Abstract
Aim
This prospective study aims to assess feasibility of helical tomotherapy (HT) for craniospinal irradiation (CSI) and perform dosimetric comparison of treatment plans for both HT and 3D conformal radiotherapy (3DCRT).
Background
CSI is a challenging procedure. Large PTV size requires field matching due to technical limitations of standard linear accelerators, which cannot irradiate such volumes as a single field. HT could help to avoid these limitations as irradiation of long fields is possible without field matching.
Materials and methods
Three adults were enrolled from 2009 to 2010. All patients received radiochemotherapy. Treatment plans in prone position for 3DCRT and in supine position for HT were generated. The superior plan was used for patients’ irradiation. Plans were compared with the application of DVH, Dx parameters – where x represents a percentage of the structure volume receiving a normalized dose and homogeneity index (HI).
Results
All patients received HT irradiation. The treatment was well tolerated. The HT plans resulted in a better dose coverage and uniformity in the PTV: HI were 5.4, 7.8, 6.8 for HT vs. 10.3, 6.6, 10.4 for 3DCRT. For most organs at risk (OARs), the D(V80) was higher for HT than for 3DCRT, whereas D(V5) was lower for HT.
Conclusions
HT is feasible for CSI, and in comparison with 3DCRT it improves PTV coverage. HT reduces high dose volumes of OARs, but larger volumes of normal tissue receive low radiation dose. HT requires further study to establish correlations between dosimetrical findings and clinical outcomes, especially with regard to late sequelae of treatment.
Keywords: Craniospinal irradiation, Helical tomotherapy, Dosimetric comparison
1. Background
Some central nervous system (CNS) tumours, e.g. medulloblastomas, primitive neuroectodermal tumours, disseminated ependymomas and germinomas have the ability to disseminate through the craniospinal fluid to the entire subarachnoid space and the CNS.1,2 The treatment of such tumours involves surgery (if possible) followed by adjuvant chemotherapy (CTx) and irradiation, the so-called craniospinal irradiation (CSI).
The clinical target volume (CTV) for CSI comprises the entire cerebrum with the spinal cord and cerebrospinal fluid space, which extends cranially into the cribriform plate, laterally encompasses the spinal roots and caudally reaches the end of the thecal sac.
In the era of 2D field arrangement, CSI consisted of two opposing lateral photon or Co-60 gamma ray beams for cranial target matched with a direct posterior spinal photon, Co-60 gamma ray or electron field(s). Patients were therefore treated in a prone position to assure precise field matching at the craniospinal junction. Later on, thanks to technical developments in radiation delivery and the advances in diagnostic and portal imaging, the three dimensional computed tomography (CT) based radiotherapy planning (so called 3D conformal radiotherapy, 3DCRT) was introduced to the everyday praxis.3 Currently, CSI patients may be treated either in prone or supine position depending on the institution's experience and preferences.4,5 Moreover, the standard 3DCRT for CSI is not uniformly performed, e.g. a number of beam directions vary from one (dorsal) to three (dorsal plus two oblique lateral) and different field matching techniques are used. Furthermore, intensity modulated dose plans using intensity modulated radiotherapy (IMRT) alone or combined with rotational dose delivery systems, the so-called volumetric modulated arc therapy (VMAT)6,7 were introduced for CSI. However, none of these approaches enables to omit field matching, which may cause unintentional underdose or overdose in the spinal cord if the minimal set-up error occurs and, thus, can contribute to disease recurrence or lead to severe side effects.8–10
Helical tomotherapy (HT), on the other hand, due to continuous helical dose delivery and synchronized movement of a treatment couch, enables irradiation of long targets without fields junction.11 This unique feature, so essential for CSI, was reported as a case sample by Bauman et al.12 and followed by dosimetric comparisons between HT, 3DCRT and IMRT and some publications summarizing treatment-based observations.13–22 However, since, as far as we know, up till now there have not been any published data comparing prone position 3DCRT (standard patient's position in our centre) to supine HT treatment.
2. Aim
We have designed a prospective single institutional study to compare prone 3DCRT plans with supine HT plans as well as asses the feasibility of HT for CSI.
3. Materials and methods
The recruitment started in September 2009 after the protocol had been approved by the Institutional Ethics Committee. Inclusion criteria encompassed a written informed consent, age over 18 years, histologically proven primary CNS tumour with confirmed indication for CSI and Karnofsky performance status ≥50. The exclusion criteria were: presence of distant metastases, pregnancy and inability to grant a consent. Three consecutive patients aged: 21, 29 and 51 admitted to our centre from September 2009 to January 2010 fulfilled the inclusion criteria and were enrolled into the study. The first patient was diagnosed with supratentorial primitive neuroectodermal tumour (PNET) localized in the thalamus region. The patient underwent stereotactic biopsy followed by induction CTx and subsequent irradiation of the primary tumour with concurrent CTx in another hospital and was referred to our institution for CSI. Two other patients were diagnosed with cerebellar tumours: medulloblastoma grade IV, and underwent subtotal resection followed by adjuvant CSI and boost to primary tumour region; concurrent CTx (vincristine, 2 mg iv) was administered weekly.
For treatment planning purpose CT (Siemens Corp.) scans were performed in both supine (for HT planning) and prone (for 3DCRT planning) positions with slice reconstruction of 3 mm. Thermoplastic head and shoulder mask with a headrest and leg fixation/knee support (CIVCO Medical Solutiones, SINMED) were used to immobilize the patients. MRI scans were performed and fused with CT scans for more precise localization of target volume.
For structure set delineation, both CT (prone and supine) and MRI image series were transferred to the treatment-planning system (Eclipse, Varian Medical Systems). The CTV encompassed the whole brain and spinal cord with the leptomeningeal space including the cribriform fossa, base of skull (emerge site of cranial nerves). The inferior limit of the CTV extended to the end of the thecal sac as determined by T2-weighted MRI. The PTV was defined by adding a 0.5 cm uniform margin to CTV. Following organs at risk (OARs) were outlined: the optic apparatus (lenses, eyeballs, optic nerves and chiasma), thyroid gland, parotid glands, heart, lungs, kidneys, spleen, liver, and intestines. The doses prescribed to the spinal PTV yielded 25.5 Gy for PNET and 35.2 Gy for medulloblastoma with dose per fraction 1.7 Gy and 1.6 Gy, respectively.
For dose calculations in supine position, CT scans with the structure set were exported in DICOM format to the TomoTherapy Planning Station 3.1.4.7 (TomoTherapy, Accuray). The treatment plans were generated using continuous arc-based intensity-modulated radiation therapy (IMRT) with 6 MV photons. Field width was 5 cm, pitch 0.287 and modulation factor yielded 1.5 and 1.8 for one and two patients, respectively.23
A conventional 3DCRT treatment plan for a linear accelerator (LINAC) in prone position was generated by the Eclipse treatment-planning system (Varian Medical Systems). 3DCRT plans consisted of two lateral opposed 6 MV fields to cover the whole brain and three fields to cover spinal target volume: one posterio-anterior 6 MV field and two oblique lateral 20 MV fields. Compensators such as blocks and wedges were used to improve OARs sparing and dose homogeneity, respectively. Because of the PTV length, three (two patients) or four (one patient) isocenters were needed since the maximum field size for LINAC was 40 cm × 40 cm at the isocenter. The upper isocenter matched two opposed lateral cranial fields with spinal fields. The spinal PTV was divided into cervical, thoracic and lumbar. Each 3DCRT CSI treatment plan consisted of two phases due to planned shifts of the field junctions to minimize the potential risk of under- or over-dose to the spinal cord. The length of the shifts was equal in both cranio-spinal and spinal–spinal junctions and yielded 1 cm.
Both HT and 3DCRT plans were optimized in line with the criteria of International Commission on Radiation Units and Measurement Report 62: the 98% of the PTV volume aimed to receive at least 95% of the prescribed dose and the near maximum dose [D(V2)] was kept lower than 107% to assure homogenous dose distribution in the target,24,25 whereas maximum cumulative dose (CSI- and boost-PTVs) to the lenses yield Dmax = 4 Gy, optic nerves and chiasm Dmax = 54 Gy and the dose to other OARs was kept as low as possible.
In order to make the plan comparison transparent, different prescribed doses were normalized to 100% for all patients and the D(Vx) parameter – where x represents the percentage of the structure volume receiving normalized dose was used for the evaluation of both PTV coverage and OARs sparing. Target coverage was analyzed using: D(V99), D(V98), D(V95) and D(V2) and homogeneity index (HI) which was calculated using the formula: HI = [D(V2) − D(V98)]/Dpres26; where D(V2) and D(V98) equal the dose to 2% and 98% of the target volume, respectively, and Dpres represents the prescribed dose. While Dpres is assumed as 100%, D(V2) and D(V98) represent dose to PTV near maximum and minimum, respectively. The lower the HI, the more homogenous the dose distribution in PTV. To analyze doses received by OARs. D(V80) (representing dose to major the part of the organ), and D(V30) and D(V5) (representing high dose burden) were chosen.
4. Results
The results of the PTV coverage are summarized in Table 1 important doses to the PTV: D(V99), D(V95), D(V1), mean dose (Dmean), maximum dose (Dmax) and minimum dose (Dmin) are listed for each patient for HT and LINAC plan, respectively. Both 3DCRT and HT plans were highly conformal. Tomotherapy plans resulted in a slightly better target coverage with higher D(V95) in all patients (100%, 98%, 98% vs. 98%, 96% and 97% in Tomo and 3DCRT, respectively) and higher D(V99) and D(V98) in patient 1 and patient 3, respectively (98%, 94% vs. 94%, 93% and 99%, 96% vs. 96%, 95%). For patient 2, D(V99) was superior in 3DCRT plan (93% vs. 95%). The HI yielded 5.4, 7.8, 6.8 for HT vs. 10.3, 6.6, 10.4 for 3DCRT. For farther evaluation of PTV differential dose volume histograms (DVH) are showed in Fig. 1.
Table 1.
PTV coverage for each patient for HT and LINAC respectively.
| Dose to PTV | D(V99) [%] |
D(V98) [%] |
D(V95) [%] |
D(V1) [%] |
Dmean [%] |
Dmax [%] |
Dmin [%] |
SD [Gy] |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Device | HT | LINAC | HT | LINAC | HT | LINAC | HT | LINAC | HT | LINAC | HT | LINAC | HT | LINAC | HT | LINAC |
| Patient 1 | 98.4 | 94.4 | 99.4 | 96.1 | 100.2 | 97.5 | 105.5 | 107.2 | 102.0 | 100.1 | 111.0 | 109.8 | 58.8 | 77.3 | 0.4 | 0.6 |
| Patient 2 | 93.1 | 94.8 | 95.5 | 95.6 | 97.5 | 96.4 | 103.8 | 102.6 | 100.3 | 100.9 | 106.8 | 109.1 | 55.7 | 76.4 | 0.7 | 0.6 |
| Patient 3 | 93.9 | 93.1 | 96.1 | 95.0 | 97.9 | 97.0 | 103.5 | 105.9 | 100.3 | 101.3 | 108.5 | 108.8 | 68.8 | 77.8 | 0.6 | 0.8 |
Fig. 1.
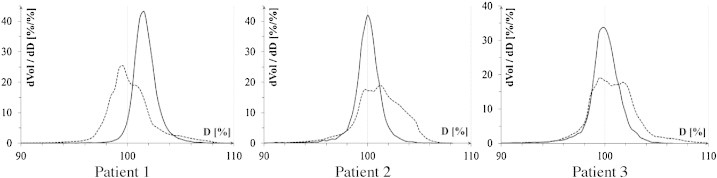
Differential DVHs for PTV for each patient. Dashed line – 3DCRT; continuous line – HT.
Table 2 summarizes the results for lungs, kidneys, intestines, liver, spleen, eyes, lenses, parotid and thyroid glands, and heart. For most of the delineated OARs: lungs, intestines, liver, eyes, heart the D(V80) was higher for the HT plans than for 3DCRT plans, whereas D(V5) and D(V1) (not shown in Table 2) were lower for the HT plans. Figs. 2 and 3 reveal cumulative DVHs of one of the patients for: eyes, lenses, parotids, thyroid gland and lungs and heart, kidneys, spleen, liver and intestines, respectively.
Table 2.
D(V80), D(V30) and D(V5) for OARs for each patient for HT and LINAC respectively. Cells marked with colours contain values < 3% in comparison to other device (for patient 1 this is 0.8 Gy, for patient 2 and 3: 1.1 Gy). Light grey: LINAC-dose < 3% than HT. Dark grey HT-dose < 3% than LINAC.
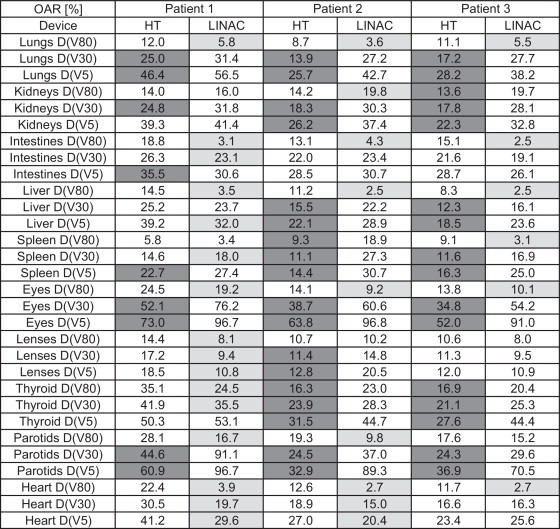
Fig. 2.
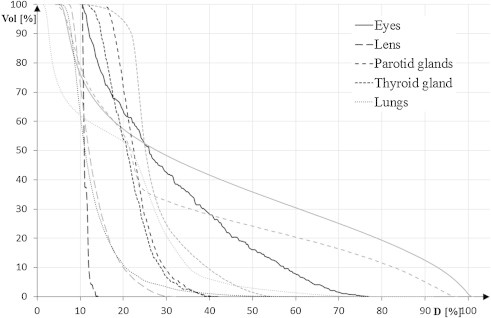
Cumulative DVH for OARs: eyes, lenses, parotids, thyroid glands and lungs. Grey lines represent 3DCRT; black lines – HT.
Fig. 3.
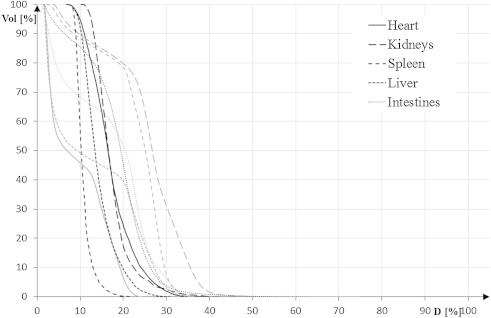
Cumulative DVH for OARs: heart, kidneys, spleen, liver and intestines. Grey lines represent 3DCRT; black lines – HT.
Figs. 4 and 5 contain dose distribution on transversal CT-images for HT and 3DCRT. Eyes sparing is possible using HT, but 80% of the eye bulbs volume receive higher dose than using 3DCRT (Fig. 4, upper row). Dose to the lenses could be reduced in the 3DCRT technique in patient 1, in patient 2 HT dose was lower; moreover, patient 3 had a similar dose in both plans (see Table 2). Middle and bottom row pictures of Fig. 4 show tomotherapy possibilities in reducing the dose to big salivary glands and thyroid, respectively. Fig. 5 reveals thoracic and abdominal OARs: the heart dose is lower in 3DCRT plans for all the patients. The kidney doses are lower for HT, except D(V80) in patient 2. This is due to the field arrangement in the conventional technique, but the other factor could be the patient's position (prone in this technique) that increases the distance between the spine and the heart. Similar phenomenon could be assumed for the intestines.
Fig. 4.
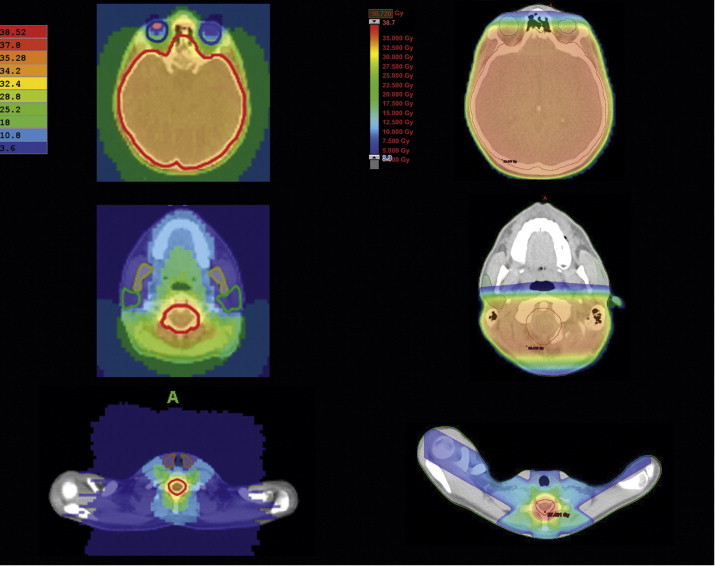
CT-transversal sections of one of the patients: HT dose distribution on the left and LINAC on the right. Sections at the level of eves, parotids and the thyroid gland. LINAC-CT-scans rotated at 180° for comparison purposes.
Fig. 5.
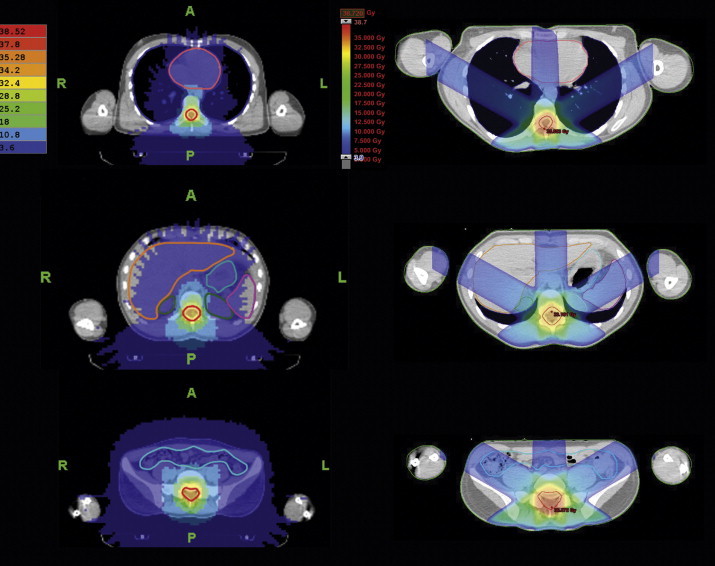
CT-transversal sections of one of the patients: HT dose distribution on the left and LINAC on the right. Sections at the level of the heart, liver, spleen and kidneys, and intestines. LINAC-CT-scans rotated at 180° for comparison purposes.
The CSI with the application of tomotherapy was well tolerated; no treatment interruptions caused by deterioration of patients’ health condition were observed. Nausea grade I (CTCAE vs. 3.0) developed in all patients but could be successfully treated with antiemetics. One patient needed platelets transfusion because of thrombocytopenia grade II (CTCAE vs. 3.0), and two of them required colony-stimulating factor to counteract leucopenia grade III (CTCAE vs. 3.0). Skin reaction grade I CTCAE vs. 3.0 was observed in all patients. The above listed acute side effects suggest that the irradiation using tomotherapy is at least as tolerable as 3DCRT CSI (compared to our centre retrospective patient cohort, data not shown).
5. Discussion
In the last few decades, indications for CSI and recommendation in line with a prescribed dose have been developed as a result of publishing a lot of data about prognostic factors for primary brain tumours with the risk of leptomeningeal seeding. However, it is important to emphasize that the majority of this data refer to paediatric patients.27 Generally, over the years, the indications for CSI have become limited to patients with cytologically confirmed presence of tumour cells in cerebrospinal fluid analysis and to subpopulations of patients with adverse prognostic factors, such as: unreselectable or residual tumour > 1.5 cm3, anaplastic or large cell medulloblastoma.28 This fact and the rare incidence of this type of primary CNS tumours in the adult population are the most important reasons for a small number of patients that could be included in this study.29,30 Each patient from our cohort had at least one of these factors present.
The advantage of this study lies in the fact that the comparison of the treatment plans was performed before the commencement of therapy to choose the best available treatment option, whereas other studies12–16,19–21 focused on retrospective analysis and did not take into account the differences in patient positioning and its impact on organ localization.
A larger number of patients would have allowed statistical data evaluation, nevertheless the results of our study are in line with the results of other studies that proved better PTV coverage for HT on the one hand, and increased volume of low dose on the other.12,14–16,18,19,22 Moreover, even with a larger number of patients, both the PTV-shape and its location would remain unchanged. Therefore, it is highly probable that the results would be similar.
It is probably of most importance to investigate a possibly different spectrum of the long term side effects caused by altered dose distribution in the body. That is essential in the therapy of children and young adults, but may be less critical in adults and elderly patients. Such long-term results are still missing. Our centre plans to summarize follow-up of these patients because only acute adverse effects have been evaluated so far. Only acute haematologic toxicity grade 2 or higher was observed, which is comparable to Petersson et al. findings.22 However, it should be noted that all patients received chemotherapy (concurrently or before the CSI). There was no treatment interruption due to toxicity, which is in line with the dosimetric findings which show good OAR sparing in HT plans. Better heart sparing in 3DCRT compared to HT is due to the field arrangement in the conventional technique. The additional factor that may play a role is patient's position (prone in this technique) that increases the distance between the spine and the heart. Similar phenomenon could be assumed for the intestines. On the other hand, HT spares the kidneys to a greater extent than 3DCRT, which is important to note as majority of CSI patients received or will receive nephrotoxic CTx.31
Last but not least, the CSI is a challenge not only for medical staff but for the patients as well as the dose delivery per fraction can take more than 20 min plus additional time for positioning and set-up verification.21 In this case, for most of the patients a supine position seems superior to prone, this constituting an additional argument for using tomotherapy for CSI.32
6. Conclusions
Helical tomotherapy is feasible for CSI. In comparison with 3DCRT, it improves PTV coverage. HT reduces high dose volumes of organs at risk, but delivers larger amount of lower doses to normal tissues. This method requires further studies to establish correlations of dosimetric findings with clinical outcomes, especially with regard to acute and late sequelae of treatment. The long-term objective of the study is to follow up the patients in order to evaluate late adverse effects. Nevertheless, since the HT treatment plans were generally superior to 3DCRT plans, tomotherapy allows to avoid field junctions and patients can find it easier to stay still during irradiation, we have decided to give up additional CT-imaging in prone position in the future.
Conflict of interest
None declared.
Financial disclosure
This study was supported by the Greater Poland Cancer Centre (grant no. 1 dated 20 May 2009).
Acknowledgement
The authors thank the RTT team for excellent technical assistance.
References
- 1.Louis D.N., Ohgaki H., Wiestler O.D. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siker L., Donahne B.R., Vogelbaum M.A., Tome W.A., Gilbert M.R., Mehta M.P. Central nervous system tumors. In: Halperin E.C., Perez C.A., Brady L.W., Wazer D.E., Freeman C., Prosnitz L.R., editors. Perez and Brady's principles and practice of radiation oncology. 5th ed. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia: 2008. [Google Scholar]
- 3.Chojnacka M., Skowronska-Gardas A., Morawska-Kaczynska M., Zygmuntowicz-Pietka A., Pedziwiatr K., Semaniak A. Craniospinal radiotherapy in children: electron- or photon-based technique of spinal irradiation. Rep Pract Oncol Radiother. 2010;15:21–24. doi: 10.1016/j.rpor.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slampa P., Zitterbart K., Dusek L. Craniospinal irradiation of medulloblastoma in the supine position. Rep Pract Oncol Radiother. 2006;11:265–272. [Google Scholar]
- 5.Meira Montenegro B., Alfaya Virzi A., Lamas Lorenzo A. Verification of positioning using a new immobilisation system for craniospinal paediatric treatment. Rep Pract Oncol Radiother. 2013;18:S384. [Google Scholar]
- 6.Lee Y.K., Brooks C.J., Bedford J.L., Warrington A.P., Saran F.H. Development and evaluation of multiple isocentric volumetric modulated arc therapy technique for craniospinal axis radiotherapy planning. Int J Radiat Oncol Biol Phys. 2012;82:1006–1012. doi: 10.1016/j.ijrobp.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 7.Luque Japon L., Lloret Saez-bravo M., Cabrera Diaz-Saavedra R., Martin Oliva R., Lara Jimenez P. Feasibility of craniospinal irradiation in supine position for medulloblastoma in children population. Comparison of different therapeutic dosimetric data. Rep Pract Oncol Radiother. 2013;18:S294. [Google Scholar]
- 8.Carrie C., Alapetite C., Mere P. Quality control of radiotherapeutic treatment of medulloblastoma in a multicentric study: the contribution of radiotherapy technique to tumour relapse. The French Medulloblastoma Group. Radiother Oncol. 1992;24:77–81. doi: 10.1016/0167-8140(92)90282-y. [DOI] [PubMed] [Google Scholar]
- 9.Carrie C., Hoffstetter S., Gomez F. Impact of targeting deviations on outcome in medulloblastoma: study of the French Society of Pediatric Oncology (SFOP) Int J Radiat Oncol Biol Phys. 1999;45:435–439. doi: 10.1016/s0360-3016(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 10.Timmermann B., Kortmann R., Kuhl J. Role of radiotherapy in the treatment of supratentorial primitive neuroectodermal tumors in childhood: results of the prospective German brain tumor trials HIT 88/89 and 91. J Clin Oncol. 2002;20:842–849. doi: 10.1200/JCO.2002.20.3.842. [DOI] [PubMed] [Google Scholar]
- 11.Mackie T.R., Holmes T., Swerdloff S. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Med Phys. 1993;20:1709–1719. doi: 10.1118/1.596958. [DOI] [PubMed] [Google Scholar]
- 12.Bauman G., Yartsev S., Coad T., Fisher B., Kron T. Helical tomotherapy for craniospinal radiation. Br J Radiol. 2005;78:548–552. doi: 10.1259/bjr/53491625. [DOI] [PubMed] [Google Scholar]
- 13.Penagaricano J.A., Papanikolaou N., Yan Y., Youssef E., Ratanatharathorn V. Feasibility of cranio-spinal axis radiation with the Hi-Art tomotherapy system. Radiother Oncol. 2005;76:72–78. doi: 10.1016/j.radonc.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Penagaricano J.A., Shi C., Ratanatharathorn V. Evaluation of integral dose in cranio-spinal axis (CSA) irradiation with conventional and helical delivery. Technol Cancer Res Treat. 2005;4:683–689. doi: 10.1177/153303460500400613. [DOI] [PubMed] [Google Scholar]
- 15.Penagaricano J.A., Yan Y., Corry P., Moros E., Ratanatharathorn V. Retrospective evaluation of pediatric cranio-spinal axis irradiation plans with the Hi-ART tomotherapy system. Technol Cancer Res Treat. 2007;6:355–360. doi: 10.1177/153303460700600413. [DOI] [PubMed] [Google Scholar]
- 16.Kunos C.A., Dobbins D.C., Kulasekere R., Latimer B., Kinsella T.J. Comparison of helical tomotherapy versus conventional radiation to deliver craniospinal radiation. Technol Cancer Res Treat. 2008;7:227–233. doi: 10.1177/153303460800700308. [DOI] [PubMed] [Google Scholar]
- 17.Penagaricano J., Moros E., Corry P., Saylors R., Ratanatharathorn V. Pediatric craniospinal axis irradiation with helical tomotherapy: patient outcome and lack of acute pulmonary toxicity. Int J Radiat Oncol Biol Phys. 2009;75:1155–1161. doi: 10.1016/j.ijrobp.2008.12.083. [DOI] [PubMed] [Google Scholar]
- 18.Parker W., Brodeur M., Roberge D., Freeman C. Standard and nonstandard craniospinal radiotherapy using helical TomoTherapy. Int J Radiat Oncol Biol Phys. 2010;77:926–931. doi: 10.1016/j.ijrobp.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Mascarin M., Giugliano F.M., Coassin E. Helical tomotherapy in children and adolescents: dosimetric comparisons, opportunities and issues. Cancers (Basel) 2011;3:3972–3990. doi: 10.3390/cancers3043972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugie C., Shibamoto Y., Ayakawa S. Craniospinal irradiation using helical tomotherapy: evaluation of acute toxicity and dose distribution. Technol Cancer Res Treat. 2011;10:187–195. doi: 10.7785/tcrt.2012.500194. [DOI] [PubMed] [Google Scholar]
- 21.Piotrowski T., Skorska M., Jodda A. Tomotherapy – a different way of dose delivery in radiotherapy. Contemp Oncol (Pozn) 2012;16:16–25. doi: 10.5114/wo.2012.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersson K., Gebre-Medhin M., Ceberg C. Haematological toxicity in adult patients receiving craniospinal irradiation – indication of a dose-bath effect. Radiother Oncol. 2014;111(1):47–51. doi: 10.1016/j.radonc.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Piotrowski T., Czajka E., Bak B. Tomotherapy: implications on daily workload and scheduling patients based on three years’ institutional experience. Technol Cancer Res Treat. 2014;13:233–242. doi: 10.7785/tcrt.2012.500374. [DOI] [PubMed] [Google Scholar]
- 24.ICRU Report 50 . International Commission on Radiation Units and Measurements; Bethesda, MD: 1993. Prescribing, recording, and reporting photon beam therapy. [Google Scholar]
- 25.ICRU Report 62 . 1999. Prescribing, recording, and reporting photon beam therapy (supplement to ICRU report 50). Bethesda, MD. [Google Scholar]
- 26.Wu Q., Mohan R., Morris M., Lauve A., Schmidt-Ullrich R. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas. I: dosimetric results. Int J Radiat Oncol Biol Phys. 2003;56:573–585. doi: 10.1016/s0360-3016(02)04617-5. [DOI] [PubMed] [Google Scholar]
- 27.CBTRUS Statistical Report . Oxford University Press; 2012. Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Comprehensive Cancer Network (NCCN). NCCN guidelines version 2.2013. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 29.Bolek-Górska M., Korab-Chrzanowska E., Czepko R., Betlej M., Adamek D., Pawlęga J. Medulloblastoma in adults. A case presentation and review of the literature. Rep Pract Oncol Radiother. 2006;11:49–54. [Google Scholar]
- 30.Lai S., Wang C., Chen Y. Medulloblastoma in adults. Treatment outcome, relapse patterns, and prognostic factors. Strahlenther Onkol. 2012;188:878–886. doi: 10.1007/s00066-012-0168-2. [DOI] [PubMed] [Google Scholar]
- 31.Chojnacka M., Skowronska-Gardas A., Pedziwiatr K., Morawska-Kaczynska M., Perek M., Perek D. Reirradiation of relapsed brain tumors in children. Rep Pract Oncol Radiother. 2011;17:32–37. doi: 10.1016/j.rpor.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hideghety K., Cserhati A., Nagy Z. A prospective study of supine versus prone positioning and whole-body thermoplastic mask fixation for craniospinal radiotherapy in adult patients. Radiother Oncol. 2012;102:214–218. doi: 10.1016/j.radonc.2011.07.003. [DOI] [PubMed] [Google Scholar]


