ and BMIZ with both D3 doses and HAZ in the 7000IU/d group were unexpected. However, the 12-week increase of 0.10 and 0.11 in weight and WAZ or BMIZ, if sustained, represents a change of 0.4 to 0.5 Z scores/year, a potentially clinically significant improvement.
Ganmaa et al [24] showed an increase in height with D3 supplementation in 120 Mongol school children (12–18y) who received 800IU/d D3 compared to placebo for six months encompassing winter. In the same region [25], a vitamin D-fortified whole milk-based supplement in 46 children (9 to 11 years) demonstrated an increase in linear growth over one-month. A seasonal growth pattern in healthy children has been demonstrated, with increased growth in sunny seasons, both in resource-rich and resource-limited environments, and a vitamin D mechanism has been postulated [26,27]. Our Batswana sample with relatively poor baseline growth status is among the first to show improvement in growth status with D3 supplementation in children with HIV. Although seasonal growth resulting from seasonal variations in food supply or infectious burden has been reported from other African settings [28], this has not been described in Botswana and the effect of season was insignificant in our analyses. Change in 25D was significantly less in our adolescents and young adults than in children or older adults. This finding was not explained by differences in sex, adherence, ART regimen, or time on ART.
Several studies have documented reduced vitamin D status in people with HIV treated with NNRTIs [2,4,5,20,29,30]. Our study is the first to document this in an African setting. Little is known regarding the effect of ART regimen on response to D3 supplementation. A 12-week D3 supplementation study of American youth (18–24y) found that efavirenz did not diminish response to D3 despite being associated with poorer vitamin D status at baseline [20]. In our sample, children and adults treated with an NNRTI did not have poorer vitamin D status at baseline, however, they had a significantly larger change in 25D with high dose D3 supplementation than those on PIs. The vitamin D response for the eight subjects on tenofovir plus NNRTIs did not differ from those on non-tenofovir based regimens. The more blunted response in those on PIs may suggest a possible alteration in metabolism of D3. Powe et al [31] demonstrated the importance of DBP status in black compared to white Americans with black participants having lower DBP and 25D, but similar bioavailable 25(OH)D. In our Botswana African sample, the DBP was even lower (1.8 μmol/L) than that seen in American blacks in the Powe et al. study (3.3 μmol/L) [31].
This pilot study included a number of limitations. The small sample size and single site experience limits generalizability and requires further study in multisite samples. Without a control group, it is not possible to evaluate if the changes measured were attributable to the intervention or were the positive effect of enrollment in the study. The mixed age population and the mixed background ART add heterogeneity to the study population and were accounted for in the analysis. To assess the effects on growth, a future study should only target those under 20 years of age.
Micronutrient supplementation which did not contain vitamin D reduced the risk of immune status decline and morbidity in ART-naïve HIV-infected adults in Botswana [32]. Our results in this pilot study suggest that high dose vitamin D supplementation is safe in the African setting. Additionally, the 7,000IU/d may convey a growth in height advantage in children and adolescents. Further study with a larger sample size and including a control group is warranted to understand mechanisms underlying differences between ART regimens in response to D3 supplementation as well as to explore the growth and age responses.
Supporting Information
(DOC)
(PDF)
(PDF)
(PDF)
(PDF)
(XLSX)
(PDF)
Acknowledgments
The authors are grateful to the subjects and their families for study participation. In addition we wish to thank our colleagues in the Ministry of Health who provide day-to-day care for these patients, as well as to the leadership of all organizations involved whose work makes cross-institutional partnerships and collaborations possible and Dr. Kelly A. Dougherty for her review of an earlier draft of the manuscript. We also thank Life Extension Vitamin D3 (Ft. Lauderdale, FL) and Vitacost Vitamin D3 (Boca Raton, FL) for the donation of supplement to the study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Jean A. Cortner Endowed Chair, Nutrition Center and the Research Institute at Children's Hospital of Philadelphia (CHOP). The project described was supported by the National Center for Research Resources, Grant UL1RR024134, and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rutstein R, Downes A, Zemel B, Schall J, Stallings V (2011) Vitamin D status in children and young adults with perinatally acquired HIV infection. Clinical nutrition 30: 624–628. 10.1016/j.clnu.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 2. Dao CN, Patel P, Overton ET, Rhame F, Pals SL, et al. (2011) Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D Levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 52: 396–405. [DOI] [PubMed] [Google Scholar]
- 3. Meyzer C, Frange P, Chappuy H, Desse B, Veber F, et al. (2013) Vitamin D deficiency and insufficiency in HIV-infected children and young adults. The Pediatric infectious disease journal 32: 1240–1244. 10.1097/INF.0b013e3182a735ed [DOI] [PubMed] [Google Scholar]
- 4. Conesa-Botella A, Florence E, Lynen L, Colebunders R, Menten J, et al. (2010) Decrease of vitamin D concentration in patients with HIV infection on a non nucleoside reverse transcriptase inhibitor-containing regimen. AIDS research and therapy 7: 40 10.1186/1742-6405-7-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Welz T, Childs K, Ibrahim F, Poulton M, Taylor CB, et al. (2010) Efavirenz is associated with severe vitamin D deficiency and increased alkaline phosphatase. AIDS 24: 1923–1928. 10.1097/QAD.0b013e32833c3281 [DOI] [PubMed] [Google Scholar]
- 6. Haug C, Muller F, Aukrust P, Froland SS (1994) Subnormal serum concentration of 1,25-vitamin D in human immunodeficiency virus infection: correlation with degree of immune deficiency and survival. The Journal of infectious diseases 169: 889–893. [DOI] [PubMed] [Google Scholar]
- 7. Teichmann J, Stephan E, Discher T, Lange U, Federlin K, et al. (2000) Changes in calciotropic hormones and biochemical markers of bone metabolism in patients with human immunodeficiency virus infection. Metabolism: clinical and experimental 49: 1134–1139. [DOI] [PubMed] [Google Scholar]
- 8. Teichmann J, Stephan E, Lange U, Discher T, Friese G, et al. (2003) Osteopenia in HIV-infected women prior to highly active antiretroviral therapy. The Journal of infection 46: 221–227. [DOI] [PubMed] [Google Scholar]
- 9. Giacomet V, Vigano A, Manfredini V, Cerini C, Bedogni G, et al. (2013) Cholecalciferol supplementation in HIV-infected youth with vitamin D insufficiency: effects on vitamin D status and T-cell phenotype: a randomized controlled trial. HIV clinical trials 14: 51–60. 10.1310/hct1402-51 [DOI] [PubMed] [Google Scholar]
- 10. Pettifor JM, Fischer PR, Thacher TD, Arnaud J, Meissner CA (2008) Dietary calcium deficiency & rickets. The Indian journal of medical research 128: 673–674; author reply 674–676. [PubMed] [Google Scholar]
- 11. Prentice A (2008) Vitamin D deficiency: a global perspective. Nutrition reviews 66: S153–164. 10.1111/j.1753-4887.2008.00100.x [DOI] [PubMed] [Google Scholar]
- 12. Mehta S, Giovannucci E, Mugusi FM, Spiegelman D, Aboud S, et al. (2010) Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PloS one 5: e8770 10.1371/journal.pone.0008770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steenhoff AP, Redwood A, Pettifor JM, Hove J, Bisson GP, et al. (2012) Vitamin D status in HIV-infected patients with and without tuberculosis: a pilot study. Journal of acquired immune deficiency syndromes 61: e21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schneider E, Whitmore S, Glynn KM, Dominguez K, Mitsch A, et al. (2008) Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years—United States, 2008. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control 57: 1–12. [PubMed] [Google Scholar]
- 15.(1993) Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control 41: 1–19. [PubMed] [Google Scholar]
- 16. Lohman TG, Roche AF, Martorell R (1988) Anthropometric standardization reference manual Champaign, IL: Human Kinetics Publishers, Inc. [Google Scholar]
- 17.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, et al. (2000) CDC growth charts: United States. Advance data: 1–27. [PubMed]
- 18. Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, et al. (2011) Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 26: 1609–1616. 10.1002/jbmr.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vieth R (2012) Implications for 25-hydroxyvitamin D testing of public health policies about the benefits and risks of vitamin D fortification and supplementation. Scandinavian journal of clinical and laboratory investigation Supplementum 243: 144–153. 10.3109/00365513.2012.682893 [DOI] [PubMed] [Google Scholar]
- 20. Havens PL, Mulligan K, Hazra R, Flynn P, Rutledge B, et al. (2012) Serum 25-hydroxyvitamin D response to vitamin D3 supplementation 50,000 IU monthly in youth with HIV-1 infection. The Journal of clinical endocrinology and metabolism 97: 4004–4013. 10.1210/jc.2012-2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kakalia S, Sochett EB, Stephens D, Assor E, Read SE, et al. (2011) Vitamin D supplementation and CD4 count in children infected with human immunodeficiency virus. The Journal of pediatrics 159: 951–957. 10.1016/j.jpeds.2011.06.010 [DOI] [PubMed] [Google Scholar]
- 22. Arpadi SM, McMahon DJ, Abrams EJ, Bamji M, Purswani M, et al. (2012) Effect of supplementation with cholecalciferol and calcium on 2-y bone mass accrual in HIV-infected children and adolescents: a randomized clinical trial. The American journal of clinical nutrition 95: 678–685. 10.3945/ajcn.111.024786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dougherty KA, Schall JI, Zemel BS, Tuluc F, Hou X, et al. (2014) Safety and efficacy of high dose daily vitamin D3 supplementation in children and young adults infected with HIV. Journal Pediatric Infect Disease Society In press. [DOI] [PMC free article] [PubMed]
- 24. Ganmaa D, Giovannucci E, Bloom BR, Fawzi W, Burr W, et al. (2012) Vitamin D, tuberculin skin test conversion, and latent tuberculosis in Mongolian school-age children: a randomized, double-blind, placebo-controlled feasibility trial. The American journal of clinical nutrition 96: 391–396. 10.3945/ajcn.112.034967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rich-Edwards JW, Ganmaa D, Pollak MN, Nakamoto EK, Kleinman K, et al. (2007) Milk consumption and the prepubertal somatotropic axis. Nutrition journal 6: 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bogin B (1979) Monthly changes in the gain and loss of growth in weight of children living in Guatemala. American journal of physical anthropology 51: 287–291. [DOI] [PubMed] [Google Scholar]
- 27. Mirwald RL, Bailey DA (1997) Seasonal height velocity variation in boys and girls 8–18 years. American journal of human biology 9: 709–715. [DOI] [PubMed] [Google Scholar]
- 28. Lindskog U, Lindskog P, Gebre-Medhin M (1987) Child health and household water supply: a longitudinal study of growth and its environmental determinants in rural Malawi. Human nutrition Clinical nutrition 41: 409–423. [PubMed] [Google Scholar]
- 29. Pasquet A, Viget N, Ajana F, de la Tribonniere X, Dubus S, et al. (2011) Vitamin D deficiency in HIV-infected patients: associated with non-nucleoside reverse transcriptase inhibitor or efavirenz use? AIDS 25: 873–874. 10.1097/QAD.0b013e32834542fa [DOI] [PubMed] [Google Scholar]
- 30. Allavena C, Delpierre C, Cuzin L, Rey D, Viget N, et al. (2012) High frequency of vitamin D deficiency in HIV-infected patients: effects of HIV-related factors and antiretroviral drugs. The Journal of antimicrobial chemotherapy 67: 2222–2230. 10.1093/jac/dks176 [DOI] [PubMed] [Google Scholar]
- 31. Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, et al. (2013) Vitamin D-binding protein and vitamin D status of black Americans and white Americans. The New England journal of medicine 369: 1991–2000. 10.1056/NEJMoa1306357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baum MK, Campa A, Lai S, Sales Martinez S, Tsalaile L, et al. (2013) Effect of micronutrient supplementation on disease progression in asymptomatic, antiretroviral-naive, HIV-infected adults in Botswana: a randomized clinical trial. JAMA: the journal of the American Medical Association 310: 2154–2163. 10.1001/jama.2013.280923 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
(PDF)
(PDF)
(PDF)
(XLSX)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.