Abstract
Background/objectives
Increased endogenous glucose production is a hallmark of type 2 diabetes. Evidence from animal models has suggested that a likely cause of this is increased mRNA expression of glucose 6-phosphatase and phosphoenolpyruvate carboxykinase (encoded by G6PC, PCK1 and PCK2). But another contributing factor may be decreased liver glucokinase (encoded by GCK).
Methods
We examined expression of these enzymes in liver biopsies from 12 nondiabetic and 28 diabetic individuals. Diabetic patients were further separated into those with HbA1c lower or higher than 7.0.
Results
In diabetic subjects with HbA1c > 7.0, we found that gluconeogenic enzymes were expressed normally, but GCK was suppressed more than 60%. Moreover, HbA1c and fasting glucose were negatively correlated with GCK, but showed no correlation with G6PC, PCK1, or PCK2.
Conclusion
These findings suggest an underlying dysregulation of hepatic GCK expression during frank diabetes, which has implications for the therapeutic use of glucokinase activators in this population.
Keywords: Humans, Diabetes, Glucokinase
1. Introduction
A characteristic feature of type 2 diabetes is increased endogenous glucose production, largely due to increased hepatic glucose production (HGP) [1–4]. Two rate-limiting enzymes of HGP are glucose 6-phosphatase catalytic subunit (encoded by G6PC) and phosphoenolpyruvate carboxykinase (encoded by PCK1 and PCK2). As the expression of these enzymes is suppressed by insulin, it has been widely held that patients with type 2 diabetes (T2D) would have increased expression of G6PC and PCK, due to hepatic insulin resistance. However, it has been challenging to correlate expression of these enzymes with diabetes or glycemia in humans [5,6].
Another determinant of hepatic glucose homeostasis is glucokinase (encoded by GCK). G6PC and GCK act in opposition to regulate the intracellular levels of free glucose; thus, the coordinated regulation of these two enzymes ultimately determines the gradient and flux of glucose into or out of the hepatocyte [7]. An increased ratio of G6PC/GCK, as occurs during fasting, causes glucose efflux to the bloodstream, whereas a decreased ratio causes increased influx.
Previous work has indicated that GCK activity is decreased in type 2 diabetes [8–12]. Although mutations in the GCK gene cause maturity onset diabetes of the young type 2 (MODY2), GCK mutations are not found in the etiology of classical T2D. Thus, the decrease in GCK activity is likely due to transcriptional or posttranslational effects. However, the expression of liver GCK during T2D has not yet been established.
In this study, we investigated the contribution of GCK, G6PC, and PCK expression to glycemia in diabetes. We examined liver tissue from 40 obese subjects: 12 had normal glucose tolerance (NGT) and 28 had type 2 diabetes.
2. Materials and methods
2.1. Subjects and liver biopsies
A liver biopsy was obtained during surgery in 28 type 2 diabetic and 12 nondiabetic subjects undergoing bariaric surgery. The liver samples were collected in RNA-Later (Ambion Inc., Applied Biosystems, Austin, TX, USA), and stored at −20 °C for total RNA extraction. Before surgery in each subject, after an overnight (12–14 h) fast, peripheral blood samples were obtained for determination of the routine blood chemistry plasma glucose, insulin and HbA1c concentrations. The diabetic patients were asked to discontinue oral antidiabetic agents (metformin ± sulfonylureas) 48–72 h before the study.
The protocol was approved by the local ethics committee. The nature and purpose of the study were carefully explained to all participants before they provided written consent to participate.
2.2. Analytical procedures
Plasma glucose was measured by the glucose-oxidase technique (Analox GM-9), plasma insulin by electro-chemiluminescence (on a COBAS e411 instrument, Roche, Indianapolis, USA).
2.3. Gene expression
RNA was extracted using Trizol (Life Technologies), cDNA was synthesized using reverse transcriptase (Applied Biosystems), and quantitative PCR was performed using SyBr Green (BioRad).
2.4. Statistical analyses
Data are given as mean ± SEM or median and [interquartile range]. Group differences were analyzed by Kruskall-Wallis and Mann–Whitney tests. P value <0.05 was considered statistically significant.
3. Results
3.1. Patient characteristics
The anthropomorphic and metabolic characteristics of the patients are shown in Table 1. T2D patients for whom HbA1c was available were further separated into two groups, those with HbA1c <7.0% (53 mmol/mol), and those >7.0%. Diabetic patients were older than NGT subjects, and the high HbA1c group had lower average BMI than the other two groups.
Table 1.
Clinical characteristics.
| NGT | All T2D | T2D HbA1c <7.0% |
T2D HbA1c >7.0% |
|
|---|---|---|---|---|
| N (F/M) | 12 (12/0) | 28 (7/21) | 7 (3/4) | 17 (3/14) |
| Age (years) | 39.5 ± 2.5 | 51.2 ± 1.3*** | 48.4 ± 1.9** | 50.9 ± 1.9** |
| BMI (kg/m2) | 50.4 ± 2.1 | 37.9 ± 1.6*** | 45.8 ± 1.4 | 36.9 ± 2.0***,§ |
| FPG (mmol/L) | 5.24 ± 0.21 | 10.46 ± 0.74*** | 6.5 ± 0.5* | 11.9 ± 0.95***,§§ |
| FPI (pmol/L) | 129.9 [45.5] | 119.1 [95.6] | 168.6 [89.3] | 111.6 [108.6] |
| HbA1C (%)a | 5.8 ± 0.2 | 8.4 ± 0.37** | 6.3 ± 0.2* | 9.3 ± 0.3**,§§§ |
*P < 0.05, **P < 0.01, ***P < 0.001 versus NGT and §P < 0.05, §§P < 0.01, §§§P < 0.001 vs T2D HbA1c<7.0%, by Mann–Whitney test.
Available for 5 out of 12 NGT subjects and 24 out of 28 T2D patients.
3.2. Glucokinase expression is reduced in T2D
In T2D patients overall, we identified a 43% reduction in GCK expression relative to NGT (0.57 ± 0.09 vs 1.00 ± 0.17, p = 0.028). However, we saw no change in expression of G6PC, PCK1, or PCK2 (data not shown). When diabetic subjects were subdivided into HbA1c categories, we found that the high HbA1c group showed a substantial reduction in GCK compared to the NGT or low HbA1c groups (Figure 1A). There were no significant changes in G6PC, PCK1, or PCK2 expression among the three groups (Figure 1A). We also examined the glucose 6-phosphate transporter (encoded by SLC37A4), which was also not different between groups (Figure 1A).
Figure 1.
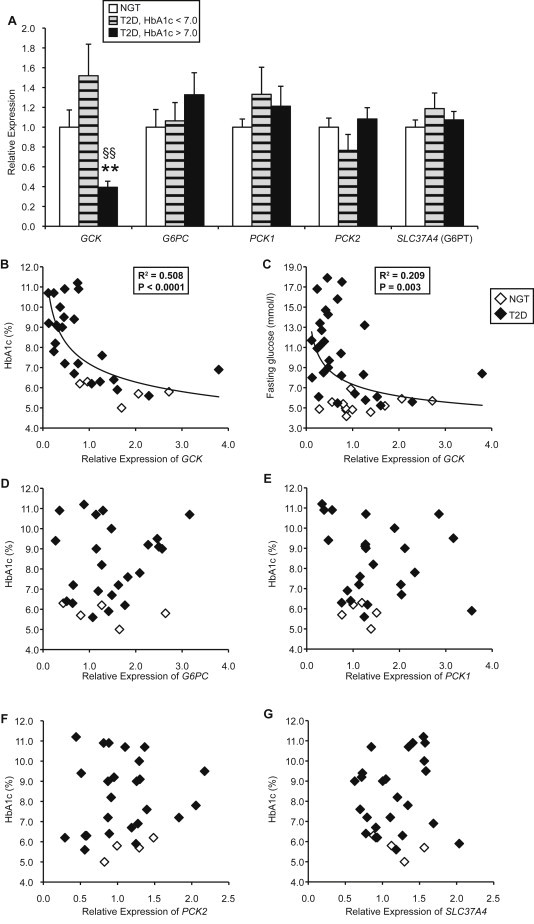
Liver biopsies. (A) Gene expression in subjects with normal glucose tolerance (NGT) (n = 12) or type 2 diabetes with HbA1c < 7.0% (n = 7) or HbA1c > 7.0% (n = 17). **p < 0.01 vs NGT and §§p < 0.01 vs T2D HbA1c < 7.0%, by Mann–Whitney test. (B–G) Correlations between gene expression and HbA1c or fasting glucose levels.
3.3. Glucokinase expression is negatively correlated with HbA1c
Next we examined the association of gene expression with measures of glycemia. We found that GCK was significantly negatively correlated with HbA1c and fasting glucose (Figure 1B–C). In contrast, G6PC, PCK1, PCK2, and SLC37A4 were not associated with HbA1c (Figure 1D–G) or fasting glucose (data not shown). Overall, these data suggest that progressive worsening of glycemia in type 2 diabetes is significantly associated with suppression of GCK, but not with expression of other gluconeogenic enzymes.
4. Discussion
This study aimed to investigate contributors to the increase in HGP during diabetes. Consistent with prior reports, we find that liver expression of gluconeogenic enzymes alone cannot explain this phenotype [5,6]. On the other hand, our data indicate that suppression of hepatic GCK in diabetes patients: (i) explains the decreased GCK activity observed in previous studies [8–12], and (ii) contributes to hyperglycemia quantitatively. The fact that defective hepatic GCK is sufficient to impair glycogen synthesis and increase gluconeogenesis was previously established in studies of liver metabolism in MODY2 patients [13]. Moreover, it has been shown that restoration of hepatic GCK activity in Zucker diabetic fatty rats causes normalization of plasma glucose and suppression of endogenous glucose production [14].
GCK is regulated by posttranslational and transcriptional mechanisms [7]. Posttranslational regulation by glucose occurs through the glucokinase regulatory protein, which binds to GCK and causes its nuclear sequestration and protein stabilization. Dissociation of the two proteins occurs at high glucose concentrations, causing cytoplasmic translocation and accelerated degradation of GCK [7,15]. One of the limitations of our study was the lack of sufficient tissue to perform immunoblots. However, we found compelling evidence that frank diabetes is associated with a disruption in the transcriptional control of GCK, which is carried out by insulin [16]. Major mediators of insulin's effect on GCK are the FOXO transcription factors. FOXOs are known for their role in promoting HGP during fasting [17]. Under normal conditions, FOXOs are inactivated by insulin, through AKT-mediated phosphorylation and nuclear exclusion. Thus, a widely held explanation for excessive glucose production during diabetes is inappropriate activation of the hepatic FOXO pathway, due to insulin resistance. Two critical aspects of how FOXO promotes glucose production are by promoting G6PC [17] and suppressing GCK [18–21]. This suggests that activated FOXOs may be responsible for excessive GCK suppression in diabetes. Consistent with this possibility, FOXO target IGFBP1 was significantly elevated in the high HbA1c patients (data not shown). How, then, can we reconcile the fact that G6PC was not also elevated? One possible explanation is that FOXOs' effect on GCK is more potent than its effect on G6PC, as we have previously observed in mice [21]. It is also possible that posttranslational modifications of FOXO that are induced by hyperglycemia, such as acetylation, affect some targets more than others [22,23].
In addition to its role in glucose homeostasis, hepatic GCK also plays a critical role in promoting de novo lipogenesis (DNL), a fact that has hindered the development of glucokinase activators as diabetes therapy [24]. Indeed, previous work has shown that liver GCK expression is positively associated with liver triglyceride content and a marker of DNL in humans [25]. Based on these findings, suppression of GCK – as we observed in our severely diabetic patients – might be expected to reduce lipogenic flux. This may seem paradoxical, as diabetic patients typically demonstrate hypertriglyceridemia. However, we observed no correlation of GCK to plasma triglyceride levels in our subjects (data not shown). A potential explanation is that DNL contributes to only a portion of circulating triglyceride levels [26–28], the major fraction originating from reesterification of free fatty acids; the latter substrate circulates in increased amounts in type 2 diabetic patients due to insulin resistance of lipolysis [29,30].
There has been a long-standing interest in developing antidiabetic glucokinase activators [24]. Given the narrow therapeutic window of these molecules, it may be important to increase our understanding of GCK dysregulation in the progression of T2D. This may be particularly valuable in designing individualized therapeutic regimens [4].
Acknowledgments
R.A.H. designed and implemented experiments, analyzed data, and wrote the manuscript. S.C., B.A., M.N., and M.A. performed experiments, contributed to discussions, and edited the manuscript. E.F. designed experiment, analyzed data, and edited the manuscript. R.A.H. and E.F. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
E.F. has served as a speaker and consultant for Boehringer Ingelheim, Merck, Sanofi, Eli Lilly and Company, Johnson & Johnson, Astellas, Daiichi Sankyo, Bristol Myers Squibb/AstraZeneca, and Novartis. No other potential conflicts of interest relevant to this article were reported.
The authors would like to acknowledge the technical assistance of J. Lee. This work was supported by the U.S. National Institute of Health grant HL111206 (to R.A.H.) the Italian Ministry for University and Research (MIUR 2010329EKE).
Conflict of interest
None declared.
References
- 1.Boden G., Chen X., Stein T.P. Gluconeogenesis in moderately and severely hyperglycemic patients with type 2 diabetes mellitus. American Journal of Physiology. Endocrinology and Metabolism. 2001;280:E23–E30. doi: 10.1152/ajpendo.2001.280.1.E23. [DOI] [PubMed] [Google Scholar]
- 2.Basu R., Barosa C., Jones J., Dube S., Carter R., Basu A. Pathogenesis of prediabetes: role of the liver in isolated fasting hyperglycemia and combined fasting and postprandial hyperglycemia. The Journal of Clinical Endocrinology and Metabolism. 2013;98:E409–E417. doi: 10.1210/jc.2012-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gastaldelli A., Toschi E., Pettiti M., Frascerra S., Quinones-Galvan A., Sironi A.M. Effect of physiological hyperinsulinemia on gluconeogenesis in nondiabetic subjects and in type 2 diabetic patients. Diabetes. 2001;50:1807–1812. doi: 10.2337/diabetes.50.8.1807. [DOI] [PubMed] [Google Scholar]
- 4.Rizza R.A. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes. 2010;59:2697–2707. doi: 10.2337/db10-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konopelska S., Kienitz T., Quinkler M. Downregulation of hepatic glucose-6-phosphatase-alpha in patients with hepatic steatosis. Obesity (Silver Spring) 2011;19:2322–2326. doi: 10.1038/oby.2011.118. [DOI] [PubMed] [Google Scholar]
- 6.Samuel V.T., Beddow S.A., Iwasaki T., Zhang X.M., Chu X., Still C.D. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12121–12126. doi: 10.1073/pnas.0812547106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochemical Journal. 2008;414:1–18. doi: 10.1042/BJ20080595. [DOI] [PubMed] [Google Scholar]
- 8.Caro J.F., Triester S., Patel V.K., Tapscott E.B., Frazier N.L., Dohm G.L. Liver glucokinase: decreased activity in patients with type II diabetes. Hormone and Metabolic Research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1995;27:19–22. doi: 10.1055/s-2007-979899. [DOI] [PubMed] [Google Scholar]
- 9.Clore J.N., Stillman J., Sugerman H. Glucose-6-phosphatase flux in vitro is increased in type 2 diabetes. Diabetes. 2000;49:969–974. doi: 10.2337/diabetes.49.6.969. [DOI] [PubMed] [Google Scholar]
- 10.Ferris S.A. Hepatic glucokinase activity in human diabetics and nondiabetics. Metabolism. 1964;13:1478–1481. doi: 10.1016/0026-0495(64)90042-3. [DOI] [PubMed] [Google Scholar]
- 11.Basu A., Basu R., Shah P., Vella A., Johnson C.M., Jensen M. Type 2 diabetes impairs splanchnic uptake of glucose but does not alter intestinal glucose absorption during enteral glucose feeding: additional evidence for a defect in hepatic glucokinase activity. Diabetes. 2001;50:1351–1362. doi: 10.2337/diabetes.50.6.1351. [DOI] [PubMed] [Google Scholar]
- 12.Basu A., Basu R., Shah P., Vella A., Johnson C.M., Nair K.S. Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes. 2000;49:272–283. doi: 10.2337/diabetes.49.2.272. [DOI] [PubMed] [Google Scholar]
- 13.Velho G., Petersen K.F., Perseghin G., Hwang J.H., Rothman D.L., Pueyo M.E. Impaired hepatic glycogen synthesis in glucokinase-deficient (MODY-2) subjects. The Journal of Clinical Investigation. 1996;98:1755–1761. doi: 10.1172/JCI118974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres T.P., Catlin R.L., Chan R., Fujimoto Y., Sasaki N., Printz R.L. Restoration of hepatic glucokinase expression corrects hepatic glucose flux and normalizes plasma glucose in zucker diabetic fatty rats. Diabetes. 2009;58:78–86. doi: 10.2337/db08-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueta K., O'Brien T.P., McCoy G.A., Kim K., Healey E.C., Farmer T.D. Glucotoxicity targets hepatic glucokinase in Zucker diabetic fatty rats, a model of type 2 diabetes associated with obesity. American Journal of Physiology. Endocrinology and Metabolism. 2014;306:E1225–E1238. doi: 10.1152/ajpendo.00507.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iynedjian P.B., Marie S., Gjinovci A., Genin B., Deng S.P., Buhler L. Glucokinase and cytosolic phosphoenolpyruvate carboxykinase (GTP) in the human liver. Regulation of gene expression in cultured hepatocytes. The Journal of Clinical Investigation. 1995;95:1966–1973. doi: 10.1172/JCI117880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin H.V., Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metabolism. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirota K., Sakamaki J., Ishida J., Shimamoto Y., Nishihara S., Kodama N. A combination of HNF-4 and Foxo1 is required for reciprocal transcriptional regulation of glucokinase and glucose-6-phosphatase genes in response to fasting and feeding. The Journal of Biological Chemistry. 2008;283:32432–32441. doi: 10.1074/jbc.M806179200. [DOI] [PubMed] [Google Scholar]
- 19.Xiong X., Tao R., DePinho R.A., Dong X.C. Deletion of hepatic FoxO1/3/4 genes in mice significantly impacts on glucose metabolism through downregulation of gluconeogenesis and upregulation of glycolysis. PLoS One. 2013;8:e74340. doi: 10.1371/journal.pone.0074340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W., Patil S., Chauhan B., Guo S., Powell D.R., Le J. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. The Journal of Biological Chemistry. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- 21.Haeusler R.A., Hartil K., Vaitheesvaran B., Arrieta-Cruz I., Knight C.M., Cook J.R. Integrated control of hepatic lipogenesis versus glucose production requires FoxO transcription factors. Nature Communications. 2014;5:5190. doi: 10.1038/ncomms6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banks A.S., Kim-Muller J.Y., Mastracci T.L., Kofler N.M., Qiang L., Haeusler R.A. Dissociation of the glucose and lipid regulatory functions of FoxO1 by targeted knockin of acetylation-defective alleles in mice. Cell Metabolism. 2011;14:587–597. doi: 10.1016/j.cmet.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haeusler R.A., Han S., Accili D. Hepatic FoxO1 ablation exacerbates lipid abnormalities during hyperglycemia. The Journal of Biological Chemistry. 2010;285:26861–26868. doi: 10.1074/jbc.M110.134023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matschinsky F.M. GKAs for diabetes therapy: why no clinically useful drug after two decades of trying? Trends in Pharmacological Sciences. 2013;34:90–99. doi: 10.1016/j.tips.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Peter A., Stefan N., Cegan A., Walenta M., Wagner S., Konigsrainer A. Hepatic glucokinase expression is associated with lipogenesis and fatty liver in humans. The Journal of Clinical Endocrinology and Metabolism. 2011;96:E1126–E1130. doi: 10.1210/jc.2010-2017. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz J.M., Linfoot P., Dare D., Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. American Journal of Clinical Nutrition. 2003;77:43–50. doi: 10.1093/ajcn/77.1.43. [DOI] [PubMed] [Google Scholar]
- 27.Vedala A., Wang W., Neese R.A., Christiansen M.P., Hellerstein M.K. Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet, and de novo lipogenesis in humans. The Journal of Lipid Research. 2006;47:2562–2574. doi: 10.1194/jlr.M600200-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Barrows B.R., Parks E.J. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. The Journal of Clinical Endocrinology and Metabolism. 2006;91:1446–1452. doi: 10.1210/jc.2005-1709. [DOI] [PubMed] [Google Scholar]
- 29.Groop L.C., Bonadonna R.C., DelPrato S., Ratheiser K., Zyck K., Ferrannini E. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. Journal of Clinical Investigation. 1989;84:205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bevilacqua S., Buzzigoli G., Bonadonna R., Brandi L.S., Oleggini M., Boni C. Operation of Randle's cycle in patients with NIDDM. Diabetes. 1990;39:383–389. doi: 10.2337/diab.39.3.383. [DOI] [PubMed] [Google Scholar]


