Abstract
Aim
To evaluate the influence of 11C-choline PET/CT on radiotherapy planning in prostate cancer patients.
Background
Precise information on the extension of prostate cancer is crucial for the choice of an appropriate therapeutic strategy.
11C-choline positron emission tomography (11C-choline PET/CT) has two roles in radiation oncology (RT): (1) patient selection for treatment and (2) target volume selection and delineation.
In conjunction with high-accuracy techniques, it might offer an opportunity of dose escalation and better tumour control while sparing healthy tissues.
Materials and methods
We carried out a retrospective study in order to analyse RT planning modification based on 11C-choline PET/CT in 16 prostate cancer patients. Patients were treated with hypofractionated step-and-shoot Intensity Modulated Radiotherapy (IMRT), or Volumetric Modulated Arc Therapy (VMAT), and a daily cone-beam CT for Image Guided Radiation Therapy (IGRT). All patients underwent a 11C-choline-PET/CT scan prior to radiotherapy.
Results
In 37.5% of cases, a re-delineation and new dose prescription occurred. Data show good preliminary clinical results in terms of biochemical control and toxicity. No gastrointestinal (GI)/genitourinary (GU) grade III toxicities were observed after a median follow-up of 9.5 months.
Conclusions
In our experience, concerning the treatment of prostate cancer (PCa), 11C-choline PET/CT may be helpful in radiotherapy planning, either for dose escalation or exclusion of selected sites.
Keywords: Prostate cancer, 11C-choline PET/CT, Radiotherapy planning
1. Background
Prostate cancer (PCa) is the most common cancer in men in the United States and the second most common type in the European Union.1,2 It is also the second leading cause of cancer death (following only lung cancer).3 The main tools to diagnose PCa include digital rectal examination, serum concentration of PSA, and transrectal ultrasonography. The definite evidence of PCa depends on the histopathological verification of adenocarcinoma in prostate biopsy cores or operative specimens.4
Magnetic resonance (MR) is particularly useful for identifying extraprostatic extension and seminal vesicle invasion (SVI), especially in the intermediate and high-risk groups5,6, while computed tomography (CT) seems to be slightly superior in the detection of pelvic lymph node metastases.7
Other recent novel prognostic tests have been developed. These measures stratify the risk of disease progression by genetic profile. It can enable professionals to better define a treatment/monitoring strategy for their patients with PCa.8–10
Until these promising tests are completely implemented in the daily work, the prognosis and decision-making are still directly linked to tumour extension.11 An accurate assessment of the primary tumour, lymph nodes involvement (common iliac, internal iliac and obturator lymph nodes), distant neoplastic involvement and tumour recurrences are essential for a proper RT treatment planning.12 Regarding the disease recurrence, within 10 years from the primary treatment, approximately 15–40% of men will experience a detectable rise in the serum PSA level (biochemical failure).13 Increases of PSA are the expression of disease relapse, but it does not contribute to differentiate between local recurrence and systemic spread of the disease.14
Positron emission tomography plus computerised tomography (PET-CT) is an emerging diagnostic modality used in PCa. Particularly, 11C-choline positron emission tomography (11C-choline PET/CT) has shown some promising results. The accuracy of 11C-choline PET/CT in defining local tumour stage has been reported to account for approximately 70%,15,16 with high values of sensitivity and specificity in visualising sites of primary disease or recurrence, especially at the lymph node level.18,19 In the setting of nodal involvement in PCa relapses, a recent meta-analysis has found high sensitivity values (100% in 3 studies).17
The use of 11C-choline PET/CT for RT treatment planning in addition to modern RT techniques, such as step-and-shoot Intensity Modulated Radiotherapy (IMRT), Volumetric Modulated Arc Therapy (VMAT), and daily Imaged Guided Radiotherapy (IGRT), might offer the opportunity of dose escalation to selected sites, thus providing a better tumour control while avoiding the unnecessary treatment of normal healthy tissues.17,20,21
IMRT and VMAT give us the ability to deliver highly conformal dose distributions. However, this requires more extensive knowledge and higher precision and accuracy in the RT planning and delivering process.22
As it occurs with 18-FDG PET CT, the use of 11C-choline in RT has many potential roles: identification of active disease that remains undetected by other techniques (CT and/or MRI); it may have an impact on treatment indication (i.e., curative or palliative); it gives us the ability to better define the RT treatment volume, it facilitates contouring and delineation (identifying areas of the GTV that might require an additional dose of RT) and, most importantly, it can improve the therapeutic efficacy.23
2. Aim
Although the value of PET-CT for RT planning has been and is still being investigated, it seems probable that this new technology will have a large, positive impact on RT treatment planning with modern techniques in the very near future. Thus, we carried out the present study with the objective of evaluating the influence of 11C-choline PET/CT on radiotherapy planning in our series of PCa patients.
3. Materials and methods
3.1. Patients
We present a retrospective study which analyses RT planning modification based on 11C-choline PET/CT in 16 patients (mean age: 64 years, range: 53–81) who had been referred to our Radiotherapy and Oncology Department. Seven patients were treated with adjuvant or salvage RT after radical prostatectomy for PCa, and other 9 were referred for exclusive RT as primary treatment. Detailed patients’ characteristics are summarised in Table 1.
Table 1.
Initial patients’ characteristics.
| Age | Mean = 64 (range 53–81) |
|---|---|
| T1–T2 | 10 |
| T3–T4 | 6 |
| N0 | 13 |
| N1 | 3 |
| M0 | 15 |
| M1 | 1 |
| Gleason < 7 | 13 |
| Gleason > 7 | 3 |
| Low risk | 3 |
| Intermediate risk | 3 |
| High risk | 10 |
| Exclusive RT | 9 |
| Adjuvant RT | 1 |
| Salvage RT | 6 |
| Initial PSA (exclusive RT) | Mean = 18.18 (range 4.90–55.40) |
| Initial PSA (adjuvant and salvage RT) | Mean = 8.73 (range 1.15–24.70) |
All patients underwent 11C-choline-PET/CT scan prior to RT. The period of inclusion was from July 2010 to April 2013.
In our series, only 4 patients had evidence of local/regional recurrence or metastatic disease in conventional clinical work-up, including digital rectal examination, transrectal ultrasonography, CT, MR and bone scan.
The mean PSA values in candidates for exclusive RT at the time of PET/CT scan were 18.18 ng/ml (4.90–55.40 ng/ml), and 8.73 ng/ml (1.15–24.70 ng/ml) for those patients with disease recurrence. The Gleason score was <7 in 8 patients; 7 in 5 patients; and >7 in 3 patients.
Three patients were classified as low risk and 13 patients as intermediate or high risk, according to the D’Amico risk criteria.24,25Androgen Deprivation Therapy (ADT) was only prescribed to 10 of them. ADT was of short duration (6 months) in 3 patients and of long duration (2 years) in the remaining patients.
All patients underwent 11C-choline PET/CT before the ADT and RT dose prescription.
SPSS 19.0 software was used for statistical analyses. We presented quantitative data in our patient group. The Spearman correlation coefficient (r) and multivariate regression analysis were used to assess the correlation of variables.
Toxicity assessment was measured with the NCI Common Terminology Criteria for Adverse Events (CTCAE) v3.0 during radiation treatment.
The study was submitted and approved by the local ethic committee.
3.2. 11C-choline-PET/CT
Patients fasted for at least 6 h before 11C-choline-PET/CT scanning.
Five minutes after the injection of 869.76 ± 94 MBq 11C-choline, patients underwent a 11C-choline-PET/CT scan from the thorax to mid-thigh in the caudo-cranial direction on a Discovery STE PET/CT scanner (GE). The PET component of the scans was acquired in a 3D mode with an acquisition time of 3 min per bed position. Emission data were corrected for random, dead time, scatter and attenuation, and were reconstructed iteratively by attenuation-weighted VUE point algorithm (3 iterations, 35 subsets) followed by a post-reconstruction smoothing Gaussian filter (6 mm full-width at half-maximum). A low-dose CT (Auto-mAs, 140 kV, 0.8 s per rotation, 3.5 mm slice thickness) was performed for attenuation correction prior to PET scan.
11C-choline PET/CT interpretation was performed by an experienced nuclear medicine physician (AG).
Image findings were interpreted as suggestive of disease in any focal tracer accumulation deviating from the physiological uptake. Maximal Standard Uptake Value (SUVmax) was measured for every lesion. The averaged SUVmax in the lesions was 5.1 ± 2.2 (ranged from 1.4 to 8.9).
3.3. Contouring
The prostate uptake was considered a “positive scan” for patients with primary treatments. The clinical target volume (CTV) included the gross tumour volume (GTV) and the anatomical prostatic gland or prostatic bed with the periprostatic tissue (in case of salvage radiotherapy), guided by the RTOG contouring atlases. If needed, according to Partin nomograms, seminal vesicles or pelvic lymph nodes (common iliac, internal iliac and obturator lymph nodes) were included.26 Partin nomograms do not establish a threshold risk value for decision-making. Such a threshold should be established by the radiation oncologist.
PET findings interpreted as metastatic disease were contoured as GTV and treated.
The PTV was generated from the CTV plus 5-mm lateral, longitudinal and ventral safety margins (3 mm dorsal for prostate).
3.4. Radiotherapy planning
All patients received step-and-shoot IMRT or Volumetric Modulated Arc Therapy (VMAT) IMRT (Elekta Synergy®) plus a cone beam CT (IGRT) before each session.
The dose prescription was different according to three possible scenarios:
-
(A)
Dose prescription to 66 Gy (3 Gy/fraction – BED 130 Gy) administered in 22 days, for radical exclusive prostate radiotherapy.
-
(B)
When other volumes (pelvis or extraprostatic disease) were included, the radiotherapy schedule was hypofractionated to 70 Gy (2.5 Gy/fraction – BED 124 Gy) in 28 days. In these cases, the prophylactic dose prescription was 49–50.4 Gy at 1.75–1.8 Gy per fraction. Concurrent with the treatment of the prophylactic PTV, the 11C-choline PET/CT positive lymph nodes, or affected bone, and the prostate were boosted to 64–70 Gy. Thereby, we used Simultaneous Integrated Boost (SIB) with 2 or 3 different PTV volumes in these cases.
-
(C)
The salvage dose prescription to the prostatic bed was 60 Gy (3 Gy/fraction) administered in 20 days.
Treatment was planned with a Monaco (Elekta®) planning system based on CT scan images.
3.5. Statistical analysis
In the univariate and multivariate analysis (bivariate Spearman correlation and Student's T test, respectively). A multiple regression model is necessary when two or more independent variables (explanatory variables) exist. The variables must be metric or dichotomous and so they can be analysed as quantitative with a Student's T test. In our series, patients with positive 11C-choline PET/CT scan did not differ from patients with a negative scan regarding T-stage, Gleason score or PSA value.
4. Results
4.1. Impact on radiotherapy planning
The patients included in our study were scheduled either for exclusive RT (9/16, 56.25%) or adjuvant/salvage RT of the prostatic bed (7/16, 43.75%).
Our study reveals PTV modification in 6 of 16 patients (37.5%). New contouring and dose prescription occurred in 4 patients intended to receive exclusive RT and, also, in 2 patients with adjuvant and salvage RT, respectively (Table 2).
Table 2.
“Re-staging” after 11C-choline PET/CT.
| Patient | Re-staging (type) | N pre PETa | N pos PETb | M pre PETc | M pos PETd |
|---|---|---|---|---|---|
| 1 | No | − | − | − | − |
| 2 | Yes (decrease) | − | − | + | − |
| 3 | No | − | − | − | − |
| 4 | Yes (increase) | − | + | − | − |
| 5 | No | − | − | − | − |
| 6 | Yes (increase) | − | + | − | − |
| 7 | Yes (increase) | − | + | − | − |
| 8 | No | − | − | − | − |
| 9 | No | − | − | − | |
| 10 | Yes (increase) | − | − | − | + |
| 11 | No | − | − | − | − |
| 12 | Yes (increase) | − | + | − | − |
| 13 | No | − | − | − | − |
| 14 | No | − | − | − | − |
| 15 | No | − | − | − | − |
| 16 | No | − | − | − | − |
N pre PET: nodal status prior 11C-choline PET/CT.
N pos PET: nodal status after 11C-choline PET/CT.
M pre PET: systemic metastatic status prior 11C-choline PET/CT.
M pos PET: systemic metastatic status after 11C-choline PET/CT.
There was an increase of the PTV extension in 5 patients (31.5%) exhibiting pathologic 11C-choline uptake in locations previously undetected by other tests, as all these patients had MR and bone scan prior to 11C-choline PET/CT. Thereby, abnormal tracer uptake in lymph nodes was identified in 4 high-risk PCa patients, and pathologic choline uptake was detected in the pubic bone of 1 high-risk patient. Examples of two RT plans are shown in Figs. 1 and 2.
Fig. 1.
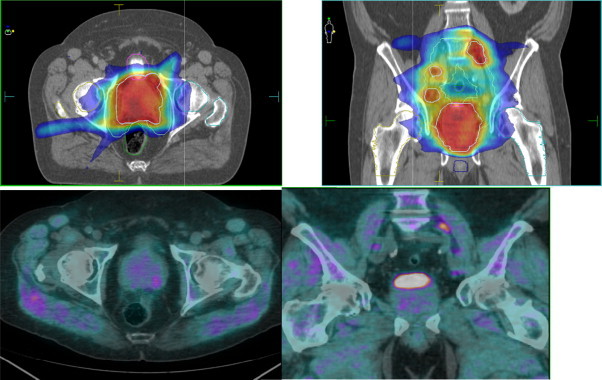
Patient a: Isodose curves of PET/CT planned IMRT and related PET/CT images in a transverse and coronal plane. Images include contours of PTV, CTV (prostate, pelvis and pathologic pelvic lymph nodes), bladder, rectum, bulb of penis and femoral heads.
Fig. 2.
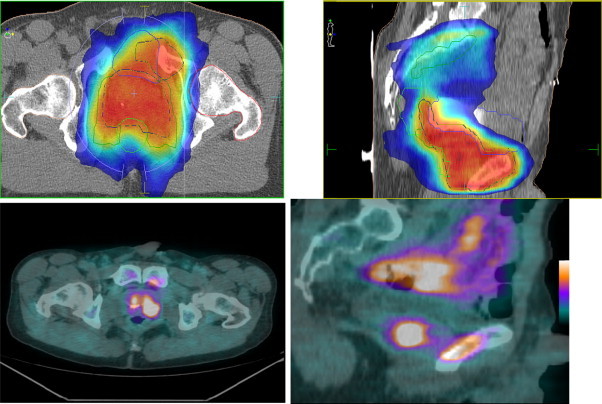
Patient b: Isodose curves of PET/CT planned IMRT and related PET/CT images in a transverse and sagital plane. Images include contours of PTV, CTV (prostate, pelvis and pubic bone metastasis), bladder, rectum and femoral heads.
On the other hand, 1 low-risk patient (6.25%) with positive bone scan at the sternal bone and negative MRI did not exhibit pathologic 11C-choline uptake, allowing the reduction of the PTV. In this case, the patient received a radical treatment (to 66 Gy, 3 Gy/fraction), instead of palliative RT or, probably, other systemic treatments. The pathologic choline uptake at the sternal bone was considered to be related to a previous trauma.
Table 3 synthesises all modifications in volume and dose prescription after 11C-choline PET/CT in these 6 patients.
Table 3.
Planning modification after 11C-choline PET/CT.a
| Patient | PET findings | CTV prior to PET | CTV after PET | Dose prescribed prior to PET | Dose prescribed after PET |
|---|---|---|---|---|---|
| 1 | Positive bone metastases | Prostate plus seminal vesicles | Pelvic lymph nodes, seminal vesicles, prostate, pubic bone | 66 Gyb | 70 Gyc |
| 2 | Negative bone metastases | Pelvic lymph nodes, seminal vesicles, prostate, vertebral bone | Prostate plus proximal seminal vesicles | No RT or 70 Gyc | 66 Gyb |
| 3 | Positive lymph nodes | Prostate plus proximal seminal vesicles | Retroperitoneal and pelvis lymph nodes, seminal vesicles, prostate | 66 Gyb | 70 Gyc (64.4 Gy, 2.3 Gy to retroperitoneal positive lymph nodes) |
| 4 | Positive lymph nodes | Prostate plus proximal seminal vesicles | Pelvic lymph nodes, seminal vesicles, prostate | 66 Gyb | 70 Gyc |
| 5 | Positive tumour bed and lymph nodes | Tumour bed | Pelvic lymph nodes, tumour bed | 60 Gyb | 70 Gyc |
| 6 | Positive tumour bed and lymph nodes | Tumour bed | Pelvic lymph nodes, tumour bed | 60 Gy | 70 Gyc |
PET/CT: positron emission tomography/computed tomography.
3 Gy/fraction.
2.5 Gy/fraction.
4.2. Therapy effectiveness
Although the objective of this study was not focused on the effectiveness of the treatment, some points regarding the results are noteworthy. Eleven patients have been, at least once, re-evaluated after radiotherapy. With a median of 13.5 months (range 9–38) of follow-up, 14 patients (87.5%) are alive without clinical or biochemical relapse (PSA <1 ng/ml).
Considering the patients with an extraprostatic positive 11C-choline PET/CT scan (6/16), the median PSA was <0.2 ng/ml at the last follow-up appointment for 5 patients (83% of these group) after radical treatment (3/5) or salvage IMRT (2/5). Only 1 patient is being currently evaluated because of an increased PSA, up to 7.31 ng/ml, after adjuvant IMRT. Regarding the 10 patients with no evidence of extraprostatic disease in 11C-choline PET/CT, 9 (90%) had a PSA <1 ng/ml. One of these patients is also expecting new PET/CT results because of PSA of 4.56 ng/ml in the last blood test after salvage radiotherapy.
Only 6 patients are still on active hormonal treatment. All of them have PSA below 1 ng/ml.
It would be much more interesting to provide tumour response information evaluated with 11C-choline PET/CT but, nowadays, it is a special and expensive test that patients have to pay for. So, we only use it for a high relapse suspicion.
4.3. Toxicity
Despite the hypofractionated scheduled treatment, no grade III or IV acute toxicity was observed. Two patients did not report any urological symptoms. In addition to this, no digestive toxicity was found in 8 patients.
The incidence of rectal acute toxicity graded I or II was 18% and 12%; while the incidence of grade I or II urological toxicity was 62% and 6%, respectively.
No late grade II toxicity has been reported with a mean follow-up of 18.6 months.
5. Discussion
PCa may metastatise to distant lymph nodes, bones and any other organ. Nevertheless, the optimal method for imaging evaluation of men with primary prostate cancer, or biochemical failure, has not been determined and remains challenging.27 This determination affects therapeutic management. A relevant step was taken with the introduction of PET/CT scans.28 The tracer most-extensively used in patients with PCa is choline labelled with 11C or 18F.29 11C-choline has shown high accuracy in defining primary lesions, local extension or detecting lymph node metastases in patients with a PSA relapse. However, it is still unclear at which PSA level choline PET/CT might have an impact on treatment decisions.30,31
The strongest predictors of PET positivity for the identification of relapse in PCa patients are a PSA value >1 ng/ml, PSAvel >1 ng/ml/year, and a PSAdt <3 months.17 Würschmidt et al. found that patients with a negative PET/CT had PSA values below 1 ng/ml.21
Also, the major weakness of these imaging devices is that up to 45% of metastatic lymph nodes are <0.4 cm in diameter, value below the spatial resolution of PET/CT.32
Nevertheless, defining local tumour stage, 11C-choline PET/CT accuracy has been reported to be approximately 70%.15,16
In addition to this, numerous studies have reported high accuracy of PET/CT in detecting lymph node metastases in patients with a PSA relapse.21 Italian researchers reported a sensitivity and specificity of 64% and 90%, respectively, for the detection of nodal metastases in men with PSA failure.33 11C-choline PET/CT demonstrated a sensitivity of 83% in the assessment of nodules of 5 mm or larger.3
In a prospective study, 22 of 36 patients had a PSA relapse and 11C-choline PET/CT showed a specificity of 100% in pelvic lymph nodes detection.34 The same group examined in a prospective study of 67 consecutive patients with histological proven PCa with 11C-choline PET/CT showed a sensitivity and specificity of 80% and 96%, respectively.35
It has also been shown that 11C-choline PET/CT, performs better than clinical nomograms, has equal sensitivity and better specificity in detecting lymph nodes, with limited capability for small lesions.36
Reinforcing these data, recent meta-analyses carried out by Evangelista et al. reported high sensitivity and specificity values. The authors found 53 complete articles related to the use of choline PET/CT in biochemical relapse which showed high sensitivity values (85.6%), 100% in 3 studies in the setting of nodal involvement. The specificity was of 92.6%, particularly when distant recurrence was suspected, underlying certain degree of false positives. There are at least 2 reasons for this specificity: firstly, frequent inflammatory changes; and secondly, artefacts or possibly small bowel activity can mime nodal positivity.17,37 Despite a possibly decreased sensitivity of choline-PET in androgen-deprived patients,38 the meta-analyses showed no association between choline PET/CT and ADT at multivariate analysis.17
Thereby, due to the high sensitivity and specificity of 11C-choline PET/CT for detecting PCa extension, it could be helpful in target volume definition and dose escalation in RT, especially for irradiation of nodal sites or localised metastases in the absence of reliable conventional imaging modalities, such as MRI and CT. Encouraging results have been found in numerous studies according to a recent review.39
Dose escalation (over 74 Gy – BED 120 Gy) has demonstrated to have a significant impact on biochemical control.40,41 Also, several studies have shown a technical feasibility and utility of radiation therapy dose painting using tumour control probability (TCP) based on the distribution derived from choline PET/CT in patients with PCa42 that could allow dose escalation.
Chang et al. reported a study of 8 patients with PCa who had 11C-choline PET/CT prior to radical prostatectomy. They compared the use in 2 IMRT plans with dose escalation (90 Gy) to dominant intraprostatic lesions (DILs) identified on 11C-choline PET/CT versus standard IMRT (78 Gy). Both plans were found to be achievable and had TCPs superior to the standard RT while not having significantly different normal tissue complication probability (NTCP).43 In another study, a high degree of correlation between 11C-choline PET/CT and prostatectomy specimens was previously demonstrated.44 However, we did not use 11C-choline PET/CT-directed dose escalation on intraprostatic lesions, only on extraprostatic disease.
In another study carried out by Vees et al., 19 high-risk PCa patients underwent 18F-choline PET/CT and SPECT/CT. A total of 27 lymph nodes were detected outside the initial CTV. Abnormal choline uptake appeared only in 2 patients, treating these node metastases with an additional boost dose. The treatment planning was modified in 40% of patients.45
Souvatzoglou studied the impact of PET/CT on the PTV extension in 37 patients with biochemical relapse. 11C-Choline PET/CT affected the treatment planning in 13% of cases (5/37).20
Würschmidt evaluated the use of 11C-choline PET/CT in 26 patients with primary or recurrent PCa (intermediate to high risk) treated with 3D conformal or intensity modulated radiotherapy. The median dose to primary tumours was 75.6 Gy and 66.6 Gy to PET-positive recurrent lymph nodal sites. Good clinical results are reported in this study, with no or mild late side effects observed in the majority of patients (84%).21
Our results show that 11C-choline PET/CT has an impact on IMRT planning (target volume contouring and dose prescription) in a considerable percentage (37.5%) of PCa patients referred for RT.
An arguable question is open-ended: the interpretation of the PET to select RT volumes. According to the recommendations of several Spanish medical societies23 for treatments with curative intent where the aim is to treat the whole tumour, it is best to use interpretative criteria in that PET-CT have a high sensitivity (assuming a low specificity), with the understanding that non-cancerous tissue (i.e., healthy tissue) will likely be included in the radiotherapy field.
For example, in our series, if anomalous tracer deposits were found in lymph nodes, these were considered positive and therefore included in the treatment volume. This also occurred in another case where one suspicious (negative bone scan) localised pubic bone metastasis was detected and included in the treatment volume.
In contrast, if our main objective were to reduce morbidity by minimising radiation to healthy tissues (i.e., salvage RT), then the interpretative criteria would have greater specificity. One example would be the case in which PET-CT did not exhibit pathologic 11C-choline uptake, allowing the reduction of the PTV (even though bone scan showed suspicious images of bone metastasis).
In our series, positive lymph nodes were treated to 64 Gy, while acute and late toxicity to small bowel was not increased compared to standard approaches with lower doses to pelvic lymph nodes. The IMRT/VMAT techniques allowed these individualised treatments. However, the influence of these techniques on the results was not an objective of the present work.
Therefore, our patients might benefit from an individualised treatment, increased loco-regional control rates or reduced normal tissue complication rates. We would need a longer follow-up in order to confirm this hypothesis.
Nevertheless, a careful interpretation of PET/CT findings and the consideration of clinical data are necessary for decision-making.
Our preliminary data suggest that this diagnostic modality might offer the opportunity of dose escalation to selected sites, thus providing a better tumour control while avoiding the unnecessary inclusion of normal healthy tissues. There are no significant statistical results, but we are aware of the limitations of this work: retrospective analysis; small and heterogeneous group (radical, adjuvant and salvage RT); no consistent cut-off SUV value of 5.1 ± 2.2 (ranged from 1.4 to 8.9); short follow-up for late toxicity (median 13.5 months) or active hormone treatment in several patients at the time of analysis.
Further studies carried out with a homogeneous patient population on a larger scale are needed to establish the final clinical role of 11C-choline PET/CT in the RT planning of PCa patients.
At the moment, the 11C-choline PET/CT has not been recommended for primary PCa detection so far due to the limitations mentioned above and the high cost of the modality.
6. Conclusions
11C-Choline PET/CT is increasingly used in many Radiotherapy and Oncology Departments for the initial staging or detection of locally recurrent or metastatic disease.
Planning guided by 11C-choline PET/CT is feasible and may be helpful to detect, contour and treat macroscopic disease in PCa patients. In addition to this, it might allow dose escalation when IMRT/VMAT and IGRT are used. Therefore, there could be a curative chance for selected patients with metastatic lymph node affection or recurrent disease.
However, further studies are needed to assess whether 11C-choline PET/CT implementation enhances the RT effectiveness.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Jemal A., Siegel R., Ward E. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Sankila R., Ferlay J., Parkin D.M. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer. 2002;38:99–166. doi: 10.1016/s0959-8049(01)00350-1. [DOI] [PubMed] [Google Scholar]
- 3.Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med. 2011;52(1):81–89. doi: 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith J.A., Jr., Scardino P.T., Resnick M.I., Hernandez A.D., Rose S.C., Egger M.J. Transrectal ultrasound versus digital rectal examination for the staging of carcinoma of the prostate: results of a prospective multi-institutional trial. J Urol. 1997;157(3):902–906. [PubMed] [Google Scholar]
- 5.Sala E., Akin O., Moskowitz C.S. Endorectal MR imaging in the evaluation of seminal vesicle invasion: diagnostic accuracy and multivariate feature analysis. Radiology. 2006;238(3):929–937. doi: 10.1148/radiol.2383050657. [DOI] [PubMed] [Google Scholar]
- 6.Wang L., Hricak H., Kattan M.W., Chen H.N., Scardino P.T., Kuroiwa K. Prediction of organ confined prostate cancer: incremental value of MRI and MRI spectroscopic imaging to staging nomograms. Radiology. 2006;238(2):597–603. doi: 10.1148/radiol.2382041905. [DOI] [PubMed] [Google Scholar]
- 7.Hövels A.M., Heesakkers R.A.M., Adang E.M. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387–395. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Matthew R., Cooperberg J.P., Simko J.E. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J Clin Oncol. 2013;31(11):1428–1434. doi: 10.1200/JCO.2012.46.4396. [DOI] [PubMed] [Google Scholar]
- 9.Cuzick J., Swanson G.P., Fisher G., Transatlantic Prostate Group Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011;12(3):245–255. doi: 10.1016/S1470-2045(10)70295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuzick J., Berney D.M., Fisher G., on behalf of the Transatlantic Prostate Group Prognostic value of a cell cycle progression signature for prostate cancer death on conservatively managed needle biopsy cohort. Br J Cancer. 2012;106(6):1095–1099. doi: 10.1038/bjc.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bubendorf L., Schöpfer A., Wagner U. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 12.Picchio M., Giovannini E., Crivellaro C., Gianolli L., di Muzio N., Messa C. Clinical evidence on PET/CT for radiation therapy planning in prostate cancer. Radiother Oncol. 2010;96:347–350. doi: 10.1016/j.radonc.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Dong J.T., Rinker-Schaeffer C.W., Ichikawa T., Barrett J.C., Isaacs J.T. Prostate cancer: biology of metastasis and its clinical implications. World J Urol. 1996;14:182–189. doi: 10.1007/BF00186898. [DOI] [PubMed] [Google Scholar]
- 14.Han M., Partin A.W., Zahurak M., Piantadosi S., Epstein J.I., Walsh P.C. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169(2):517–523. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 15.Rinnab L., Mottaghy F.M., Blumstein N.M. Evaluation of [11C]-choline positron-emission/computed tomography in patients with increasing prostate-specific antigen levels after primary treatment for prostate cancer. BJU Int. 2007;100(4):786–793. doi: 10.1111/j.1464-410X.2007.07083.x. [DOI] [PubMed] [Google Scholar]
- 16.Giovacchini G., Picchio M., Coradeschi E. [(11)C]choline uptake with PET/CT for the initial diagnosis of prostate cancer: relation to PSA levels, tumour stage and anti-androgenic therapy. Eur J Nucl Med Mol Imaging. 2008;35(6):1065–1073. doi: 10.1007/s00259-008-0716-2. [DOI] [PubMed] [Google Scholar]
- 17.Evangelista L., Zattoni F., Guttilla A. Biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med. 2013;38(5):305–314. doi: 10.1097/RLU.0b013e3182867f3c. [DOI] [PubMed] [Google Scholar]
- 18.Cimitan M., Bortolus R., Morassut S. 18F-Fluorocholine PET/CT imaging for the detection of recurrent prostate cancer at PSA relapse: experience in 100 consecutive patients. Eur J Nucl Med Mol Imaging. 2006;33:1387–1398. doi: 10.1007/s00259-006-0150-2. [DOI] [PubMed] [Google Scholar]
- 19.Rinnab L., Simon J., Hautmann R.E. 11C-Choline PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy. World J Urol. 2009;27:619–625. doi: 10.1007/s00345-009-0371-7. [DOI] [PubMed] [Google Scholar]
- 20.Souvatzoglou M., Krause B.J., Pürschel A. Influence of (11)C-choline PET/CT on the treatment planning for salvage radiation therapy in patients with biochemical recurrence of prostate cancer. Radiother Oncol. 2011;99(2):193–200. doi: 10.1016/j.radonc.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Würschmidt F., Cordula P., Wahl A., Dahle J., Kretschmer M. 18F-Fluoroethylcholine-PET/CT imaging for radiation treatment planning of recurrent and primary prostate cancer with dose escalation to PET/CT-positive lymph nodes. Radiat Oncol. 2011;6:44–51. doi: 10.1186/1748-717X-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malicki J. The importance of accurate treatment planning, delivery, and dose verification. Rep Pract Oncol Radiother. 2012;17:63–65. doi: 10.1016/j.rpor.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caballero Perea B., Villegas A.C., Rodríguez J.M. Recommendations of the Spanish Societies of Radiation Oncology (SEOR) Nuclear Medicine & Molecular Imaging (SEMNiM), and Medical Physics (SEFM) on 18F-FDG PET-CT for radiotherapy treatment planning. Rep Pract Oncol Radiother. 2012;17(6):298–318. doi: 10.1016/j.rpor.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Amico A.V., Whittington R., Malkowicz S.B. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 25.D’Amico A.V., Cote K., Loffredo M., Renshaw A.A., Schultz D. Determinants of prostate cancer specific survival after radiation therapy for patients with clinically localized prostate cancer. J Clin Oncol. 2002;20(23):4567–4613. doi: 10.1200/JCO.2002.03.061. [DOI] [PubMed] [Google Scholar]
- 26.Partin A.W., Mangold L.A., Lamm D.M., Walsh P.C., Epstein J.I., Pearson J.D. Contemporary update of the prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58(6):843–848. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 27.Jadvar H., Alavi A. Role of imaging in prostate cancer. PET Clin. 2009;4:135–138. doi: 10.1016/j.cpet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelosi E., Messa C., Sironi S. Value of integrated PET/CT for lesion localisation in cancer patients: a comparative study. Eur J Nucl Med Mol Imaging. 2004;31(7):932–939. doi: 10.1007/s00259-004-1483-3. [DOI] [PubMed] [Google Scholar]
- 29.Skanjeti A., Ettore P. Lymph node staging with choline PET/CT in patients with prostate cancer: a review. ISRN Oncol. 2011;2011:2190–2264. doi: 10.5402/2011/219064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vees H., Buchegger F., Albrecht S. 18F-Choline and/or 11C-acetate positron emission tomography: detection of residual or progressive subclinical disease at very low prostate-specific antigen values (<1 mg/ml) after radical prostatectomy. BJU Int. 2007;99:1415–1420. doi: 10.1111/j.1464-410X.2007.06772.x. [DOI] [PubMed] [Google Scholar]
- 31.Schilling D., Schelemmer H.P., Wagner P.H. Histological verification of 11C-choline-positron emission/computed tomography-positive lymph nodes in patients with biochemical failure after treatment for localized prostate cancer. BJU Int. 2008;102:446–451. doi: 10.1111/j.1464-410X.2008.07592.x. [DOI] [PubMed] [Google Scholar]
- 32.Evangelista L., Gutilla A., Zattoni F., Muzzio P.C., Zattoni F. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur Urol. 2012;63(6):1040–1048. doi: 10.1016/j.eururo.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 33.Scattoni V., Picchio M., Suardi N. Detection of lymph-node metastases with integrated 11C-choline PET/CT in patients with PSA failure after radical retropubic prostatectomy: results confirmed by open pelvic-retroperitoneal lymphadenectomy. Eur Radiol. 2007;52:423–429. doi: 10.1016/j.eururo.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 34.De Jong I.J., Pruim J., Elsinga P.H., Vaalburg W., Mensink H.J.A. 11C-Choline positron emission tomography for the evaluation after treatment of localized prostate cancer. Eur Urol. 2003;44:32–38. doi: 10.1016/s0302-2838(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 35.De Jong I.J., Pruim J., Elsinga P.H., Vaalburg W., Mensink H.J.A. Preoperative staging of pelvic lymph nodes in prostate cancer by 11C-choline PET. J Nucl Med. 2003;44:331–335. [PubMed] [Google Scholar]
- 36.Schiavina R., Scattoni V., Castellucci P. (11)C-Choline positron emission tomography/computerized tomography for preoperative lymph-node staging in intermediate-risk and high-risk prostate cancer: comparison with clinical staging nomograms. Eur Urol. 2008;54(2):392–401. doi: 10.1016/j.eururo.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 37.Juweid M.E., Cheson B.D. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 2006;354:496–507. doi: 10.1056/NEJMra050276. [DOI] [PubMed] [Google Scholar]
- 38.DeGrado T.R., Coleman R.E., Wang S. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: initial findings in prostate cancer. Cancer Res. 2001;61:110–117. [PubMed] [Google Scholar]
- 39.Schwarzenböck S.M., Kurth J., Gocke Ch., Kuhnt T., Hildebrandt G., Krause B.J. Role of choline PET/CT in guiding target volume delineation for irradiation of prostate cancer. Eur J Nucl Med Mol Imaging. 2013;40(Suppl. 1):S28–S35. doi: 10.1007/s00259-013-2404-0. [DOI] [PubMed] [Google Scholar]
- 40.Viani G.A., Stefano E.J., Afonso S.L. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009;74(5):1405–1418. doi: 10.1016/j.ijrobp.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 41.Kuban D.A., Tucker S.L., Dong L. Long-term results of the M.D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(1):67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 42.Dirscherl T., Rickhey M., Bogner L. Feasibility of TCP-based dose painting by numbers applied to a prostate case with 18F-choline PET imaging. Z Med Phys. 2012;22(1):48–57. doi: 10.1016/j.zemedi.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Chang J.H., Lim Joon D., Lee S.T. Intensity modulated radiation therapy dose painting for localized prostate cancer using 11C-choline positron emission tomography scans. Int J Radiat Oncol Biol Phys. 2012;1(5):83. doi: 10.1016/j.ijrobp.2012.01.087. [DOI] [PubMed] [Google Scholar]
- 44.Chang J.H., Joon D.L., Lee S.T. Histopathological correlation of 11C-choline PET scans for target volume definition in radical prostate radiotherapy. Radiother Oncol. 2011;99:187–192. doi: 10.1016/j.radonc.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Vees H., Steiner C., Dipasquale G. Target volume definition in high-risk prostate cancer patients using node SPET/CT and 18F-choline PET/CT. Radiat Oncol. 2012;7:134. doi: 10.1186/1748-717X-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]


